Abstract
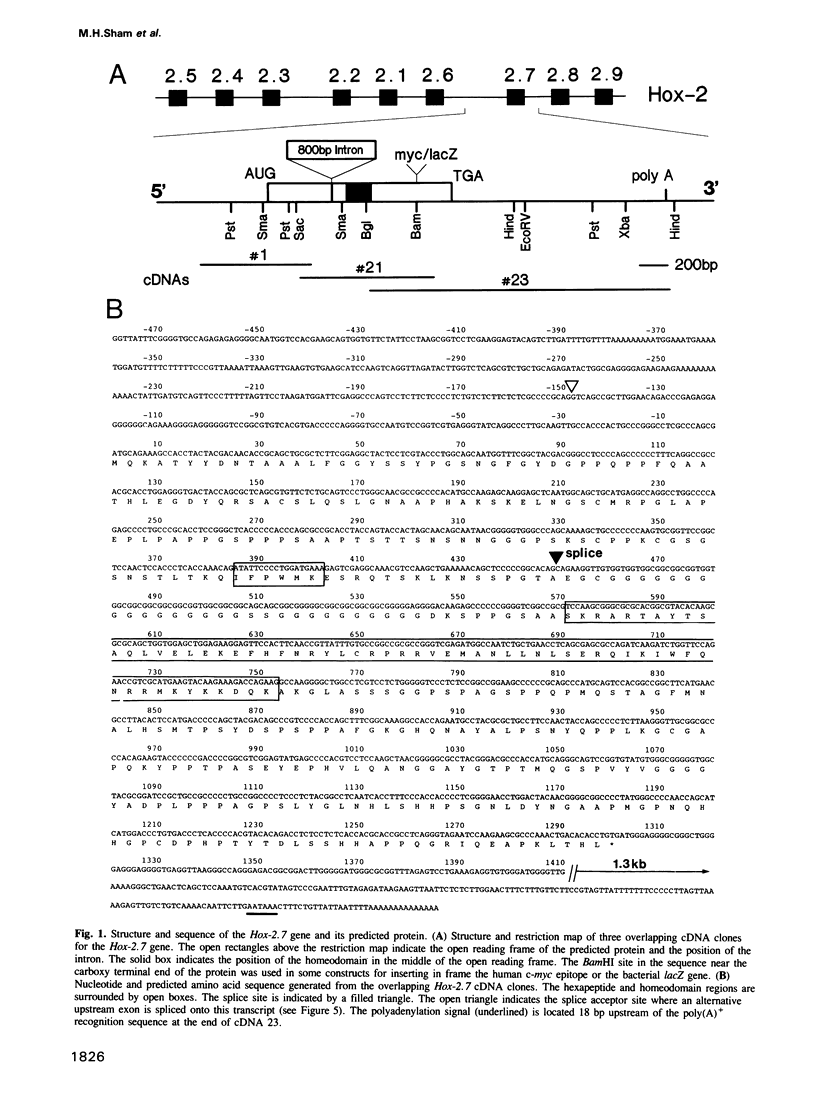
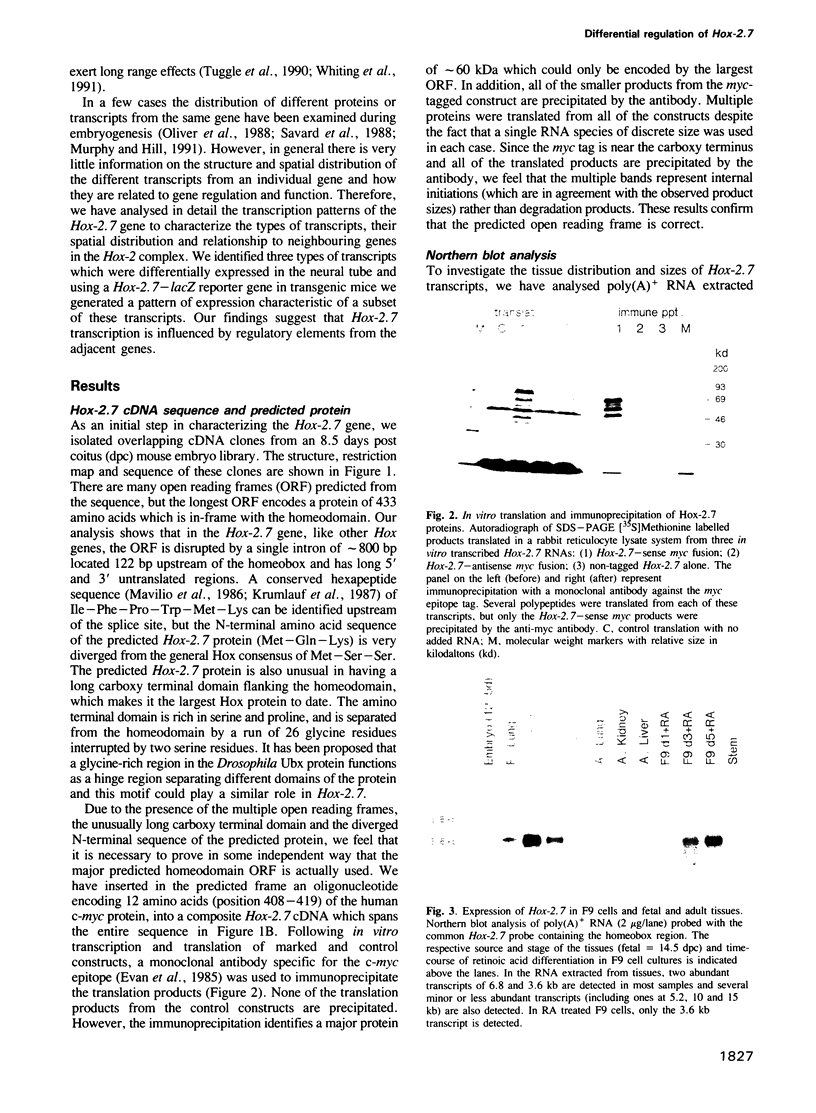
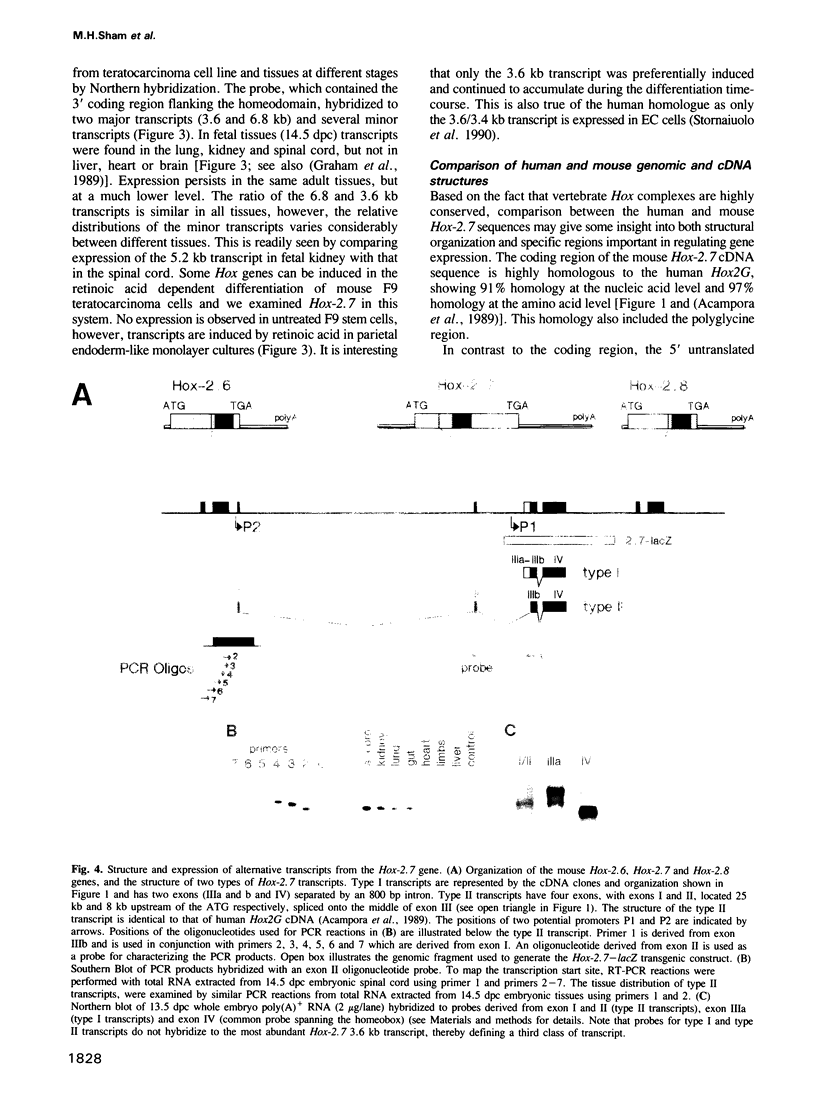
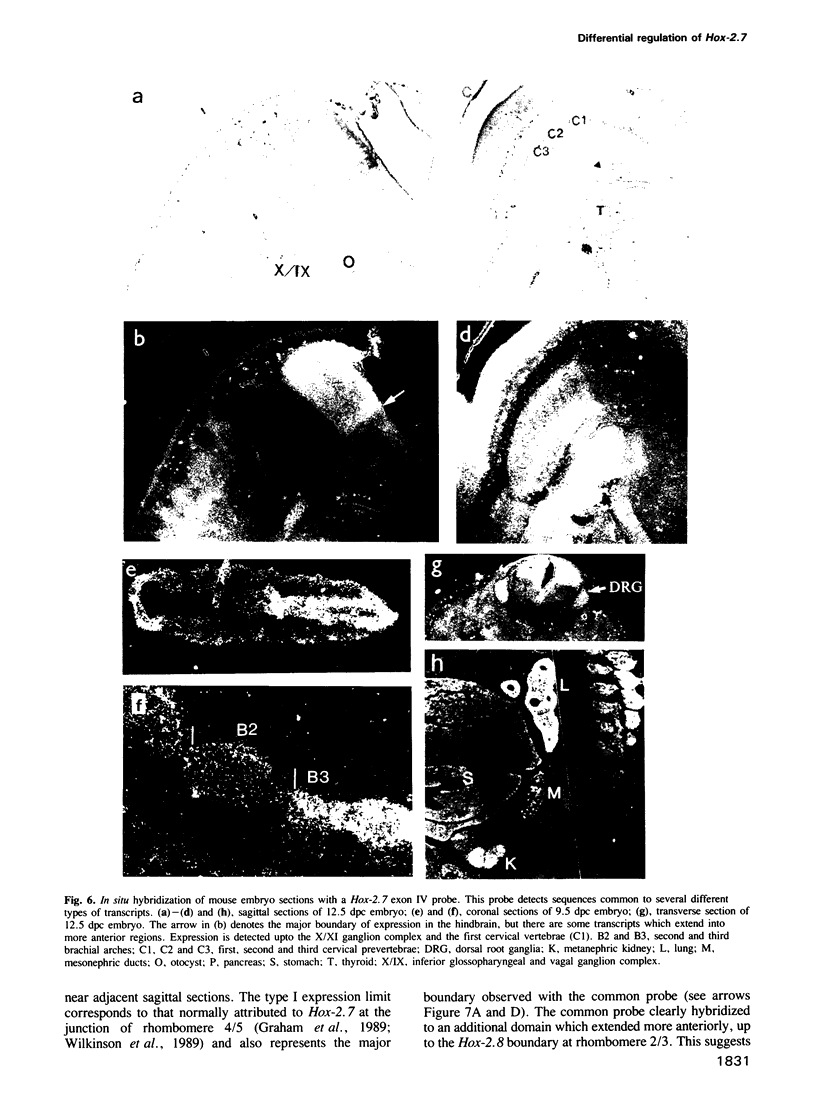
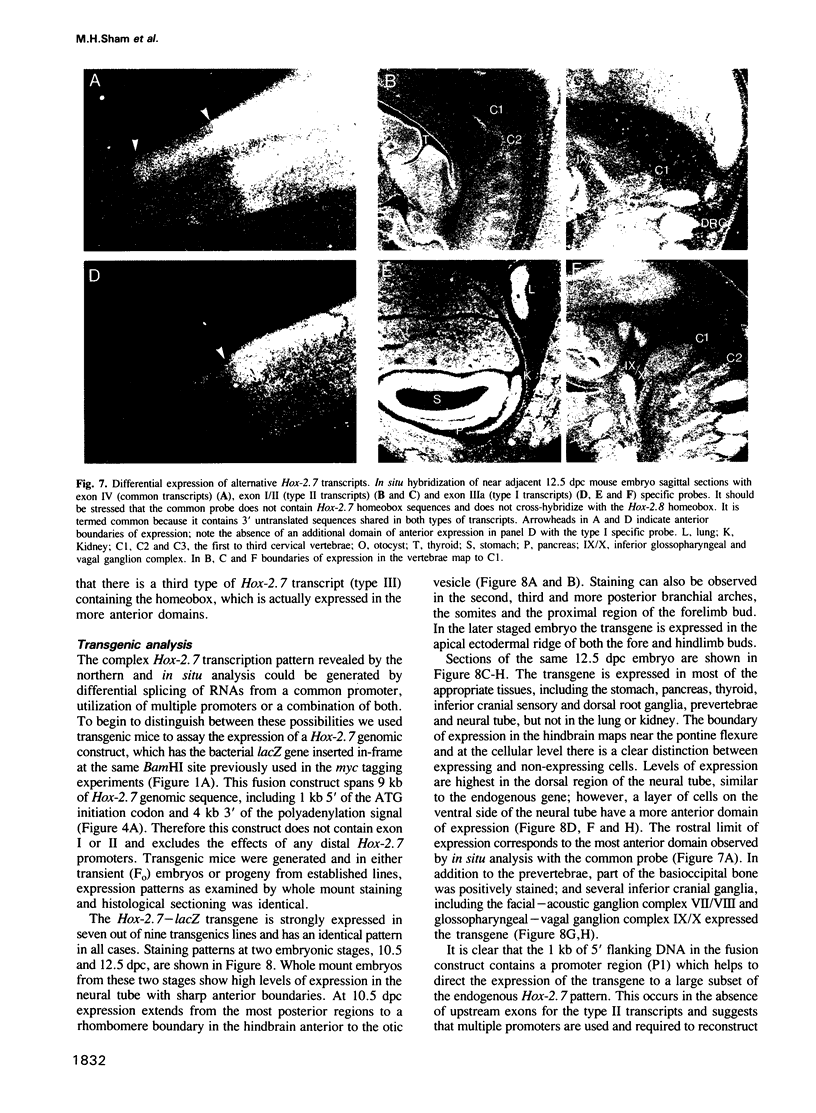
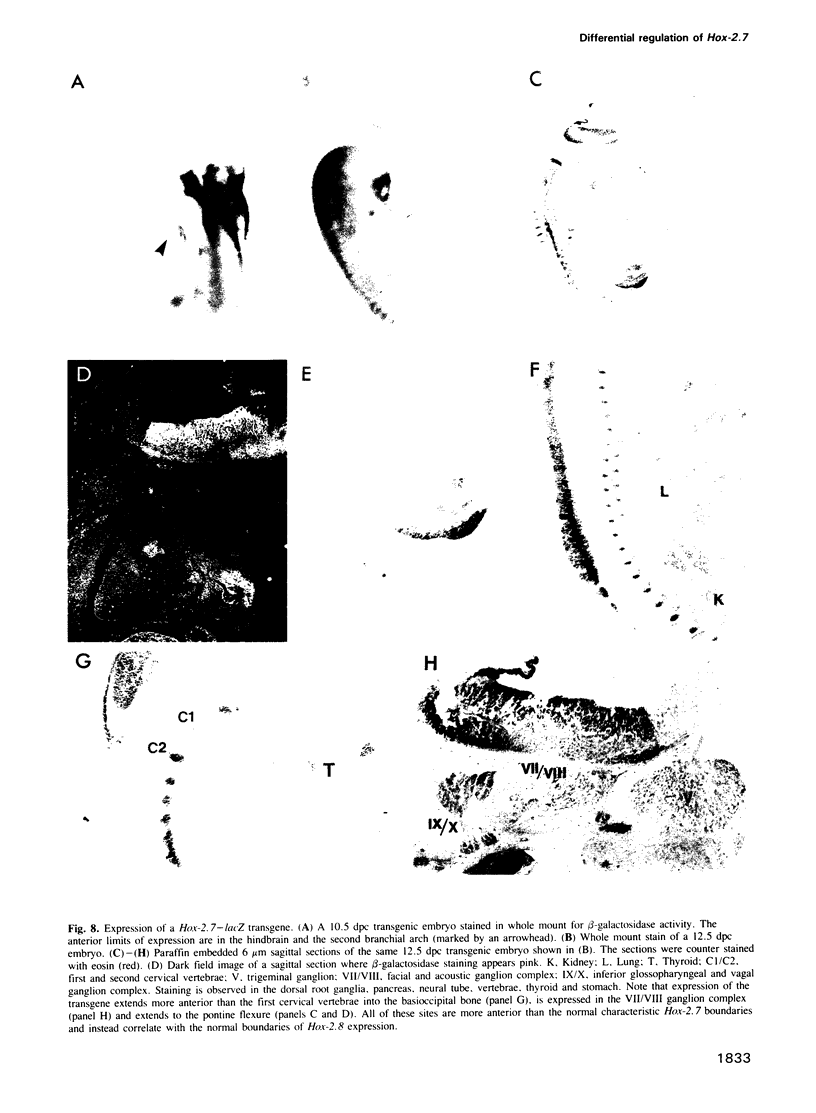
In this study we have investigated the organization and regulation of the mouse Hox-2.7 gene. There are several alternative transcripts some of which are conserved between mouse and humans. By Northern and in situ analysis we are able to identify at least three types of transcripts which are different in size and splicing pattern and have distinctly different boundaries of expression in the nervous system. One subset of the endogenous transcripts has a boundary of expression that corresponds to the adjacent Hox-2.8 gene instead of Hox-2.7. In another type of transcript there is an alternative reading frame which predicts a protein that has homology to an enzyme ATPase and suggests that a non-homeobox containing gene may be located in the Hox-2 cluster. A Hox-2.7-lacZ transgene is expressed in a similar pattern to the endogenous gene in that spatially-restricted domains of expression are seen in the branchial arches, neural tube, paraxial mesoderm (somites), cranial ganglia, neural crest and gut. However, the anterior boundaries of transgene expression only correspond to the subset of Hox-2.7 transcripts which map to the Hox-2.8 boundary. The proximity of a Hox-2.7 promoter to regions which regulate the adjacent Hox-2.6 gene and the expression of transgenic and endogenous transcripts in a Hox-2.8 pattern, suggest that regulatory elements may be shared by neighbouring genes to establish the complete expression pattern.