Abstract
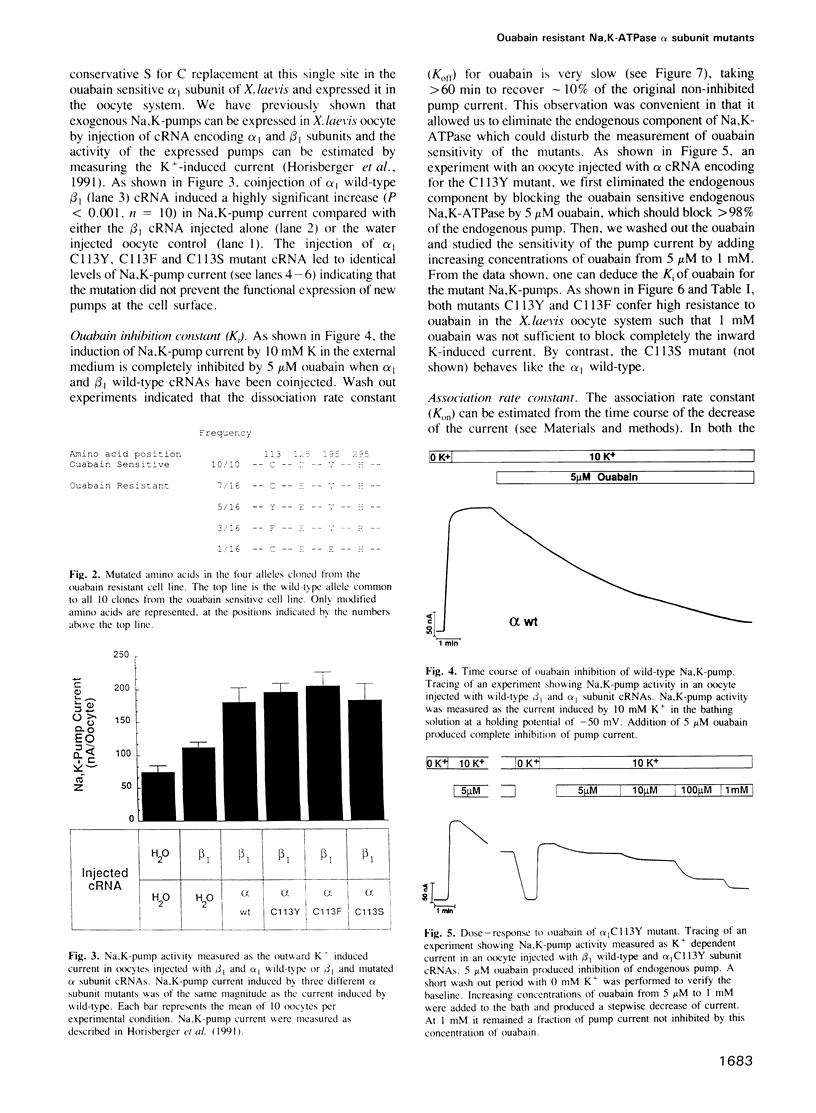
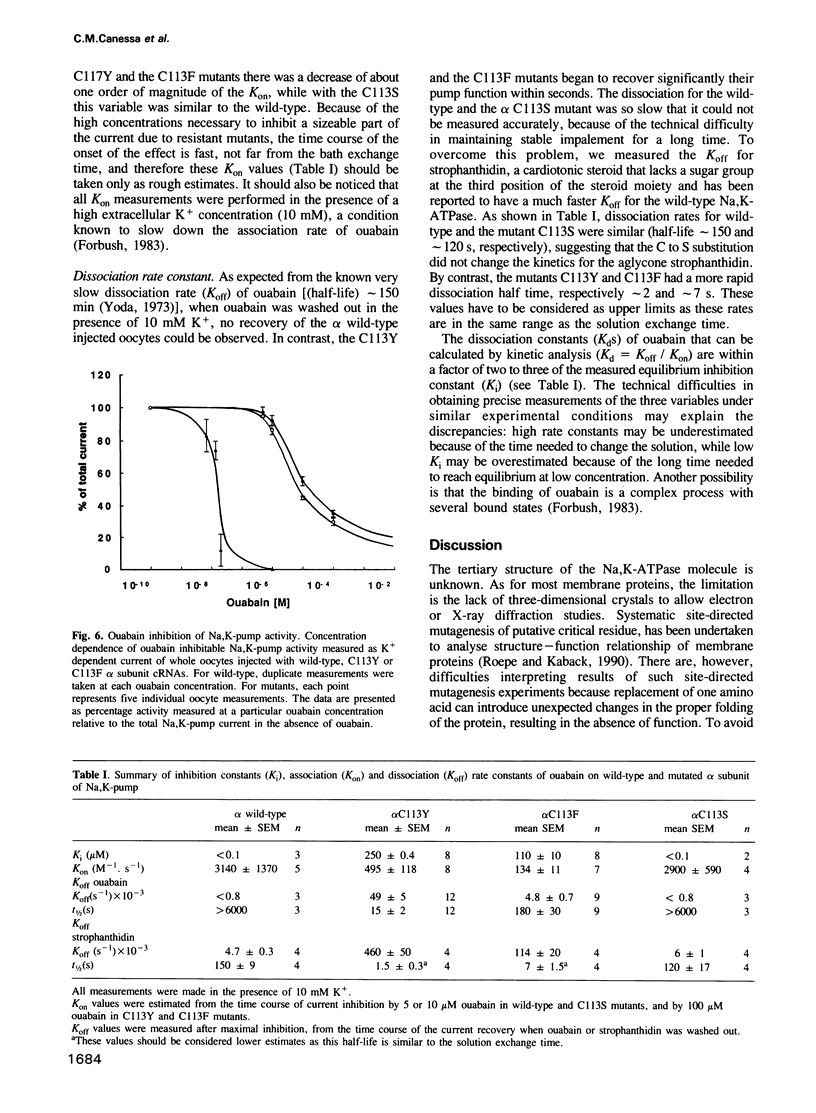
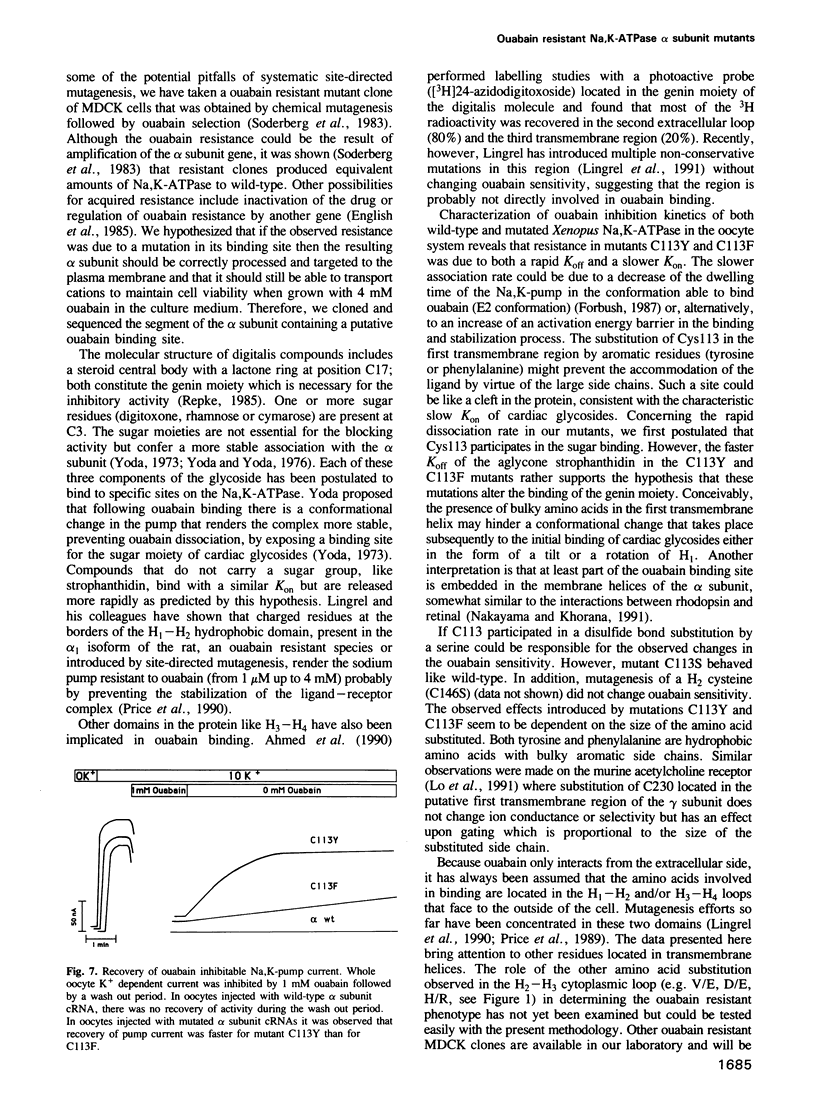
The cardiac glycoside ouabain inhibits Na,K-ATPase by binding to the alpha subunit. In a highly ouabain resistant clone from the MDCK cell line, we have found two alleles of the alpha subunit in which the cysteine, present in the wild-type first transmembrane segment, is replaced by a tyrosine (Y) or a phenylalanine (F). We have studied the kinetics of ouabain inhibition by measuring the current generated by the Na,K-pump in Xenopus oocytes injected with wild-type and mutated alpha 1 and wild-type beta 1 subunit cRNAs. When these mutations, alpha 1C113Y and alpha 1C113F [according to the published sequence [Verrey et al. (1989) Am. J. Physiol., 256, F1034] were introduced in the alpha 1 subunit of the Na,K-ATPase from Xenopus laevis, the inhibition constant (Ki) of ouabain increased greater than 1000-fold compared with wild-type. A more conservative mutation, serine alpha 1C113S did not change the Ki. We observed that the decreased affinity for ouabain was mainly due to a faster dissociation, but probably also to a slower association. Thus we propose that an amino acid residue of the first transmembrane segment located deep in the plasma membrane participates in the structure and the function of the ouabain binding site.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- English L. H., Epstein J., Cantley L., Housman D., Levenson R. Expression of an ouabain resistance gene in transfected cells. Ouabain treatment induces a K+-transport system. J Biol Chem. 1985 Jan 25;260(2):1114–1119. [PubMed] [Google Scholar]
