Abstract
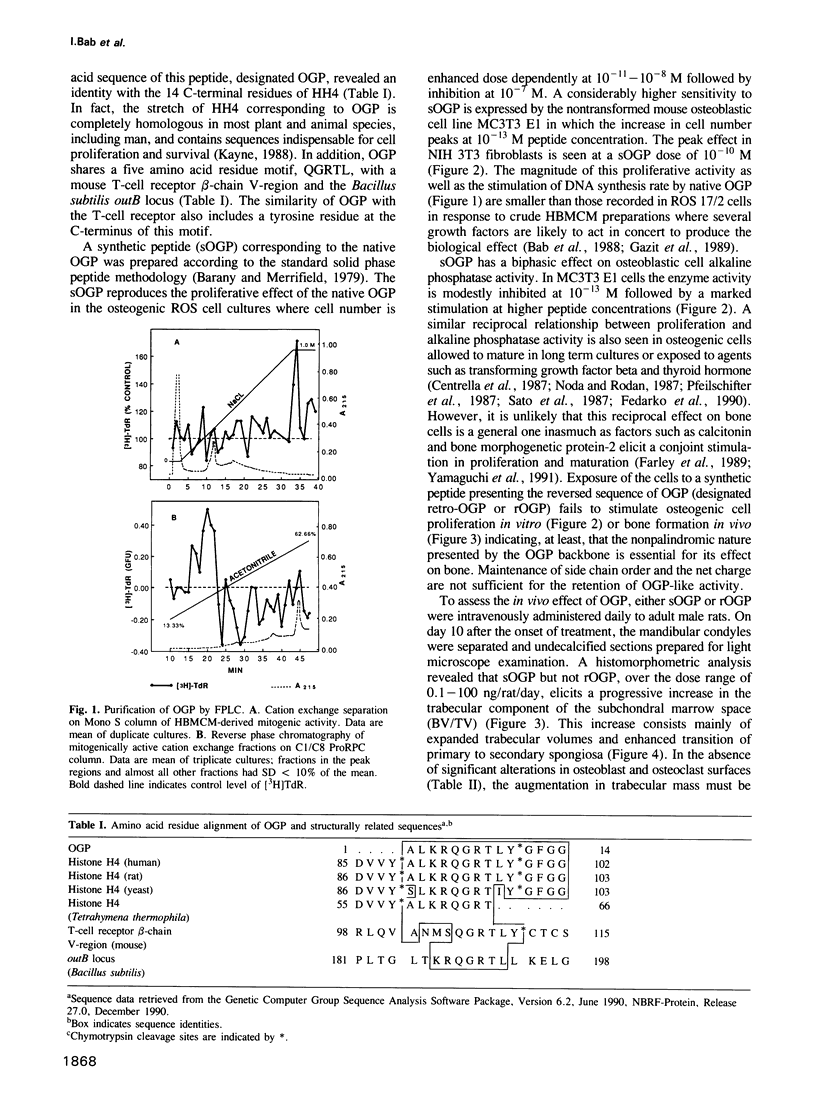
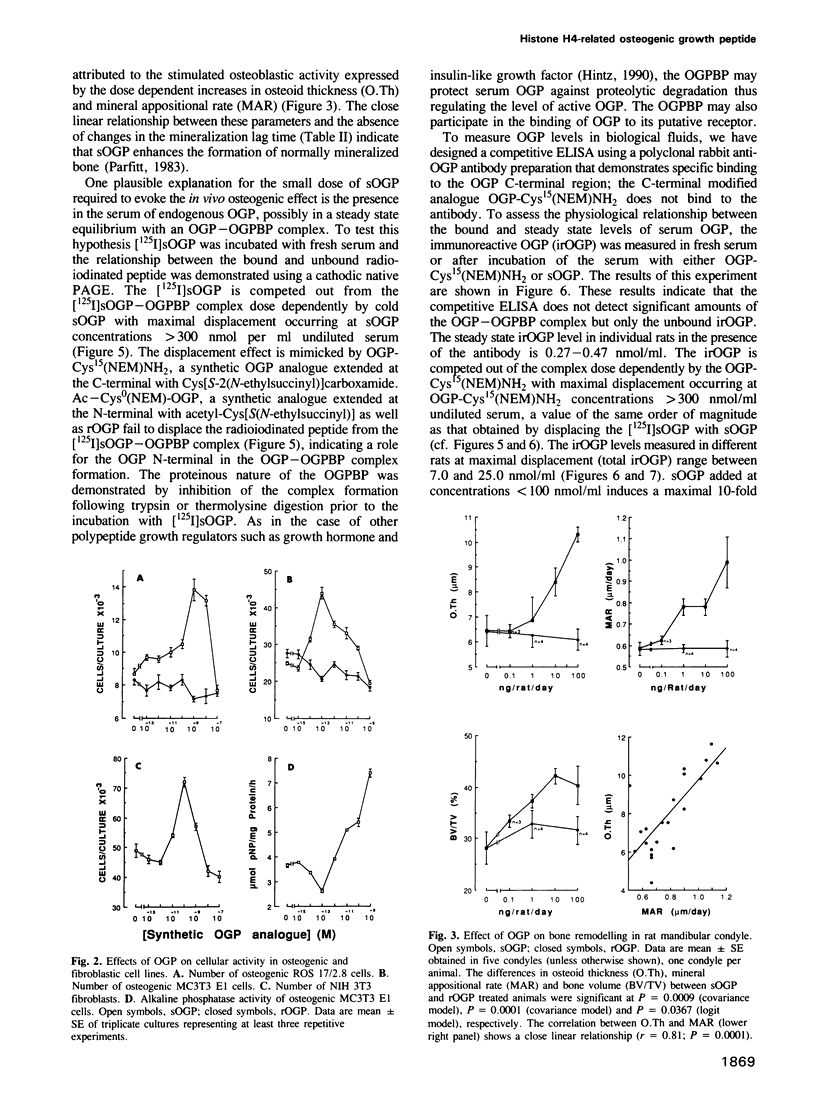
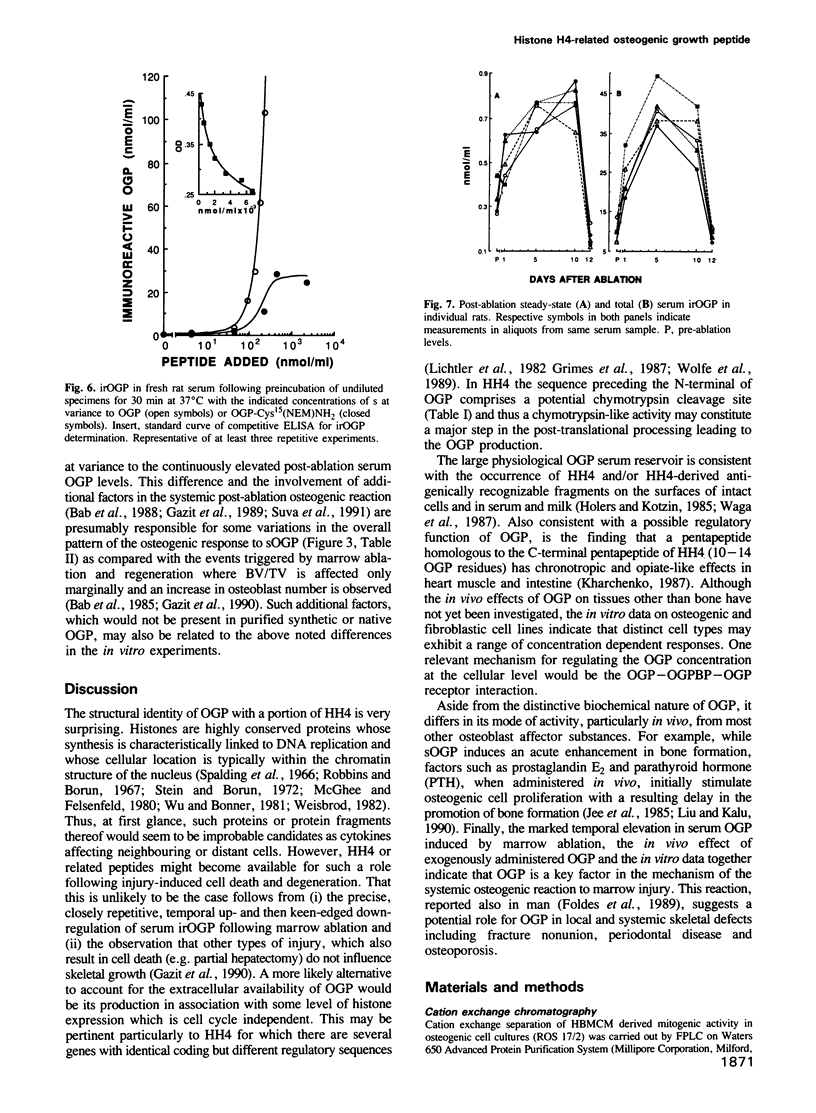
It has been established that regenerating marrow induces an osteogenic response in distant skeletal sites and that this activity is mediated by factors released into the circulation by the healing tissue. In the present study we have characterized one of these factors, a 14 amino acid peptide named osteogenic growth peptide (OGP). Synthetic OGP, identical in structure to the native molecule, stimulates the proliferation and alkaline phosphatase activity of osteoblastic cells in vitro and increases bone mass in rats when injected in vivo. Immunoreactive OGP in high abundance is present physiologically in the serum, mainly in the form of an OGP-OGP binding protein complex. A marked increase in serum bound and unbound OGP accompanies the osteogenic phase of post-ablation marrow regeneration and associated systemic osteogenic response. Authentic OGP is identical to the C-terminus of histone H4 and shares a five residue motif with a T-cell receptor beta-chain V-region and the Bacillus subtilis outB locus. Since these latter proteins have not been implicated previously in the control of cell proliferation or differentiation, OGP may belong to a novel, heretofore unrecognized family of regulatory peptides. Perhaps more importantly, OGP appears to represent a new class of molecules involved in the systemic control of osteoblast proliferation and differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsel S., Maniatis A., Tavassoli M., Crosby W. H. The significance of intramedullary cancellous bone formation in the repair of bone marrow tissue. Anat Rec. 1969 May;164(1):101–111. doi: 10.1002/ar.1091640107. [DOI] [PubMed] [Google Scholar]
- Ashton B. A., Allen T. D., Howlett C. R., Eaglesom C. C., Hattori A., Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980 Sep;(151):294–307. [PubMed] [Google Scholar]
- Bab I., Gazit D., Massarawa A., Sela J. Removal of tibial marrow induces increased formation of bone and cartilage in rat mandibular condyle. Calcif Tissue Int. 1985 Sep;37(5):551–555. doi: 10.1007/BF02557840. [DOI] [PubMed] [Google Scholar]