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. The human HOX gene family. Nucleic Acids Res. 1989 Dec 25;17(24):10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balling R., Mutter G., Gruss P., Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989 Jul 28;58(2):337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Baron A., Featherstone M. S., Hill R. E., Hall A., Galliot B., Duboule D. Hox-1.6: a mouse homeo-box-containing gene member of the Hox-1 complex. EMBO J. 1987 Oct;6(10):2977–2986. doi: 10.1002/j.1460-2075.1987.tb02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich C. J., Utset M. F., Awgulewitsch A., Ruddle F. H. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncinelli E., Somma R., Acampora D., Pannese M., D'Esposito M., Faiella A., Simeone A. Organization of human homeobox genes. Hum Reprod. 1988 Oct;3(7):880–886. doi: 10.1093/oxfordjournals.humrep.a136802. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Goetz J., Wright C. V., Fritz A., Hardwicke J., De Robertis E. M. Differential utilization of the same reading frame in a Xenopus homeobox gene encodes two related proteins sharing the same DNA-binding specificity. EMBO J. 1988 Jul;7(7):2139–2149. doi: 10.1002/j.1460-2075.1988.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Falkenstein H., Renucci A., Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989 Dec 14;342(6251):767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Dressler G. R., Gruss P. Anterior boundaries of Hox gene expression in mesoderm-derived structures correlate with the linear gene order along the chromosome. Differentiation. 1989 Sep;41(3):193–201. doi: 10.1111/j.1432-0436.1989.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner K., Hogan B. L., Flavell R. A. Transcription of H-2 and Qa genes in embryonic and adult mice. EMBO J. 1987 May;6(5):1265–1271. doi: 10.1002/j.1460-2075.1987.tb02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Maden M., Krumlauf R. The murine Hox-2 genes display dynamic dorsoventral patterns of expression during central nervous system development. Development. 1991 May;112(1):255–264. doi: 10.1242/dev.112.1.255. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Lorimer J., McVey J. H., Tuddenham E. G., Krumlauf R. Characterization of a murine homeo box gene, Hox-2.6, related to the Drosophila Deformed gene. Genes Dev. 1988 Nov;2(11):1424–1438. doi: 10.1101/gad.2.11.1424. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Hunt P., Gulisano M., Cook M., Sham M. H., Faiella A., Wilkinson D., Boncinelli E., Krumlauf R. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991 Oct 31;353(6347):861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- Hunt P., Krumlauf R. Deciphering the Hox code: clues to patterning branchial regions of the head. Cell. 1991 Sep 20;66(6):1075–1078. doi: 10.1016/0092-8674(91)90029-x. [DOI] [PubMed] [Google Scholar]