- Haber E., Haupert G. T., Jr The search for a hypothalamic Na+,K+-ATPase inhibitor. Hypertension. 1987 Apr;9(4):315–324. doi: 10.1161/01.hyp.9.4.315. [DOI] [PubMed] [Google Scholar]
- Hamlyn J. M., Blaustein M. P., Bova S., DuCharme D. W., Harris D. W., Mandel F., Mathews W. R., Ludens J. H. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger J. D., Jaunin P., Good P. J., Rossier B. C., Geering K. Coexpression of alpha 1 with putative beta 3 subunits results in functional Na+/K+ pumps in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8397–8400. doi: 10.1073/pnas.88.19.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Noguchi S., Noda M., Takahashi H., Ohta T., Kawamura M., Nojima H., Nagano K., Hirose T., Inayama S. Primary structure of the alpha-subunit of Torpedo californica (Na+ + K+)ATPase deduced from cDNA sequence. Nature. 1985 Aug 22;316(6030):733–736. doi: 10.1038/316733a0. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Ohta T., Nojima H., Nagano K. Primary structure of the alpha-subunit of human Na,K-ATPase deduced from cDNA sequence. J Biochem. 1986 Aug;100(2):389–397. doi: 10.1093/oxfordjournals.jbchem.a121726. [DOI] [PubMed] [Google Scholar]
- Lebovitz R. M., Takeyasu K., Fambrough D. M. Molecular characterization and expression of the (Na+ + K+)-ATPase alpha-subunit in Drosophila melanogaster. EMBO J. 1989 Jan;8(1):193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D. C., Pinkham J. L., Stevens C. F. Role of a key cysteine residue in the gating of the acetylcholine receptor. Neuron. 1991 Jan;6(1):31–40. doi: 10.1016/0896-6273(91)90119-k. [DOI] [PubMed] [Google Scholar]