- Bab I., Gazit D., Muhlrad A., Shteyer A. Regenerating bone marrow produces a potent growth-promoting activity to osteogenic cells. Endocrinology. 1988 Jul;123(1):345–352. doi: 10.1210/endo-123-1-345. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Einhorn T. A., Simon G., Devlin V. J., Warman J., Sidhu S. P., Vigorita V. J. The osteogenic response to distant skeletal injury. J Bone Joint Surg Am. 1990 Oct;72(9):1374–1378. [PubMed] [Google Scholar]
- Farley J. R., Hall S. L., Tarbaux N. M. Calcitonin (but not calcitonin gene-related peptide) increases mouse bone cell proliferation in a dose-dependent manner, and increases mouse bone formation, alone and in combination with fluoride. Calcif Tissue Int. 1989 Oct;45(4):214–221. doi: 10.1007/BF02556040. [DOI] [PubMed] [Google Scholar]
- Fedarko N. S., Bianco P., Vetter U., Robey P. G. Human bone cell enzyme expression and cellular heterogeneity: correlation of alkaline phosphatase enzyme activity with cell cycle. J Cell Physiol. 1990 Jul;144(1):115–121. doi: 10.1002/jcp.1041440115. [DOI] [PubMed] [Google Scholar]
- Foldes J., Naparstek E., Statter M., Menczel J., Bab I. Osteogenic response to marrow aspiration: increased serum osteocalcin and alkaline phosphatase in human bone marrow donors. J Bone Miner Res. 1989 Aug;4(4):643–646. doi: 10.1002/jbmr.5650040424. [DOI] [PubMed] [Google Scholar]
- Friedenstein A. J. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Gazit D., Karmish M., Holzman L., Bab I. Regenerating marrow induces systemic increase in osteo- and chondrogenesis. Endocrinology. 1990 May;126(5):2607–2613. doi: 10.1210/endo-126-5-2607. [DOI] [PubMed] [Google Scholar]
- Gazit D., Shteyer A., Bab I. Further characterization of osteogenic-cell growth promoting activity derived from healing bone marrow. Connect Tissue Res. 1989;23(2-3):153–161. doi: 10.3109/03008208909002415. [DOI] [PubMed] [Google Scholar]
- Grimes S., Weisz-Carrington P., Daum H., 3rd, Smith J., Green L., Wright K., Stein G., Stein J. A rat histone H4 gene closely associated with the testis-specific H1t gene. Exp Cell Res. 1987 Dec;173(2):534–545. doi: 10.1016/0014-4827(87)90293-x. [DOI] [PubMed] [Google Scholar]
