Abstract
Manipulation of cellular motility using a target signal can facilitate the development of biosensors or microbe-powered biorobots. Here, we engineered signal-dependent motility in Escherichia coli via the transcriptional control of a key motility gene. Without manipulating chemotaxis, signal-dependent switching of motility, either on or off, led to population-level directional movement of cells up or down a signal gradient. We developed a mathematical model that captures the behaviour of the cells, enables identification of key parameters controlling system behaviour, and facilitates predictive-design of motility-based pattern formation. We demonstrated that motility of the receiver strains could be controlled by a sender strain generating a signal gradient. The modular quorum sensing-dependent architecture for interfacing different senders with receivers enabled a broad range of systems-level behaviours. The directional control of motility, especially combined with the potential to incorporate tuneable sensors and more complex sensing-logic, may lead to tools for novel biosensing and targeted-delivery applications.
Introduction
Cellular motility is a key microbial behaviour with a broad range of functions in natural systems, including navigation of the environment1, biofilm formation2, and control of biodiversity in consortia3. Bacteria move in a self-propelled manner by drawing energy from their surroundings and have developed mechanisms to effectively navigate their environments. Bacteria also monitor their environment and respond to changes therin4, 5. Controlling cellular motility in response to an external signal can facilitate the development of biosensors6–8 or micromachines that use microbes to enable movement in microfluidic environments9, 10, with potential applications as targeted-delivery agents.
Escherichia coli swim through their environment powered by the rotation of their flagella11. The flagella are self-assembled structures made up of a hook, filament and motor12. The hook is flexible while the filament is rigid and its shape is determined by the direction of flagellar rotation. The motor is powered by a proton gradient that generates the torque required for flagellar rotation13, 14. In the absence of attractants or repellents to guide the direction of movement, bacteria follow a random walk pattern involving a series of runs and tumbles determined by the direction of flagellar rotation1. Chemoreceptors bind to attractants resulting in a change in the phosphorylation state of proteins that control the direction of flagellar rotation15, 16, reducing the tumbling frequency of cells, and allowing cells to run in more direct paths towards attractants17, 18.
Different strategies have been pursued to engineer motility in E. coli in response to target signal molecules19. Several efforts have used E. coli strains rendered non-motile via deletion of motility proteins and then restored motility via inducible expression of the deleted gene from a plasmid20, 21. For example, control of motility was achieved in a cheZ-deletion E. coli strain by using a theophylline-sensitive riboswitch to control expression of CheZ, a protein that controls cellular tumbling rate22. Control of directional motility in response to target compounds has been achieved by engineering E. coli chemoreceptors to recognize target compounds via directed evolution23, rational design of the chemoreceptor specificity24, and designing hybrid chemoreceptors consisting of an E. coli signalling domain and a sensory domain from other species that recognizes a target compound25. While such strategies for controlling directional movement targeting E. coli’s chemotactic network have led to some success, the limited number of natural chemoreceptor scaffolds imposes constraints on ligands that can be targeted. Such engineering challenges have led to alternative approaches, such as converting the desired target to a compound recognized by E. coli’s native chemotactic machinery26.
Engineering directional motility in response to signal molecules non-native to E. coli’s sensing machinery, without manipulation of chemotaxis, would expand the use of bacteria in sensing and actuation applications. Interestingly, some enzymes have been observed to exhibit an increase in diffusivity that correlates to increasing concentrations of their substrate. The substrate concentration-dependent enhancement in diffusivity enables directional movement of the enzymes up gradients of their cognate signals26. For example, urease exhibits an increase in diffusivity with increasing concentrations of its substrate urea, and this enables directional movement of urease up a substrate gradient27, 28. Similar directional migration was observed with catalase molecules in a hydrogen peroxide gradient28. This ability of enzymes to enable directed self-propulsion has been harnessed to drive polystyrene beads coated with urease or catalase up the gradients of their cognate substrates29. This mechanism of directional movement resulting from substrate concentration-dependent enhanced diffusivity could be applied to engineer directional movement of cells in a signal gradient by enhancing cellular diffusivity in the presence of a signal. We hypothesized that transcriptional control of a key motility gene in response to a signal would allow signal-dependent manipulation of cellular diffusivity and enable population-level directional movement of cells in a signal gradient.
Natural quorum sensing (QS) systems enable cell density-dependent control of gene expression in bacteria based on the production and detection of QS signal molecules30, 31. QS systems have been used by synthetic biologists for tuneable transcriptional control of gene expression32, 33, and the expression of a QS-signal synthase in E. coli has been shown to generate a signal gradient across a petri dish34. QS systems have been widely used for construction of genetic circuits in individual cells35, 36 and to enable communication in synthetic consortia37, 38. Previous work has used QS regulatory elements to control motility in E. coli strains lacking cheZ 20 or motB 21, where the missing motility gene was expressed from a QS-signal inducible promoter. The ability to reliably control gene expression and manipulate cells in cell-generated gradients make QS regulatory components ideal tools for examining transcriptional control of motility in E. coli in a signal gradient.
Here, we engineered E. coli strains where motility is tightly regulated by transcriptional control of the motor protein, MotA, and is induced by a QS signal molecule. We demonstrate robust directional control of motility in the engineered ‘receiver’ cells that was not only achieved in a gradient of exogenously added signal but also in a bio-generated gradient of the signal produced by ‘sender’ cells. We show that our sender-receiver architecture is modular and can be used to generate a range of sensitivity and responses to the signal. Further, we describe a mathematical model that provides insight into key aspects of system behaviour and enables predictive-design of motility-based pattern formation by cells.
