Full text
PDF


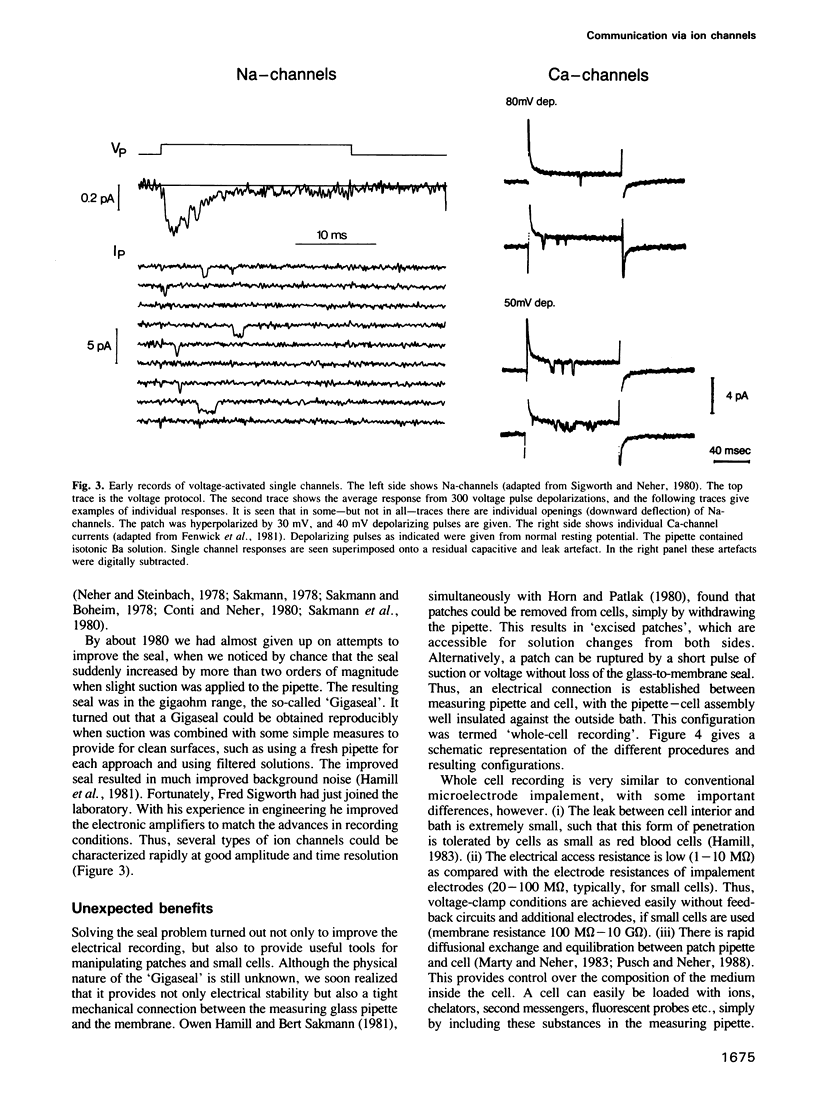
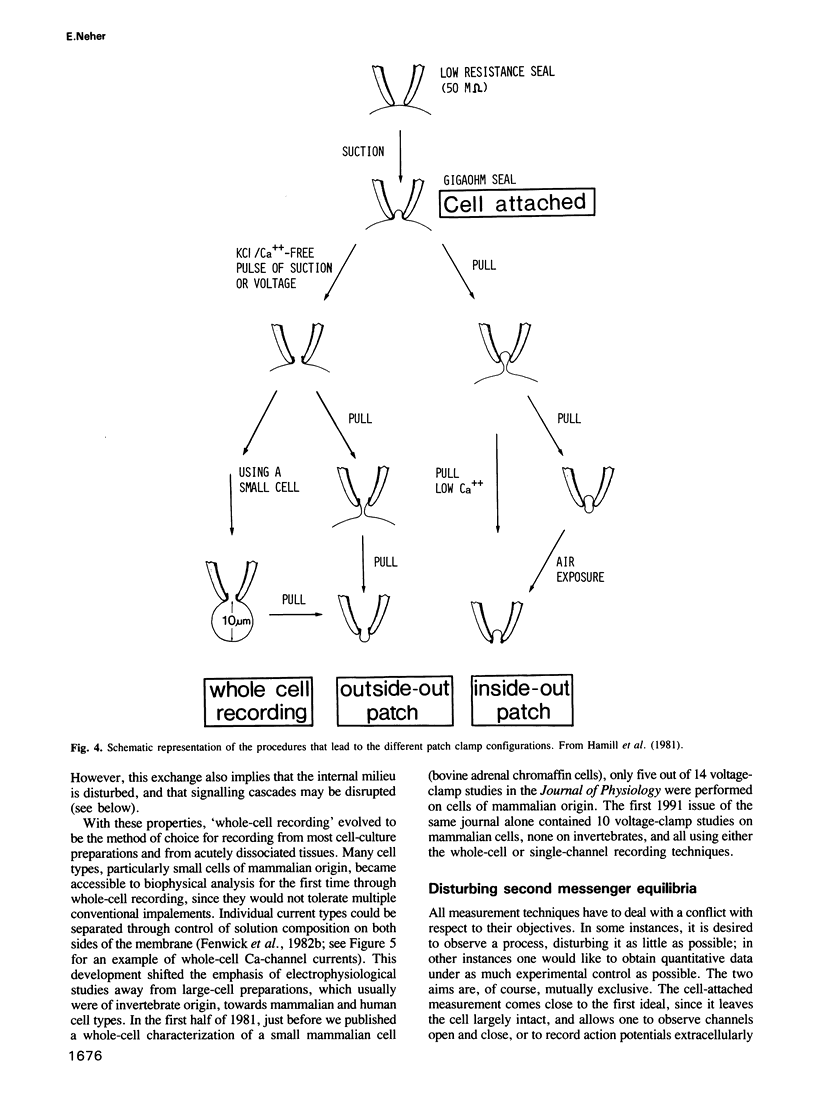
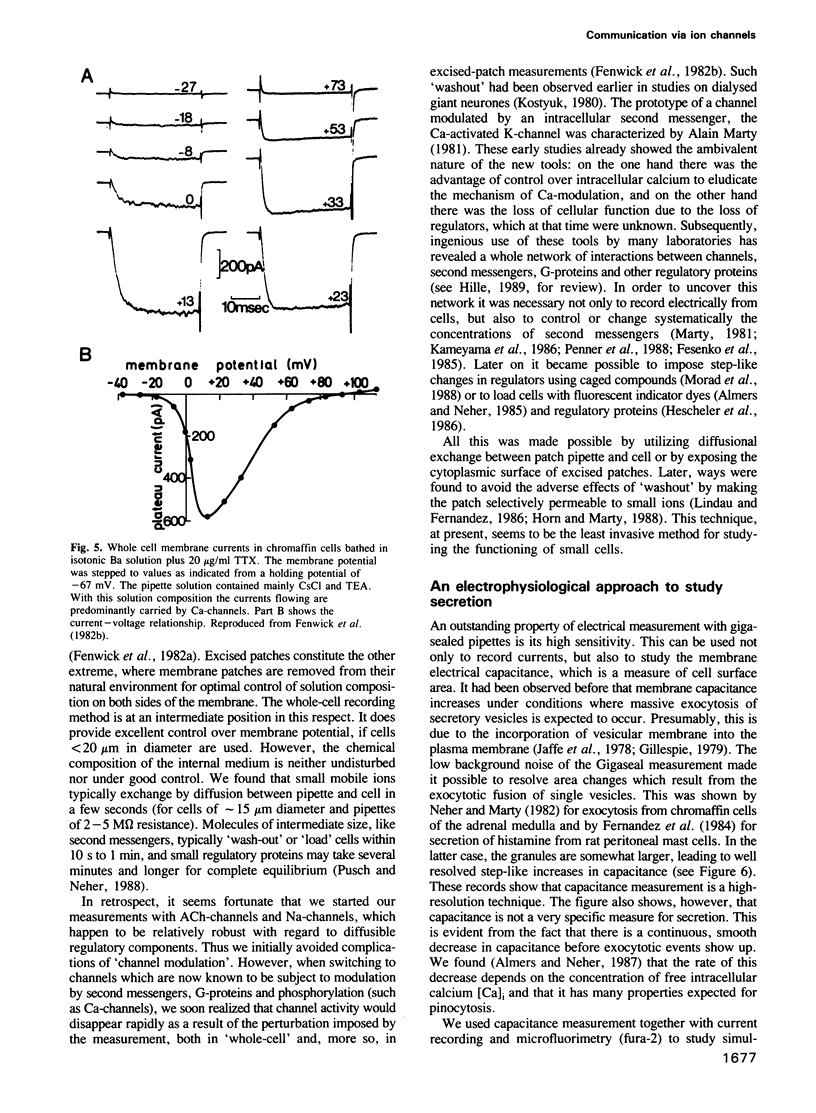
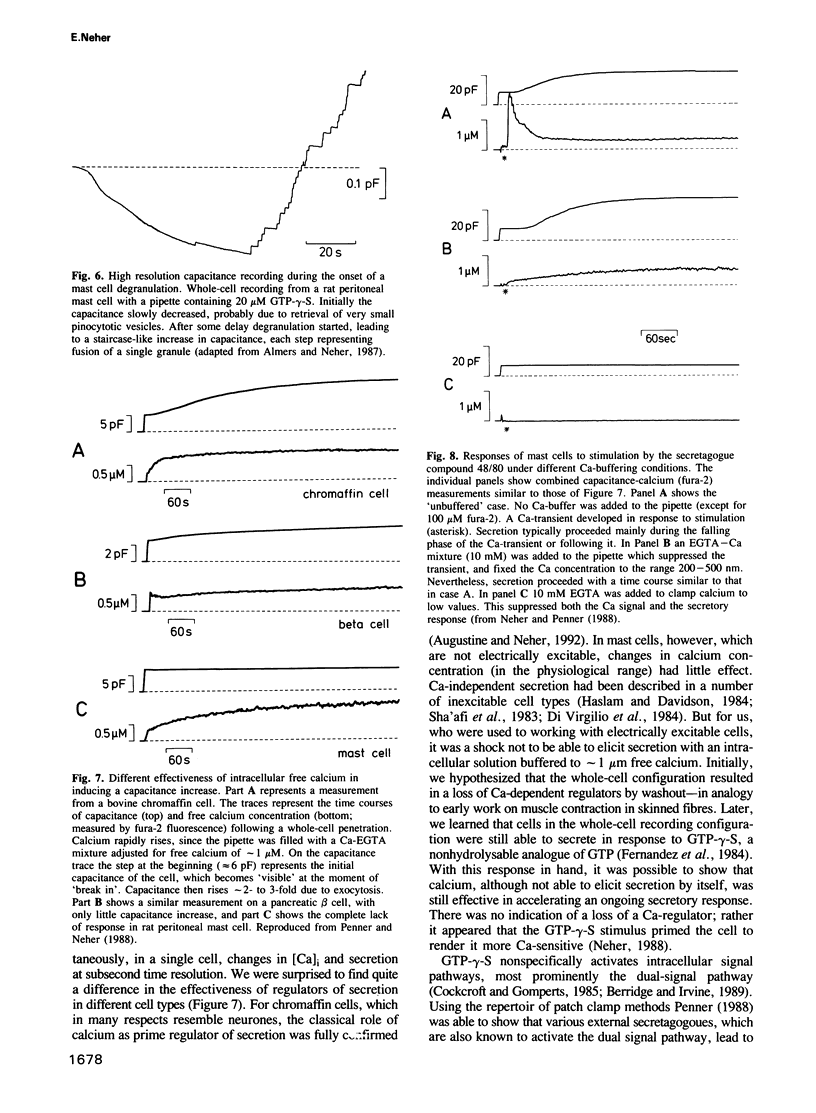

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J Physiol. 1987 May;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean R. C., Shepherd W. C., Chan H., Eichner J. Discrete conductance fluctuations in lipid bilayer protein membranes. J Gen Physiol. 1969 Jun;53(6):741–757. doi: 10.1085/jgp.53.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Fishman H. M. Relaxation spectra of potassium channel noise from squid axon membranes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):876–879. doi: 10.1073/pnas.70.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I. The effect of repetitive stimulation on the passive electrical properties of the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):293–306. doi: 10.1098/rspb.1979.0106. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Barrowman M. M., Cockcroft S. Dual role for guanine nucleotides in stimulus-secretion coupling. Fed Proc. 1986 Jun;45(7):2156–2161. [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Guanine nucleotides decrease the free [Ca2+] required for secretion of serotonin from permeabilized blood platelets. Evidence of a role for a GTP-binding protein in platelet activation. FEBS Lett. 1984 Aug 20;174(1):90–95. doi: 10.1016/0014-5793(84)81084-4. [DOI] [PubMed] [Google Scholar]
- Heiman A. S., Crews F. T. Characterization of the effects of phorbol esters on rat mast cell secretion. J Immunol. 1985 Jan;134(1):548–555. [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels in nerve membranes. Prog Biophys Mol Biol. 1970;21:1–32. doi: 10.1016/0079-6107(70)90022-2. [DOI] [PubMed] [Google Scholar]
- Hille B. The Sharpey-Schafer Lecture. Ionic channels: evolutionary origins and modern roles. Q J Exp Physiol. 1989 Nov;74(6):785–804. doi: 10.1113/expphysiol.1989.sp003349. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J. Single channel currents from excised patches of muscle membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6930–6934. doi: 10.1073/pnas.77.11.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A., Hagiwara S., Kado R. T. The time course of cortical vesicle fusion in sea urchin eggs observed as membrane capacitance changes. Dev Biol. 1978 Nov;67(1):243–248. doi: 10.1016/0012-1606(78)90314-7. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G. Calcium ionic channels in electrically excitable membrane. Neuroscience. 1980;5(6):945–959. doi: 10.1016/0306-4522(80)90178-5. [DOI] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. IgE-mediated degranulation of mast cells does not require opening of ion channels. Nature. 1986 Jan 9;319(6049):150–153. doi: 10.1038/319150a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Morad M., Davies N. W., Kaplan J. H., Lux H. D. Inactivation and block of calcium channels by photo-released Ca2+ in dorsal root ganglion neurons. Science. 1988 Aug 12;241(4867):842–844. doi: 10.1126/science.2457253. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Induced excitability in reconstituted cell membrane structure. J Theor Biol. 1963 May;4(3):268–280. doi: 10.1016/0022-5193(63)90006-7. [DOI] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Voltage clamp on Helix pomatia neuronal membrane; current measurement over a limited area of the soma surface. Pflugers Arch. 1969;311(3):272–277. doi: 10.1007/BF00590532. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. VII. Another voltage dependent process beside Ca entry controls the time course of phasic release. Pflugers Arch. 1986 Feb;406(2):121–130. doi: 10.1007/BF00586672. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E. The patch-clamp technique in the study of secretion. Trends Neurosci. 1989 Apr;12(4):159–163. doi: 10.1016/0166-2236(89)90059-3. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Sakmann B. Acetylcholine-induced ionic channels in rat skeletal muscle. Fed Proc. 1978 Oct;37(12):2654–2659. [PubMed] [Google Scholar]
- Sakmann B., Boheim G. Alamethicin-induced single channel conductance fluctuations in biological membranes. Nature. 1979 Nov 15;282(5736):336–339. doi: 10.1038/282336a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., White J. R., Molski T. F., Shefcyk J., Volpi M., Naccache P. H., Feinstein M. B. Phorbol 12-myristate 13-acetate activates rabbit neutrophils without an apparent rise in the level of intracellular free calcium. Biochem Biophys Res Commun. 1983 Jul 29;114(2):638–645. doi: 10.1016/0006-291x(83)90828-8. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Strickholm A. Impedance of a Small Electrically Isolated Area of the Muscle Cell Surface. J Gen Physiol. 1961 Jul 1;44(6):1073–1088. doi: 10.1085/jgp.44.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]