- Hintz R. L. Role of growth-hormone and insulin-like growth-factor-binding proteins. Horm Res. 1990;33(2-4):105–110. doi: 10.1159/000181492. [DOI] [PubMed] [Google Scholar]
- Holers V. M., Kotzin B. L. Human peripheral blood monocytes display surface antigens recognized by monoclonal antinuclear antibodies. J Clin Invest. 1985 Sep;76(3):991–998. doi: 10.1172/JCI112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee W. S., Ueno K., Deng Y. P., Woodbury D. M. The effects of prostaglandin E2 in growing rats: increased metaphyseal hard tissue and cortico-endosteal bone formation. Calcif Tissue Int. 1985 Mar;37(2):148–157. doi: 10.1007/BF02554834. [DOI] [PubMed] [Google Scholar]
- Kayne P. S., Kim U. J., Han M., Mullen J. R., Yoshizaki F., Grunstein M. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell. 1988 Oct 7;55(1):27–39. doi: 10.1016/0092-8674(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Kharchenko E. P., Bagrov A. Ia, Sokolova T. V. Farmakologicheskoe vliianie ékzogennykh gistonov na myshtsy. Biull Eksp Biol Med. 1987 Apr;103(4):418–420. [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtler A. C., Sierra F., Clark S., Wells J. R., Stein J. L., Stein G. S. Multiple H4 histone mRNAs of HeLa cells are encoded in different genes. Nature. 1982 Jul 8;298(5870):195–198. doi: 10.1038/298195a0. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Kalu D. N. Human parathyroid hormone-(1-34) prevents bone loss and augments bone formation in sexually mature ovariectomized rats. J Bone Miner Res. 1990 Sep;5(9):973–982. doi: 10.1002/jbmr.5650050911. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Mueller M., Schilling T., Minne H. W., Ziegler R. A systemic acceleratory phenomenon (SAP) accompanies the regional acceleratory phenomenon (RAP) during healing of a bone defect in the rat. J Bone Miner Res. 1991 Apr;6(4):401–410. doi: 10.1002/jbmr.5650060412. [DOI] [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Type beta transforming growth factor (TGF beta) regulation of alkaline phosphatase expression and other phenotype-related mRNAs in osteoblastic rat osteosarcoma cells. J Cell Physiol. 