Results
Design and characterization of signal-molecule dependent motility in E. coli
To build a system where the motility of E. coli is transcriptionally regulated by QS components, we used the esa QS system to control expression of MotA in an E. coli motA deletion strain (∆motA)39. MotA is a motor protein that provides a channel for the proton gradient required for generation of torque40. ∆motA strains can build flagella but are non-motile because they are unable to generate the torque required for flagellar rotation14. Expression of motA from a plasmid has been shown to restore motility in ∆motA strains41. Previous efforts to regulate motility using the activation-based lux QS system faced challenges in achieving tight regulation of the target gene and basal expression of the gene was sufficient to restore motility in the absence of the signal21. The esa QS system is from the plant pathogen Pantoea stewartii 42, and has been shown to provide tight regulation of genes downstream of the esaR promoter (PesaR)43. The QS regulator EsaR represses PesaR expression by binding to the promoter. The addition of acyl-homoserine lactone QS signal molecule, 3-oxo-hexanoyl homoserine lactone (3OC6HSL)43, induces gene expression from PesaR by triggering EsaR to release the promoter and allow RNA polymerase to bind and initiate transcription. Here, we constructed a two-plasmid system in ∆motA cells consisting of an EsaR-expression plasmid and a plasmid in which motA is placed under the control of PesaR. As shown in Fig. 1a, expression of motA is repressed by the transcriptional repressor (EsaR) in the absence of 3OC6HSL. In the presence of 3OC6HSL, EsaR is expected to dissociate from the promoter triggering expression of motA, thereby restoring motility in ∆motA cells. The green fluorescent protein was also placed downstream of PesaR to allow characterization of expression from PesaR, if motA expression was not sufficient to provide detectable motility in our assays. This strain is designated as the Communication-dependent Motility (CoMot) strain.
Figure 1.
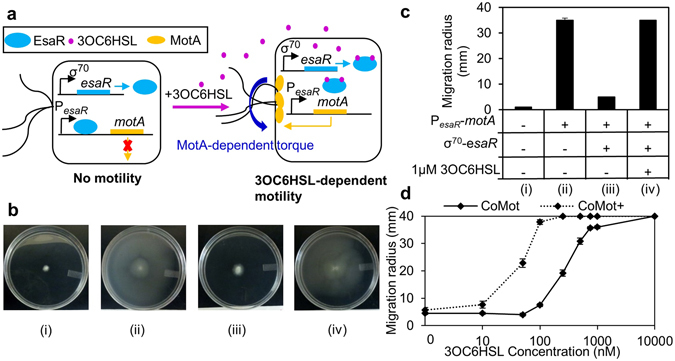
Engineered CoMot strains display 3OC6HSL-dependent motility: (a) Illustration of 3OC6HSL-dependent motA expression in the CoMot strain. Expression of motA is under the control of the PesaR promoter. esaR is constitutively expressed from a σ70-dependent promoter and represses PesaR. In the absence of 3OC6HSL, motA expression is repressed and the cell is non-motile. Following addition of 3OC6HSL, PesaR is de-repressed and motA is expressed. MotA generates the torque required to rotate the flagella and cellular motility is restored. (b) Plates were inoculated with (i) ΔmotA, (ii) ΔmotA transformed with plasmids containing PesaR-motA (iii) CoMot cells (ΔmotA transformed with plasmids containing PesaR-motA and Pσ70-esaR) on plates without 3OC6HSL and (iv) CoMot cells on plates with 1 μM 3OC6HSL and incubated at 30 °C for 36 h. Representative plate images are shown. (c) Migration radius was measured as the distance between the inoculation point and the visible edge of migration of cells on the plate for cases (i–iv). Error bars represent one standard deviation from the mean migration radius of three biological replicates. (d) Plates with 3OC6HSL concentration ranging from 0 to 10 μM were inoculated with CoMot or CoMot+ (ΔmotA transformed with plasmids containing PesaR-motA and Pσ70-esaR-D91G). The migration radius was measured after 36 h at 30 °C. Error bars represent one standard deviation from the mean migration radius of three biological replicates.
A motility assay using semi-solid agar plates that allow cells, which were inoculated by stabbing 1 μL of cells into the agar, to migrate through the media, was used to quantify motility. The migration radius was measured as the distance between the inoculation point and the visible edge of the migrating cells on the plate. As expected, migration was not observed on plates inoculated with ∆motA cells (Fig. 1b and c; case i). A migration radius of 35 mm was observed on plates inoculated with ∆motA cells transformed with a plasmid containing PesaR -motA (case ii), indicating that constitutive expression of MotA from PesaR was sufficient to restore motility in ∆motA cells. To assess if 3OC6HSL-inducible motility could be achieved in CoMot cells, they were inoculated on plates with and without 3OC6HSL. As seen in case iii, a migration radius of only 5 mm was observed in the absence of 3OC6HSL. This was comparable to the migration radius of ∆motA cells, demonstrating that motility in the absence of 3OC6HSL is minimal. A seven-fold increase in the migration radius exhibited by CoMot cells was observed in the presence of micromolar concentrations of 3OC6HSL (case iv), indicating that engineered cells exhibit signal-molecule dependent motility.
CoMot cells were inoculated on plates with 0, 10, 50, 100, 250, 500, 750, 1000 and 10000 nM 3OC6HSL, to assess their sensitivity to the signal molecule. As shown in Fig. 1d, an increase in migration radius was observed with increasing 3OC6HSL concentrations, where 250 nM 3OC6HSL was required to observe a migration radius larger than the background migration radius observed in the absence of 3OC6HSL (p = 0.0017). To increase the 3OC6HSL sensitivity of the cells, we replaced the transcriptional repressor EsaR with a variant, EsaR-D91G and designated this strain as CoMot+. E. coli cells with EsaR-D91G have been reported to display a 100-fold higher sensitivity to 3OC6HSL compared to wild-type EsaR in a luminescence-based promoter assay43. As seen in Fig. 1d, CoMot+ cells required 50 nM 3OC6HSL to display a migration radius above background (p = 0.0028), demonstrating that the CoMot+ strain does exhibit increased sensitivity to 3OC6HSL. In addition, 10000 nM of 3OC6HSL was required for CoMot cells to reach the edge of the plate in 36 hours, while only 250 nM was required for CoMot+ cells (Fig. 1d).