- Hunt P., Wilkinson D., Krumlauf R. Patterning the vertebrate head: murine Hox 2 genes mark distinct subpopulations of premigratory and migrating cranial neural crest. Development. 1991 May;112(1):43–50. doi: 10.1242/dev.112.1.43. [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Falkenstein H., Dollé P., Renucci A., Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991 Aug;10(8):2279–2289. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Tickle C., Dollé P., Wolpert L., Duboule D. Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. Nature. 1991 Apr 18;350(6319):585–589. doi: 10.1038/350585a0. [DOI] [PubMed] [Google Scholar]
- Kappen C., Schughart K., Ruddle F. H. Two steps in the evolution of Antennapedia-class vertebrate homeobox genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5459–5463. doi: 10.1073/pnas.86.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Balling R., Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990 Apr 20;61(2):301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991 Oct 4;67(1):89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Kress C., Vogels R., De Graaff W., Bonnerot C., Meijlink F., Nicolas J. F., Deschamps J. Hox-2.3 upstream sequences mediate lacZ expression in intermediate mesoderm derivatives of transgenic mice. Development. 1990 Aug;109(4):775–786. doi: 10.1242/dev.109.4.775. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Holland P. W., McVey J. H., Hogan B. L. Developmental and spatial patterns of expression of the mouse homeobox gene, Hox 2.1. Development. 