1987 Dec;133(3):426–437. doi: 10.1002/jcp.1041330303. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987 Dec;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Patt H. M., Maloney M. A. Bone marrow regeneration after local injury: a review. Exp Hematol. 1975 Apr;3(2):135–148. [PubMed] [Google Scholar]
- Pfeilschifter J., D'Souza S. M., Mundy G. R. Effects of transforming growth factor-beta on osteoblastic osteosarcoma cells. Endocrinology. 1987 Jul;121(1):212–218. doi: 10.1210/endo-121-1-212. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Han D. C., Fujii Y., Tsushima T., Shizume K. Thyroid hormone stimulates alkaline phosphatase activity in cultured rat osteoblastic cells (ROS 17/2.8) through 3,5,3'-triiodo-L-thyronine nuclear receptors. Endocrinology. 1987 May;120(5):1873–1881. doi: 10.1210/endo-120-5-1873. [DOI] [PubMed] [Google Scholar]
- Schneider G. B., Relfson M., Nicolas J. Pluripotent hemopoietic stem cells give rise to osteoclasts. Am J Anat. 1986 Dec;177(4):505–511. doi: 10.1002/aja.1001770408. [DOI] [PubMed] [Google Scholar]
- Spalding J., Kajiwara K., Mueller G. C. The metabolism of basic proteins in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1535–1542. doi: 10.1073/pnas.56.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waga S., Tan E. M., Rubin R. L. Identification and isolation of soluble histones from bovine milk and serum. Biochem J. 1987 Jun 15;244(3):675–682. doi: 10.1042/bj2440675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. A., Anderson J. V., Grimes S. R., Stein G. S., Stein J. S. Comparison of the structural organization and expression of germinal and somatic rat histone H4 genes. Biochim Biophys Acta. 1989 Mar 1;1007(2):140–150. doi: 10.1016/0167-4781(89)90032-8. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Katagiri T., Ikeda T., Wozney J. M., Rosen V., Wang E. A., Kahn A. J., Suda T., Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991 May;113(3):681–687. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]