Characterization of directional movement of the CoMot variants in a 3OC6HSL gradient
To assess if CoMot and CoMot+ cells display directional movement in a signal gradient, 0.02 μmoles of 3OC6HSL was added on a membrane (3OC6HSL source), placed 1.25 cm from the edge of the plate, and allowed to diffuse and establish a gradient for 8 h prior to inoculation of cells at the centre of the plate. 1 μM of 3OC6HSL would be the final concentration if the 0.02 μmoles diffused uniformly through the 25 mL plate. As shown in Fig. 2, both CoMot variants reached the edge of the plate (40 mm) in the forward direction towards the 3OC6HSL source by 36 h. Here, we define forward migration distance as distance between the inoculation point and the visible edge of cells that have migrated up the signal gradient. The reverse migration distance (distance between the inoculation point and the visible edge of cells that have migrated down the signal gradient) was 10 mm for CoMot and 18 mm for CoMot+. Therefore, directional movement of both CoMot variants up the 3OC6HSL gradient was observed. Directional movement was not displayed by ∆motA cells that contain PesaR-motA and constitutively express motA (Fig. 2), indicating that regulation by EsaR or EsaR-D91G in the 3OC6HSL gradient is required for directional movement.
Figure 2.
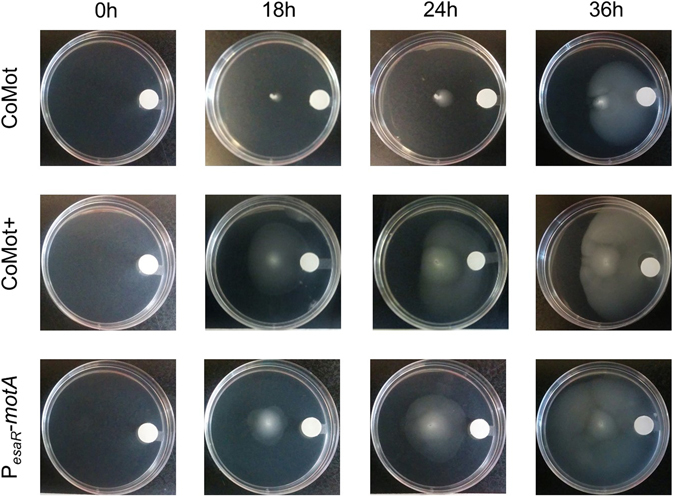
CoMot and CoMot+ cells in a 3OC6HSL gradient show directional movement towards the 3OC6HSL source: A 3OC6HSL gradient was established by adding 0.02 μmoles of 3OC6HSL to a Whatmann membrane and allowing it to diffuse for 8 h. 1 μM of 3OC6HSL would be the final concentration if 0.02 μmoles of 3OC6HSL diffused uniformly through the plate. CoMot, CoMot+ or cells that constitutively express motA (∆motA transformed with a plasmid containing PesaR-motA) were then inoculated at the centre of the plate. Images were obtained following 0, 18, 24 and 36 h of incubation at 30 °C. The assay was run in triplicate for each strain and representative images are shown.
We then examined the sensitivity of CoMot and CoMot+ cells in gradients established using varying amounts of 3OC6HSL. As seen in Fig. 3a, both CoMot variants showed an increase in the forward migration distance with increasing 3OC6HSL concentrations. Similar to the uniform 3OC6HSL-titration results, a lower 3OC6HSL concentration was required to observe forward migration distances greater than background levels with the CoMot+ (100 nM) than CoMot cells (500 nM). Both strains displayed directional movement towards the 3OC6HSL source (Fig. 3b). We also observed that the forward migration distance decreased when cells were inoculated at increasing distances from the 3OC6HSL source indicating that motility response is affected by the spatial arrangement of the signal and cells (Supplementary Fig. S1).
Figure 3.
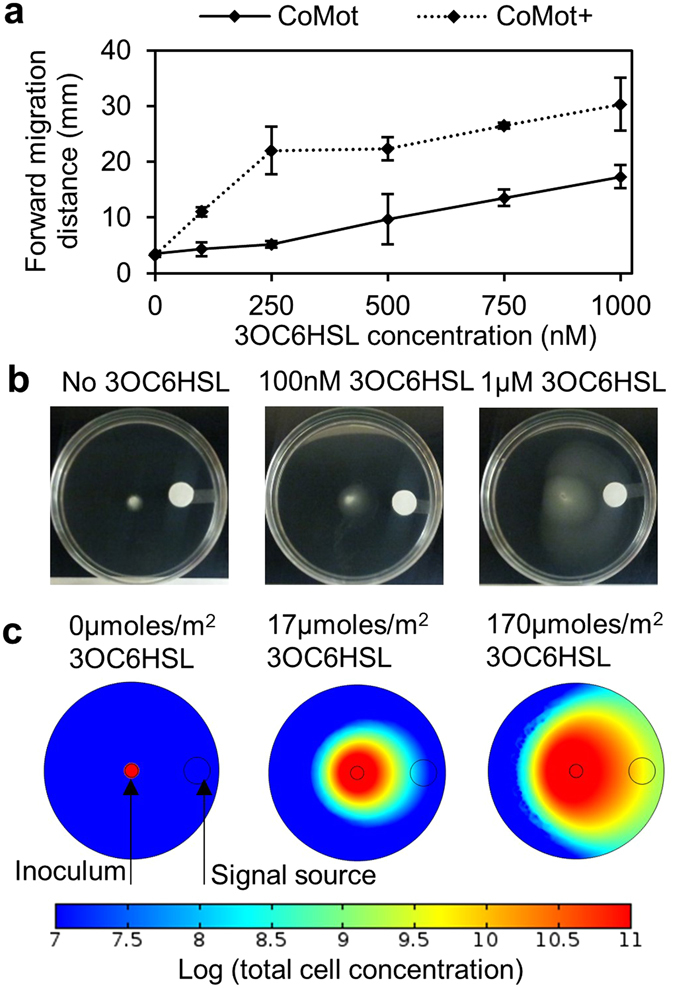
Motility assays and simulations show 3OC6HSL-dependent directional movement of cells in a 3OC6HSL gradient: (a) 3OC6HSL gradients were established by adding 0–0.02 μmoles of 3OC6HSL on a Whatmann membrane and allowing it to diffuse for 8 h. CoMot and CoMot+ cells were then inoculated at the centre of the plate. The forward migration distance was measured as the distance between the inoculation point and the visible edge of migration of cells up the signal gradient after 24 h of incubation at 30 °C. Error bars represent one standard deviation from the mean forward migration distance of three biological replicates. (b) Representative images of plates inoculated with CoMot+ cells. (c) Results from simulation of the migration response of cells in a similar set up as in (b). Signal gradients were simulated by using 0, 17 or 170 μmoles/m2 of the signal near the edge of the plate. 3.5*107 CoMot+ cells/m2 was used as the inoculum at the centre of the plate. The log10 of the total cell concentration after a simulation time of 24 h is shown in the images.