- Nakayama T. A., Khorana H. G. Mapping of the amino acids in membrane-embedded helices that interact with the retinal chromophore in bovine rhodopsin. J Biol Chem. 1991 Mar 5;266(7):4269–4275. [PubMed] [Google Scholar]
- Nelson R. M., Long G. L. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase chain reaction. Anal Biochem. 1989 Jul;180(1):147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Modyanov N. N., Broude N. E., Petrukhin K. E., Grishin A. V., Arzamazova N. M., Aldanova N. A., Monastyrskaya G. S., Sverdlov E. D. Pig kidney Na+,K+-ATPase. Primary structure and spatial organization. FEBS Lett. 1986 Jun 9;201(2):237–245. doi: 10.1016/0014-5793(86)80616-0. [DOI] [PubMed] [Google Scholar]
- Price E. M., Lingrel J. B. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988 Nov 1;27(22):8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- Price E. M., Rice D. A., Lingrel J. B. Site-directed mutagenesis of a conserved, extracellular aspartic acid residue affects the ouabain sensitivity of sheep Na,K-ATPase. J Biol Chem. 1989 Dec 25;264(36):21902–21906. [PubMed] [Google Scholar]
- Price E. M., Rice D. A., Lingrel J. B. Structure-function studies of Na,K-ATPase. Site-directed mutagenesis of the border residues from the H1-H2 extracellular domain of the alpha subunit. J Biol Chem. 1990 Apr 25;265(12):6638–6641. [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Smith T. W. Digitalis. Mechanisms of action and clinical use. N Engl J Med. 1988 Feb 11;318(6):358–365. doi: 10.1056/NEJM198802113180606. [DOI] [PubMed] [Google Scholar]
- Soderberg K., Rossi B., Lazdunski M., Louvard D. Characterization of ouabain-resistant mutants of a canine kidney cell line, MDCK. J Biol Chem. 1983 Oct 25;258(20):12300–12307. [PubMed] [Google Scholar]
- Takeyasu K., Tamkun M. M., Renaud K. J., Fambrough D. M. Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem. 1988 Mar 25;263(9):4347–4354. [PubMed] [Google Scholar]
- Verrey F., Kairouz P., Schaerer E., Fuentes P., Geering K., Rossier B. C., Kraehenbuhl J. P. Primary sequence of Xenopus laevis Na+-K+-ATPase and its localization in A6 kidney cells. Am J Physiol. 1989 Jun;256(6 Pt 2):F1034–F1043. doi: 10.1152/ajprenal.1989.256.6.F1034. [DOI] [PubMed] [Google Scholar]
- Yoda A. Structue-activity relationships of cardiotonic steroids for the inhibition of sodium- and potassium-dependent adenosine triphosphatase. I. Dissociation rate constants of various enzyme-cardiac glycoside complexes formed in the presence of magnesium and phosphate. Mol Pharmacol. 1973 Jan;9(1):51–60. [PubMed] [Google Scholar]
- Yoda A., Yoda S. Association and dissociation rate constants of the complexes between various cardiac aglycones and sodium- and potassium-dependent adenosine triphosphatase formed in the presence of magnesium and phosphate. Mol Pharmacol. 1977 Mar;13(2):352–361. [PubMed] [Google Scholar]


