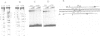Abstract
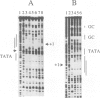
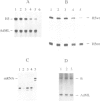
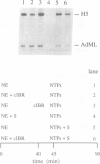
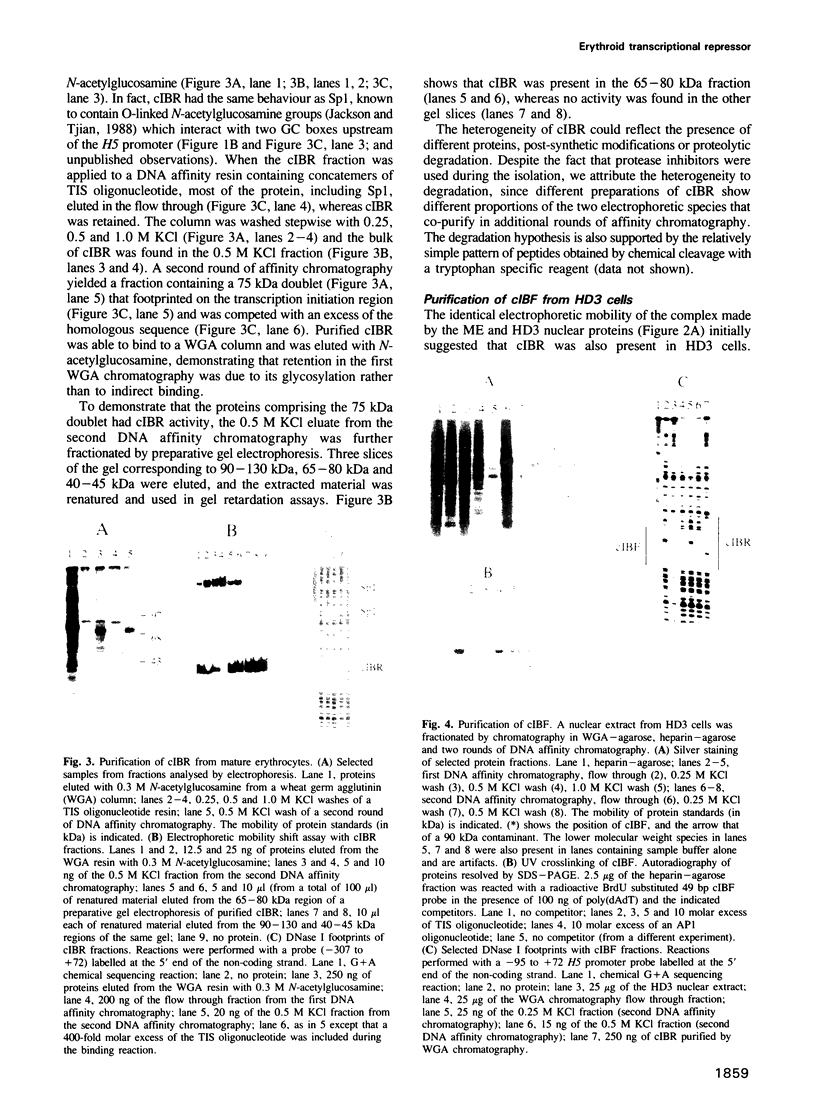
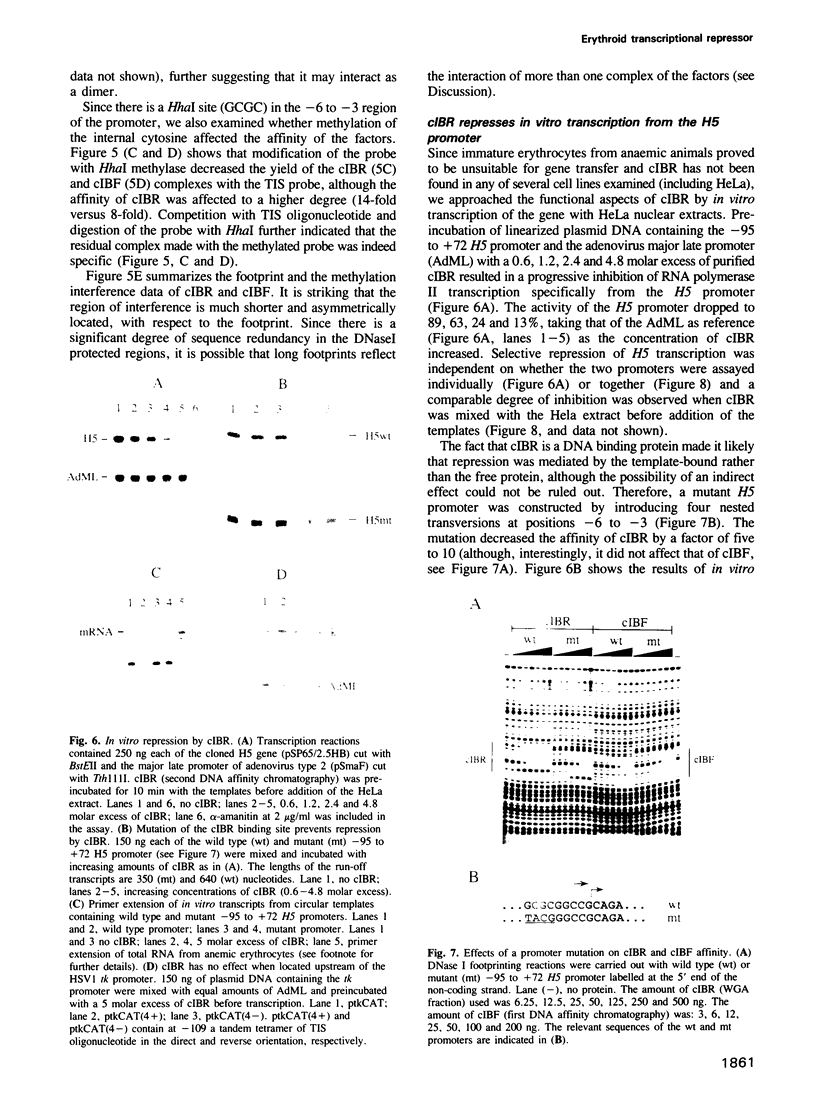
Expression of histone H5, like that of other erythrocyte specific proteins, declines during the latter stages of erythroid maturation because of a decrease in the rate of gene transcription. Here, we report the isolation of cIBR (chicken initiation binding repressor), a 75 kDa DNA binding glycoprotein from mature chicken erythrocytes that recognizes sequences spanning the transcription start sites of the H5 gene. cIBR was found to repress transcription from the H5 promoter in vitro and this effect could be relieved by mutations that lowered the affinity of the factor for its cognate sequence. cIBR inhibited transcription by interfering with assembly of the initiation complex, but it did not affect transcription from pre-assembled complexes. Consistent with this, binding of bacterially expressed human TFIID to the TATA element prevented subsequent binding of cIBR, although the opposite was not true. This, and the fact that cIBR had no effect when bound in a location upstream from the promoter, suggests that binding of cIBR to the start site region causes repression by direct interference with general transcription factors other than TFIID, possibly TFIIB. cIBR was found in mature and relatively late erythrocytes but not in early erythroid cells which actively transcribe the H5 gene; the transcriptionally active cells contain instead cIBF (chicken initiation binding factor). Purified cIBF is a non-glycosylated 68-70 kDa DNA binding protein(s) which also recognizes the region of transcription initiation of the H5 gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Côté J., Renaud J., Ruiz-Carrillo A. Regulation of histone and beta A-globin gene expression during differentiation of chicken erythroid cells. Mol Cell Biol. 1987 Oct;7(10):3663–3672. doi: 10.1128/mcb.7.10.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Ayer D. E., Dynan W. S. A downstream-element-binding factor facilitates assembly of a functional preinitiation complex at the simian virus 40 major late promoter. Mol Cell Biol. 1990 Jul;10(7):3635–3645. doi: 10.1128/mcb.10.7.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaupain D., Eléouët J. F., Roméo P. H. Initiation of transcription of the erythroid promoter of the porphobilinogen deaminase gene is regulated by a cis-acting sequence around the cap site. Nucleic Acids Res. 1990 Nov 25;18(22):6509–6515. doi: 10.1093/nar/18.22.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman M. G., Wawra E., Winge M. Chicken histone H5 inhibits transcription and replication when introduced into proliferating cells by microinjection. J Cell Sci. 1988 Oct;91(Pt 2):201–209. doi: 10.1242/jcs.91.2.201. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Carcamo J., Buckbinder L., Reinberg D. The initiator directs the assembly of a transcription factor IID-dependent transcription complex. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8052–8056. doi: 10.1073/pnas.88.18.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S., Thompson J. A., Jacob W. F., Cohen R., Safer B. Identification and characterization of an adenovirus 2 major late promoter CAP sequence DNA-binding protein. J Biol Chem. 1990 Jun 25;265(18):10309–10319. [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Luciw P. A., Duchange N. Structural arrangements of transcription control domains within the 5'-untranslated leader regions of the HIV-1 and HIV-2 promoters. Genes Dev. 1988 Sep;2(9):1101–1114. doi: 10.1101/gad.2.9.1101. [DOI] [PubMed] [Google Scholar]
- Kato H., Horikoshi M., Roeder R. G. Repression of HIV-1 transcription by a cellular protein. Science. 1991 Mar 22;251(5000):1476–1479. doi: 10.1126/science.2006421. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Robins A. J., D'Andrea R., Wells J. R. The chicken H5 gene is unlinked to core and H1 histone genes. Nucleic Acids Res. 1983 Feb 11;11(3):619–627. doi: 10.1093/nar/11.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Means A. L., Farnham P. J. Transcription initiation from the dihydrofolate reductase promoter is positioned by HIP1 binding at the initiation site. Mol Cell Biol. 1990 Feb;10(2):653–661. doi: 10.1128/mcb.10.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Wall L., deBoer E., Grosveld F., Romeo P. H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989 Jan 11;17(1):37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Horikoshi M., Brenner M., Yamamoto T., Besnard F., Roeder R. G., Freese E. A downstream initiation element required for efficient TATA box binding and in vitro function of TFIID. Nature. 1990 Nov 1;348(6296):86–88. doi: 10.1038/348086a0. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Horikoshi M., Roeder R. G., Desplan C. Engrailed, a homeodomain protein, can repress in vitro transcription by competition with the TATA box-binding protein transcription factor IID. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2289–2293. doi: 10.1073/pnas.87.6.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S. K., Zinkel S. S., Kiessling A. A., Cooper G. M. c-mos expression in mouse oocytes is controlled by initiator-related sequences immediately downstream of the transcription initiation site. Mol Cell Biol. 1991 Oct;11(10):5190–5196. doi: 10.1128/mcb.11.10.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J., Ruiz-Carrillo A. Fine analysis of the active H5 gene chromatin of chicken erythroid cells at different stages of differentiation. J Mol Biol. 1986 May 5;189(1):217–226. doi: 10.1016/0022-2836(86)90392-x. [DOI] [PubMed] [Google Scholar]