1987 Apr;99(4):603–617. doi: 10.1242/dev.99.4.603. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Giampaolo A., Faiella A., Zappavigna V., Acampora D., Poiana G., Russo G., Peschle C., Boncinelli E. Differential and stage-related expression in embryonic tissues of a new human homoeobox gene. Nature. 1986 Dec 18;324(6098):664–668. doi: 10.1038/324664a0. [DOI] [PubMed] [Google Scholar]
- Murphy P., Hill R. E. Expression of the mouse labial-like homeobox-containing genes, Hox 2.9 and Hox 1.6, during segmentation of the hindbrain. Development. 1991 Jan;111(1):61–74. doi: 10.1242/dev.111.1.61. [DOI] [PubMed] [Google Scholar]
- Nohno T., Noji S., Koyama E., Ohyama K., Myokai F., Kuroiwa A., Saito T., Taniguchi S. Involvement of the Chox-4 chicken homeobox genes in determination of anteroposterior axial polarity during limb development. Cell. 1991 Mar 22;64(6):1197–1205. doi: 10.1016/0092-8674(91)90274-3. [DOI] [PubMed] [Google Scholar]
- Ohta S., Kagawa Y. Human F1-ATPase: molecular cloning of cDNA for the beta subunit. J Biochem. 1986 Jan;99(1):135–141. doi: 10.1093/oxfordjournals.jbchem.a135452. [DOI] [PubMed] [Google Scholar]
- Oliver G., Wright C. V., Hardwicke J., De Robertis E. M. Differential antero-posterior expression of two proteins encoded by a homeobox gene in Xenopus and mouse embryos. EMBO J. 1988 Oct;7(10):3199–3209. doi: 10.1002/j.1460-2075.1988.tb03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Püschel A. W., Balling R., Gruss P. Position-specific activity of the Hox1.1 promoter in transgenic mice. Development. 1990 Mar;108(3):435–442. doi: 10.1242/dev.108.3.435. [DOI] [PubMed] [Google Scholar]