Modelling of signal molecule-guided bacterial motility
To gain insight into the key parameters controlling motility in the engineered strains and to identify factors contributing to the observed directional movement, we developed a mathematical model. The distribution of CoMot cells in response to the signal molecule was modelled using Equations (1–3). We used a Michaelis Menten-type term (term III) to model the rate of switching from static (s) to motile cells (m) in the presence of the signal molecule (A) and an inhibition-kinetics equation to capture the switching from motile to static cells (term IV). Parameters k 1 and γ capture the maximum rate of switching from static to motile and motile to static, respectively. K 2 and K 4 define the sensitivity of cells to A. The displacement of motile cells is modelled via a diffusion term (term I), where D m is the effective diffusivity of cells. We used Monod kinetics to capture the exponential growth of cells. λ represents growth rate in term II. Diffusion of the signal molecule is captured in term V, where D a represents the diffusivity of A. A two-dimensional version of the experiment was modelled using geometry (plate size and location of signal and cells) similar to the experimental setup. The model was simulated using parameters estimated experimentally or from the literature (Supplementary Table S1). When experimental quantification was not possible and when quantitative values were unavailable in literature parameter values were chosen based on educated guesses in biologically feasible regimes. These values were then tuned to fit experimental findings when required.
| 1 |
| 2 |
| 3 |
We started by simulating the migration response of CoMot+ cells in gradients established using 0, 17 or 170 µmole/m2 of the signal molecule. In the motility assays, 0.02 μmoles of 3OC6HSL were added to the membrane and used to generate a gradient equivalent to 1 μM. In the 2D simulations, 0.02 μmoles was initially distributed across the area of the membrane (1.13*10−4 m2). Therefore, an initial signal concentration of 170 μmoles/m2 (0.02 μmoles/1.13*10−4 m2) on the membrane was used, where 3.8 μmoles/m2 would be the final concentration if the signal diffused uniformly across the simulated area of the plate. Images after a simulated time of 24 h are shown in Fig. 3c. In the absence of the signal, simulated cells remained at the inoculation point. Similar to experimental observations (Fig. 3b), an increase in movement of simulated cells towards the signal source was observed with increase in signal concentration. To model the difference in 3OC6HSL sensitivity of CoMot and CoMot+ cells, we increased the sensitivity parameter, K 2 from 1 to 100 nmole/cm2. Time course simulations of CoMot and CoMot+ cells in a gradient established using 170 µmole/m2 of the signal are shown in Supplementary Fig. S2. CoMot+ cells displayed approximately 2-fold higher forward migration distance than CoMot cells after a simulation time of 24 h, where a cell concentration ≥108 was used as the cut off for the migration distance in the simulations. Thus, our model is representative of the system and captures key system properties - signal-molecule dependent directional movement in a gradient and the difference in the 3OC6HSL sensitivity between CoMot and CoMot+.
To understand the effect of the two switching rates on system behaviour, we varied k 1 and γ and plotted the ratio of motile to static cells (Fig. 4a and supplementary Fig. S3a). An increase in the motile to static cell ratio was observed with increasing k 1 and decreasing γ. The increase in this ratio leads to an increase in both the forward and reverse migration distances. Thus, varying the switching rates allow for tuning of the magnitude of motility response of the cells. Our simulations showed that m/s increases as cells move towards the 3OC6HSL source, indicating that motile cells dominate the population up the signal gradient and static cells dominate down the gradient. Thus, a cell once motile, though capable of moving in any random direction, remains motile if it happens to move up the gradient, but switches to static if it migrates down the gradient and into a region of low signal. This static population continues to accumulate and grow. Directional movement then results from population-level movement of motile cells towards the signal source.
Figure 4.
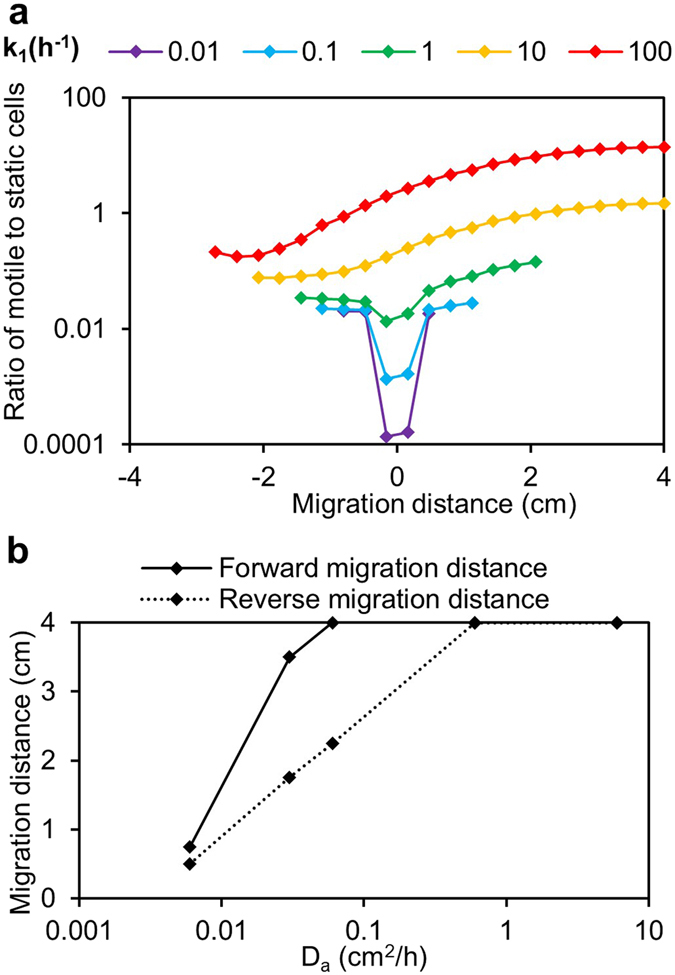
The rate of switching from static to motile (k 1) and the diffusivity of the signal (D a) are keys parameters controlling migration of cells: In the simulations, the gradient was established using 85 μmoles/m2 of the signal near edge of the plate (migration distance = 4 cm). 3.5*107 static cells/m2 was used as the inoculum at the centre of the plate (migration distance = 0 cm). (a) k 1 was varied from 0.01–100 h−1 while all other parameters were held constant at values defined in the base parameter set. For each value of k 1, the ratio of motile to static cells (m/s) across the diameter of the plate (migration distance = −4 to 4 cm) was plotted after a simulation time of 24 h. The ratio was only calculated at points with a total cell concentration ≥108 cells/m2. (b) Simulations were run varying Da from 0.01−10 cm2/h while holding all other parameters constant. Forward and reverse migration distances were measured as distances from the inoculation point (migration distance = 0) towards and away from the signal source at which a total cell concentration ≥108 cells/m2 was observed after a simulation time of 24 h.