- Renkawitz R. Transcriptional repression in eukaryotes. Trends Genet. 1990 Jun;6(6):192–197. doi: 10.1016/0168-9525(90)90176-7. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Tjian R. SV40 T antigen binding site mutations that affect autoregulation. Cell. 1983 Apr;32(4):1227–1240. doi: 10.1016/0092-8674(83)90305-7. [DOI] [PubMed] [Google Scholar]
- Rousseau S., Renaud J., Ruiz-Carrillo A. Basal expression of the histone H5 gene is controlled by positive and negative cis-acting sequences. Nucleic Acids Res. 1989 Sep 25;17(18):7495–7511. doi: 10.1093/nar/17.18.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Affolter M., Renaud J. Genomic organization of the genes coding for the six main histones of the chicken: complete sequence of the H5 gene. J Mol Biol. 1983 Nov 15;170(4):843–859. doi: 10.1016/s0022-2836(83)80191-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Littau V. C., Allfrey V. G. Changes in histone acetyl content and in nuclear non-histone protein composition of avian erythroid cells at different stages of maturation. J Biol Chem. 1974 Nov 25;249(22):7358–7368. [PubMed] [Google Scholar]
- Rupp R. A., Kruse U., Multhaup G., Göbel U., Beyreuther K., Sippel A. E. Chicken NFI/TGGCA proteins are encoded by at least three independent genes: NFI-A, NFI-B and NFI-C with homologues in mammalian genomes. Nucleic Acids Res. 1990 May 11;18(9):2607–2616. doi: 10.1093/nar/18.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Schaffner W. How do different transcription factors binding the same DNA sequence sort out their jobs? Trends Genet. 1989 Feb;5(2):37–39. doi: 10.1016/0168-9525(89)90017-6. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Sun J. M., Ali Z., Lurz R., Ruiz-Carrillo A. Replacement of histone H1 by H5 in vivo does not change the nucleosome repeat length of chromatin but increases its stability. EMBO J. 1990 May;9(5):1651–1658. doi: 10.1002/j.1460-2075.1990.tb08285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. M., Wiaderkiewicz R., Ruiz-Carrillo A. Histone H5 in the control of DNA synthesis and cell proliferation. Science. 1989 Jul 7;245(4913):68–71. doi: 10.1126/science.2740916. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Stamler S. J., Engel J. D. Erythroid-specific transcription of the chicken histone H5 gene is directed by a 3' enhancer. 1987 Aug 27-Sep 2Nature. 328(6133):827–830. doi: 10.1038/328827a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wigley P. L., Sturm R. A., Wells J. R. The tissue-specific chicken histone H5 gene is transcribed with fidelity in Xenopus laevis oocytes. J Mol Biol. 1985 Feb 5;181(3):449–452. doi: 10.1016/0022-2836(85)90231-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]