Abstract
The subclass of catalytic RNAs termed ribozymes cleave specific target RNA sequences in vitro. Only circumstantial evidence supports the idea that ribozymes may also act in vivo. In this study, ribozymes with a hammerhead motif directed against a target sequence within the mRNA of the neomycin phosphotransferase gene (npt) were embedded into a functional chimeric gene. Two genes, one containing the ribozyme and the other producing the target, were cotransfected into plant protoplasts. Following in vivo expression, a predefined cleavage product of the target mRNA was detected by ribonuclease protection. Expression of both the ribozyme gene and the target gene was driven by the CaMV 35S promoter. Concomitant with the endonucleolytic cleavage of the target mRNA, a complete reduction of NPT activity was observed. An A to G substitution within the ribozyme domain completely inactivates ribozyme-mediated hydrolysis but still shows a reduction in NPT activity, albeit less pronounced. Therefore, the reduction of NPT activity produced by the active ribozyme is best explained by both hydrolytic cleavage and an antisense effect. However, the mutant ribozyme--target complex was more stable than the wildtype ribozyme--target complex. This may result in an overestimation of the antisense effect contributing to the overall reduction of gene expression.
Full text
PDF
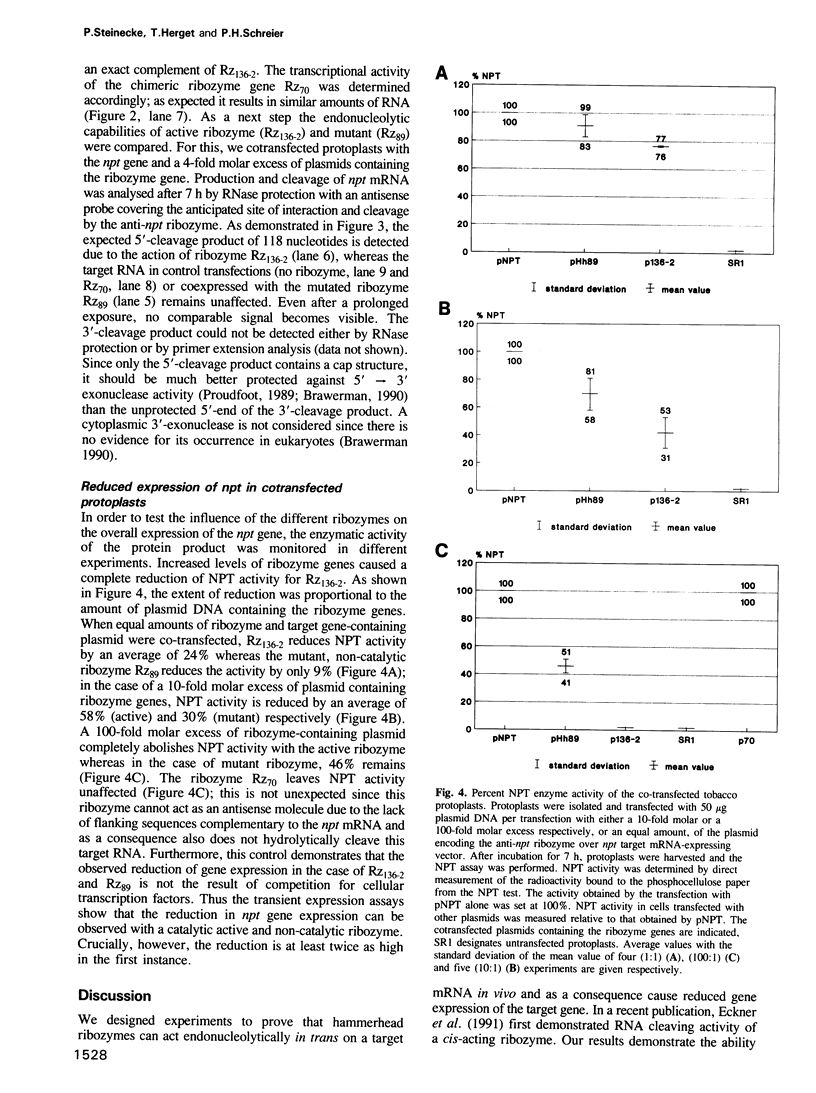


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Mechanisms of mRNA decay. Trends Biotechnol. 1990 Jul;8(7):171–174. doi: 10.1016/0167-7799(90)90167-v. [DOI] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Specific gene suppression by engineered ribozymes in monkey cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9139–9143. doi: 10.1073/pnas.86.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Cheong C., Varani G., Tinoco I., Jr Solution structure of an unusually stable RNA hairpin, 5'GGAC(UUCG)GUCC. Nature. 1990 Aug 16;346(6285):680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Schaffner G., Birnstiel M. L. Ribozyme, antisense RNA, and antisense DNA inhibition of U7 small nuclear ribonucleoprotein-mediated histone pre-mRNA processing in vitro. Mol Cell Biol. 1989 Oct;9(10):4479–4487. doi: 10.1128/mcb.9.10.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M. The in vivo application of ribozymes. Trends Biotechnol. 1990 Jul;8(7):174–178. doi: 10.1016/0167-7799(90)90168-w. [DOI] [PubMed] [Google Scholar]
- Davies C., Haseloff J., Symons R. H. Structure, self-cleavage, and replication of two viroid-like satellite RNAs (virusoids) of subterranean clover mottle virus. Virology. 1990 Jul;177(1):216–224. doi: 10.1016/0042-6822(90)90475-7. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R., Ellmeier W., Birnstiel M. L. Mature mRNA 3' end formation stimulates RNA export from the nucleus. EMBO J. 1991 Nov;10(11):3513–3522. doi: 10.1002/j.1460-2075.1991.tb04915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz C. C., Herget T., Wolter F. P., Schell J., Schreier P. H. Reduced steady-state levels of rbcS mRNA in plants kept in the dark are due to differential degradation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4458–4462. doi: 10.1073/pnas.88.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Iwai S., Ohtsuka E. Cleavage of specific sites of RNA by designed ribozymes. FEBS Lett. 1988 Nov 7;239(2):285–288. doi: 10.1016/0014-5793(88)80935-9. [DOI] [PubMed] [Google Scholar]