Simulations varying the signal diffusivity (D a) were run to assess its effect on the established gradient on migration response. The signal concentration across the plate (Supplementary Fig. S3b) and the forward and reverse migration distances were examined (Fig. 4b) for each simulated D a. Directional movement, as indicated by a greater forward compared to reverse migration distance, was only observed with D a sufficient to establish a gradient (D a < 0.6 cm2/h). It is thus evident that the established signal gradient drives the directional movement of the cells.
K 2 and K 4 capture the 3OC6HSL-sensitivity of cells when switching from static to motile and motile to static, respectively. Our simulations showed that a 104-fold increase in K 2 resulted in a 1000-fold increase in the signal concentration required to achieve a forward migration distance equivalent to reaching the edge of the plate in 24 hours, while a 108-fold increase in K 4 resulted in only a 50-fold increase (Supplementary Fig. S3c,d). Thus, the sensitivity of the cells to 3OC6HSL when switching from static to motile has a larger effect on overall system behaviour than the sensitivity when switching from motile to static. At high K 4 (K 4 > 2500 nmole/cm2), where switching from motile to static becomes independent of 3OC6HSL, directional movement of cells was still observed. Here, dilution of MotA as cells grow and divide leads to switching from motile to static if the local concentration of 3OC6HSL is not sufficient to induce additional motA expression. However, in simulations with low K 4 (K 4 < 0.0025 nmole/cm2), directional behaviour was not observed (Supplementary Fig. 3e,f). In this case, cells never switch back to static once they become motile and thus continue migrating across the plate. Overall, these simulations indicate that while it is essential that cells are able to switch from motile to static (term IV), observed experimental behaviour and directional movement can be captured if term IV is modelled as dependent or independent of signal concentration.
We observed that both the forward and reverse migration distances increased when the effective diffusivity of cells (D m) was increased (Supplementary Fig. S3g). Directional movement towards the signal was not observed at values of D m 10-fold greater than the experimentally estimated 0.1 cm2/h. This could be because in the simulated geometry-scale at high D m, once motile, the diffusivity of cells was sufficient to keep them motile regardless of the local signal concentration. These studies have shown that the distribution of cells depends both on the parameters regulating motility and the established gradient.
Model-guided pattern formation
We next explored if our model can be used to predict cell distribution patterns formed in response to changing the spatial arrangement of the signal and cells. Representative patterns of cell distribution that were simulated under different spatial arrangements of signal and cells and tested in similar experimental set ups are shown in Fig. 5. In case (i), the pattern predicted to be formed by cells inoculated diametrically opposite to each other in a plate with a signal source at its centre is in agreement with the pattern experimentally observed on a petri dish with CoMot+ cells inoculated in a similar set up. In case (ii), we were able to successfully predict the pattern formed by cells inoculated at the centre of a square petri dish with signal sources along a diagonal. We were thus able to predict the distribution of cells in response to different initial arrangements of the signal and cells, thereby enabling predictive-design of pattern formation by cells.
Figure 5.
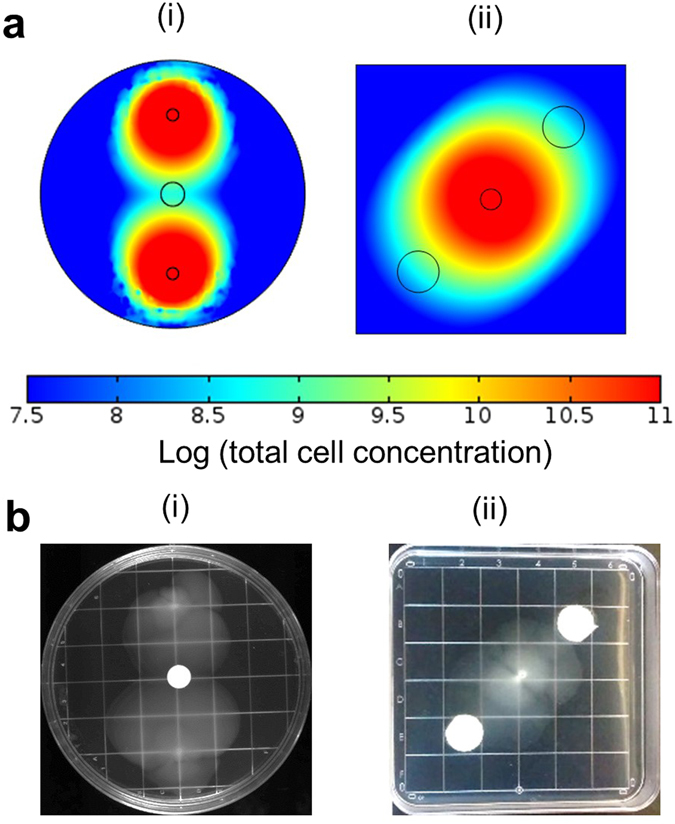
Predictive design and experimental verification of motility-induced pattern formation: (a) Results from simulation of the patterns formed by cells in response to different spatial arrangements of signals and starting inoculum of cells. (i) 3.5*107 CoMot+ cells/m2 were used as the inoculum at opposite edges of a circular plate and a gradient was established using 85 μmoles/m2 of the signal at the centre of the plate. 85 μmoles/m2 of the signal is equivalent to an experimental 3OC6HSL concentration of 500 nM. Images were obtained after a simulation time of 36 h. (ii) 3.5*107 CoMot+ cells/m2 were used as the inoculum at the centre of a square plate in which the signal gradient was established using two signal sources along a diagonal. 85 μmoles/m2 of the signal was used for each source to establish the gradient. Images were obtained after a simulation time of 36 h. (b) Experimental verification of patterns predicted by the model. (i) The equivalent of 500 nM of 3OC6HSL was added on a membrane 12 h prior to inoculation of CoMot+ cells at opposite edges of the plate. (ii) Membranes with the equivalent of 500 nM of 3OC6HSL were placed along the diagonal of the plate 12 h prior to inoculation of CoMot+ cells at the centre. Each pattern was experimentally verified in triplicate and representative images obtained after 36 h of incubation at 30 °C are shown.