- Püschel A. W., Balling R., Gruss P. Separate elements cause lineage restriction and specify boundaries of Hox-1.1 expression. Development. 1991 May;112(1):279–287. doi: 10.1242/dev.112.1.279. [DOI] [PubMed] [Google Scholar]
- Savard P., Gates P. B., Brockes J. P. Position dependent expression of a homeobox gene transcript in relation to amphibian limb regeneration. EMBO J. 1988 Dec 20;7(13):4275–4282. doi: 10.1002/j.1460-2075.1988.tb03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schughart K., Bieberich C. J., Eid R., Ruddle F. H. A regulatory region from the mouse Hox-2.2 promoter directs gene expression into developing limbs. Development. 1991 Jul;112(3):807–811. doi: 10.1242/dev.112.3.807. [DOI] [PubMed] [Google Scholar]
- Seeger M. A., Haffley L., Kaufman T. C. Characterization of amalgam: a member of the immunoglobulin superfamily from Drosophila. Cell. 1988 Nov 18;55(4):589–600. doi: 10.1016/0092-8674(88)90217-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Mavilio F., Acampora D., Giampaolo A., Faiella A., Zappavigna V., D'Esposito M., Pannese M., Russo G., Boncinelli E. Two human homeobox genes, c1 and c8: structure analysis and expression in embryonic development. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4914–4918. doi: 10.1073/pnas.84.14.