- Koncz C., Martini N., Mayerhofer R., Koncz-Kalman Z., Körber H., Redei G. P., Schell J. High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8467–8471. doi: 10.1073/pnas.86.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. W., Hay R. T. Ribozymes that cleave potato leafroll virus RNA within the coat protein and polymerase genes. J Gen Virol. 1990 Oct;71(Pt 10):2257–2264. doi: 10.1099/0022-1317-71-10-2257. [DOI] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. How RNA polymerase II terminates transcription in higher eukaryotes. Trends Biochem Sci. 1989 Mar;14(3):105–110. doi: 10.1016/0968-0004(89)90132-1. [DOI] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Schaller H. Protein fusions with the kanamycin resistance gene from transposon Tn5. EMBO J. 1984 Dec 20;3(13):3317–3322. doi: 10.1002/j.1460-2075.1984.tb02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Stormo G. D., Uhlenbeck O. C. Sequence requirements of the hammerhead RNA self-cleavage reaction. Biochemistry. 1990 Nov 27;29(47):10695–10702. doi: 10.1021/bi00499a018. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Saxena S. K., Ackerman E. J. Ribozymes correctly cleave a model substrate and endogenous RNA in vivo. J Biol Chem. 1990 Oct 5;265(28):17106–17109. [PubMed] [Google Scholar]
- Schreier P. H., Seftor E. A., Schell J., Bohnert H. J. The use of nuclear-encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts. EMBO J. 1985 Jan;4(1):25–32. doi: 10.1002/j.1460-2075.1985.tb02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Drlica K. Prevention of human immunodeficiency virus type 1 integrase expression in Escherichia coli by a ribozyme. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7303–7307. doi: 10.1073/pnas.88.16.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Hofmann H., Förtsch J., Gross H. J., Randles J. W., Sänger H. L., Riesner D. Conformational transitions in viroids and virusoids: comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984 Dec;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Self-cleavage of RNA in the replication of small pathogens of plants and animals. Trends Biochem Sci. 1989 Nov;14(11):445–450. doi: 10.1016/0968-0004(89)90103-5. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. Tetraloops and RNA folding. Nature. 1990 Aug 16;346(6285):613–614. doi: 10.1038/346613a0. [DOI] [PubMed] [Google Scholar]
- Werntges H., Steger G., Riesner D., Fritz H. J. Mismatches in DNA double strands: thermodynamic parameters and their correlation to repair efficiencies. Nucleic Acids Res. 1986 May 12;14(9):3773–3790. doi: 10.1093/nar/14.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F. P., Fritz C. C., Willmitzer L., Schell J., Schreier P. H. rbcS genes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci U S A. 1988 Feb;85(3):846–850. doi: 10.1073/pnas.85.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]