Engineering CoMot variants to migrate down a 3OC6HSL gradient
We sought to engineer a strain that migrates down a 3OC6HSL gradient. The PesaS promoter, which is activated by EsaR in the absence of 3OC6HSL44, was used to control expression of MotA. We predicted that this new strain, CoMot-S, would express MotA and be motile in the absence of 3OC6HSL and that motility would decrease with increasing 3OC6HSL due to dissociation of 3OC6HSL-bound EsaR from PesaS (Fig. 6a). CoMot-S and CoMot-S+ cells with wild-type EsaR and EsaR-D91G, respectively, were inoculated on plates with uniform 3OC6HSL concentrations ranging from 0–10 µM (Supplementary Fig. S4). In the absence of 3OC6HSL, migration was observed for both strains. Approximately 1 µM of 3OC6HSL was required to observe a migration radius significantly smaller (CoMot p = 0.02, CoMot+ p = 0.016) than that observed in the absence of 3OC6HSL after 24 h of incubation at 30 °C. However, no difference in the migration radius of CoMot-S cells was observed in the presence or absence of 3OC6HSL after 36 h of incubation. The migration radius of CoMot-S+ cells, on the other hand, decreased 2.7-fold in the presence of 10 μM 3OC6HSL. Leaky expression of motA from the activation-based PesaS-controlled system was likely sufficient to restore motility in the CoMot-S strains even in the presence of 3OC6HSL. Despite the incomplete control of motility, we decided to assess whether we would observe directional motility of CoMot-S and CoMot-S+ down a 3OC6HSL gradient. We inoculated CoMot-S strains on plates with gradients established using 3OC6HSL concentrations equivalent to 0, 100, 250, 500, 1,000 and 10,000 nM. As seen in Fig. 6b, in the absence of 3OC6HSL, the forward and reverse migration distances are similar for the CoMot-S strains. In the presence of 3OC6HSL, migration to the edge of the plate away from the source (reverse migration distance) was observed under all conditions except for CoMot-S+ at 10 μM 3OC6HSL. A decrease in the forward migration distance with increasing 3OC6HSL concentration was observed with CoMot-S+, while 10 µM 3OC6HSL was required to see a decrease in forward migration distance with CoMot-S cells after 24 h of incubation (Fig. 6c). The directional movement away from the source was observed even after 36 h of incubation despite the previously observed migration of the CoMot-S strains in the uniform 3OC6HSL motility assays. The CoMot-S strains are capable of migrating down a 3OC6HSL gradient, thereby adding to our repertoire of ways to control the directional motility of E. coli.
Figure 6.
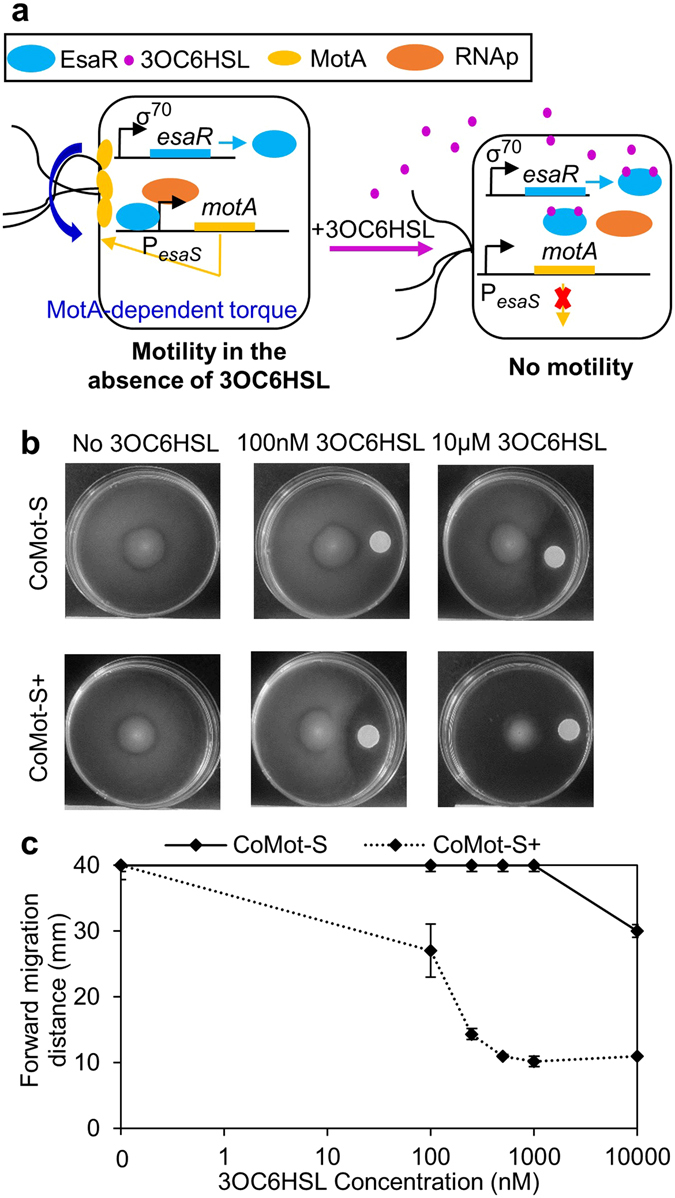
Characterization of the migration response of CoMot-S and CoMot-S+ cells in a 3OC6HSL gradient shows directional movement of cells away from a 3OC6HSL source: (a) Illustration of 3OC6HSL-dependent motA expression in the CoMot-S strain. Expression of motA is under the control of the PesaS promoter. esaR is constitutively expressed from a σ70-dependent promoter and activates expression from PesaS. In the absence of 3OC6HSL, motA is expressed and motility is restored in the ΔmotA cells. Following addition of 3OC6HSL, EsaR unbinds from PesaS. motA expression decreases and the cells become non-motile. (b) 3OC6HSL gradients were established by adding 0–53 μg of 3OC6HSL on a Whatmann membrane and allowing it to diffuse for 8 h. 10 μM of 3OC6HSL would be the final concentration if 53 µg of 3OC6HSL diffused uniformly through the plate. CoMot-S (ΔmotA transformed with plasmids containing PesaS-motA and Pσ70-esaR) or CoMot-S+ (ΔmotA transformed with plasmids containing PesaS-motA and Pσ70-esaR-D91G) cells were then inoculated at the centre of the plate. Representative plate images obtained after 24 h of incubation at 30 °C. (c) Forward migration distance was measured as the distance between the inoculation point and the visible edge of migration of cells up the signal gradient after 24 h of incubation at 30 °C. Error bars represent one standard deviation from the mean forward migration distance of three biological replicates.
Design and characterization of a sender-receiver system