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Pannese M., Acampora D., D'Esposito M., Boncinelli E. At least three human homeoboxes on chromosome 12 belong to the same transcription unit. Nucleic Acids Res. 1988 Jun 24;16(12):5379–5390. doi: 10.1093/nar/16.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornaiuolo A., Acampora D., Pannese M., D'Esposito M., Morelli F., Migliaccio E., Rambaldi M., Faiella A., Nigro V., Simeone A. Human HOX genes are differentially activated by retinoic acid in embryonal carcinoma cells according to their position within the four loci. Cell Differ Dev. 1990 Aug;31(2):119–127. doi: 10.1016/0922-3371(90)90015-o. [DOI] [PubMed] [Google Scholar]
- Tuggle C. K., Zakany J., Cianetti L., Peschle C., Nguyen-Huu M. C. Region-specific enhancers near two mammalian homeo box genes define adjacent rostrocaudal domains in the central nervous system. Genes Dev. 1990 Feb;4(2):180–189. doi: 10.1101/gad.4.2.180. [DOI] [PubMed] [Google Scholar]
- Whiting J., Marshall H., Cook M., Krumlauf R., Rigby P. W., Stott D., Allemann R. K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991 Nov;5(11):2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y., Sasaki H., Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991 Oct 3;353(6343):443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Zakany J., Tuggle C. K., Nguyen-Huu C. M. The use of lacZ gene fusions in the studies of mammalian development: developmental regulation of mammalian homeobox genes in the CNS. J Physiol (Paris) 1990;84(1):21–26. [PubMed] [Google Scholar]