To test if motility in CoMot strains can be regulated in a 3OC6HSL gradient generated by a second population of cells, we engineered non-motile E. coli sender strains that constitutively express a 3OC6HSL synthase (EsaI). Sender strains that produce different amounts of 3OC6HSL were designed by modifying the strength of the ribosome-binding site (RBS) upstream of esaI. We used two luminescent E. coli reporter strains with different dynamic ranges to quantify 3OC6HSL production by each sender strain. We observed that the sender strain with a weak RBS (weak-sender) produced approximately 10 µM 3OC6HSL and strain with the strong RBS (strong-sender) produced approximately 100 µM (Supplementary Fig. S5).
We used motility assays to assess if the different senders are able to induce motility in the CoMot and CoMot-S strains (receivers). Sender strains were added on a membrane, placed on the plate surface, and incubated at 30 °C for 8 h prior to inoculation of a CoMot variant. As shown in Fig. 7a, the forward migration distance of CoMot cells inoculated onto plates with control cells that do not produce EsaI was comparable to levels of background migration observed in the absence of 3OC6HSL. CoMot cells inoculated onto plates with either sender strain were observed to be motile, and the forward migration distance with the strong-sender cells was significantly higher (p = 0.0028) than with the weak-sender cells. As expected, the more-sensitive CoMot+ cells showed a greater forward migration distance in response to both senders than the CoMot cells (Fig. 7b). No significant difference between the forward migration distances of CoMot+ cells was observed in response to the two sender cell strains. However, a higher density of CoMot+ cells was observed in response to the strong senders, indicating that motility was likely turned on in a larger population of CoMot+ cells (Fig. 7a). With the CoMot-S+ strain, migration to the edge of the plate was observed with the control cells. A decrease in forward migration distance was observed in the presence of both senders, where the decrease in forward migration distance was significantly higher in the presence of the strong-sender cells. With the weak senders, CoMot cells displayed a 1.9-fold (p = 0.0081) and CoMot+ cells a 1.6-fold (p = 0.00086) higher forward migration distance than reverse migration distance (distance between the inoculation point and the visible edge of cells that have migrated away from the senders). Similarly, CoMot and CoMot+ cells displayed 1.6-fold (p = 0.0025) and 1.5-fold (p = 0.035) higher forward than reverse migration distance with the strong senders. On the other hand, CoMot-S+ cells displayed a 1.9-fold (p = 0.015) lower forward migration distance than reverse migration distance in response to the weak senders and 2.6-fold lower (p = 0.015) with the strong senders. These observations indicate directional movement of CoMot and CoMot+ cells towards the senders and movement of CoMot-S+ away from the senders. The 3OC6HSL-insensitive strains (ΔmotA transformed with plasmids containing PesaR-motA or PesaS-motA) did not display directional movement (Supplementary Fig. S6). Further, the modularity of the engineered sender-receiver architecture enables tuning of the 3OC6HSL gradient, motility response and the overall direction in which the population of cells migrates.
Figure 7.
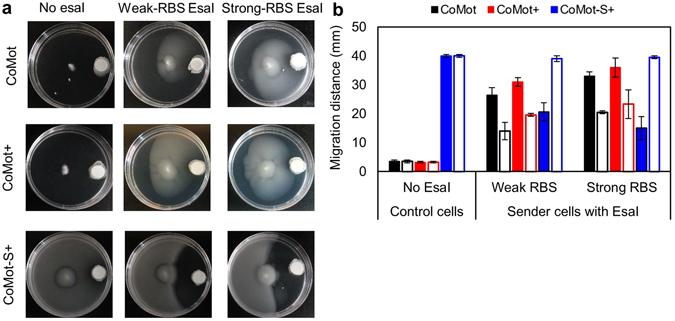
CoMot, CoMot+ and CoMot-S+ display directional movement in 3OC6HSL gradients generated by sender strains: (a) Control cells with no plasmid for EsaI expression, or sender cells where EsaI expression is controlled by a weak or strong RBS were used. Control, weak-sender and strong-sender cells were added on a Whatmann membrane and the plates were incubated for 8 h at 30 °C. CoMot, CoMot+ or CoMot-S+ were then inoculated at the centre of the plate and incubated at 30 °C for 36 h. Representative plate images are shown. (b) Plot of forward (solid bars) and reverse (open bars) migration distances for each sender/receiver combination. Error bars represent one standard deviation from the mean forward migration distance of three biological replicates.
Discussion
We have engineered QS signal-dependent motility in E. coli via transcriptional control of the motor protein, motA. In the absence of a gradient, the migration of cells was positively affected by the 3OC6HSL concentration and directional movement towards the signal source was observed in the presence of a signal gradient. Simulations of the migration response of CoMot cells further indicate that a gradient is essential for directional behaviour. The enhanced migration exhibited by cells in a signal gradient is remarkably similar to the behaviour of enzyme-based systems in their substrate gradients. For example, the increase in diffusivity of urease with increasing urea concentrations enables directed-propulsion of urease-coated beads up a gradient27–29. In our system, the motility of CoMot cells increases with increasing 3OC6HSL concentrations leading to enhanced diffusivity of cells that migrate to locations with signal concentrations sufficient to induce motA expression. This enhanced diffusivity enables population-level movement of cells up a signal gradient.
We also engineered CoMot-S cells that move down a 3OC6HSL gradient by changing the promoter controlling motA expression from the PesaR to PesaS. Such a system, where cells can be manipulated to move up or down a signal gradient by switching one regulatory element, would be difficult to engineer with chemotaxis-based control, where a signal typically behaves as either an attractant or repellant45, 46. Although chemotaxis was not manipulated in CoMot cells, it is possible that chemotaxis enhances their directional behaviour in our experimental setup. At the beginning of the motility assays, any cell leaving the point of inoculation will move to a region of the plate that is nutrient rich. However, as the population distributes itself up and down the 3OC6HSL gradient, the CoMot cells that move up the 3OC6HSL gradient will continue to enter nutrient-rich regions, while those migrating down the gradient may enter regions potentially depleted of nutrients by the accumulated static cells. Therefore, chemotaxis may enhance migration towards nutrient-rich regions that are available up the gradient and increase the forward migration distance.
While chemotaxis-enabled directional movement of cells occurs at the individual-cell level15, the directional movement of CoMot cells occurs at the population-level. Further studies are required to explore if CoMot cells will exhibit observable levels of enhanced-diffusivity up a 3OC6HSL gradient at the microscopic-level and whether this type of control can be used for applications in microfluidic devices. The time required for non-uniform distribution of CoMot cells in signal gradient, which requires transcription, translation and cell movement, is also expected to be much longer than a chemotaxis-based system, which requires only protein phosphorylation47, 48. These two time-scales may be advantageous for different applications, and could potentially be combined for precise cellular movement in response to signal molecules. Previously, a population of Serratia cells was attached to a 10 μm-sized piece of polymer to generate a micro-bio-robot. The motility of the cells was used to propel the robot. However, the direction of movement was controlled using external magnetic fields49. An understanding of the behaviour of CoMot cells under microfluidic conditions could enable their use as actuators of both motion and direction for micro-bio-robots or targeted-delivery systems.
Pairing our QS-responsive receiver strains, CoMot, CoMot+ and CoMot-S+, with 3OC6HSL-producing sender strains demonstrated that our motility-control system is both modular and tuneable. As described above, the use of EsaR variants and esa promoter variants enabled the engineering of receiver strains that display a range of behaviours in terms of signal sensitivity, whether motility is turned on or off in the presence of 3OC6HSL, and the directionality of movement in a signal gradient. The amount of 3OC6HSL produced by the sender cells was tuned by varying the strength of the RBS upstream of esaI. The ability to manipulate motility in CoMot cells using sender cells could enable the use of our sender-receiver system to detect other signals of interest. Detection of target signals using the sender-receiver system can be achieved by swapping the constitutive promoter controlling esaI expression to one regulated in response to any target signal of interest. Advantages of this architecture for biosensing-system design include modularity and signal amplification via QS50. Further, logic gate-type behaviours in the receivers may enable more complex sensing systems51, 52. For example, multiple senders engineered to detect different target molecules and produce 3OC6HSL could be combined to generate a modular, tuneable OR-type sensor system that is turned on by any one of the target molecules. Multiple intermediary QS signals could be used to generate a broader range of signal integration circuits. These systems can be applied for biosensing and bioactuation in complex environments, such as a tumour, soil or the human gastrointestinal tract, where recognition, integration and reporting of multiple signal inputs is advantageous.
We have demonstrated that transcriptional regulation of a motility gene allows for control of cell motility and enables directional movement in gradients generated by exogenously added signal or in-situ bio-production. The strategy used here presents a robust mechanism for controlling the directional movement of a population of cells without the manipulation of chemotaxis. We anticipate that these engineered cells will find application as actuators for micro-bio-robots and as drivers of motility-dependent pattern formation. The modular sender-receiver architecture, combined with the population-level control of directional movement, sets the stage for development of biosensing frameworks where senders are engineered to detect target stimuli and produce a QS molecule, and cellular motility in the receivers serves as novel biosensing output.
Methods
Plasmid Construction and strains
The motA-deletion strain (∆motA), E. coli RP666639, was used in this study. Plasmids and primers used in this study are listed in Supplementary Tables S2 and S3. Sequences of the promoters and ribosome binding sites (RBS) used in this study are provided in Supplementary Table S4 and S5. To engineer the receiver strains, we constructed a two-plasmid system consisting of a motA-expression plasmid and an esaR-expression plasmid. pCS-PesaR -motA-gfp and pCS-PesaS -motA-gfp were used for expression of motA. To construct pCS-PesaR -motA-gfp, the PesaR promoter, motA and gfp were cloned between the XhoI and NotI sites of a low-copy, pCS26 plasmid. We tuned the strength of the RBS upstream of motA and gfp using the RBS calculator53, 54 and the 5′ primer was used to add the modified RBSs to the genes. motA was PCR-amplified from pDFB3641 using the primers 5′-SMotA-KpnI and 3′-MotA-BamHI. PesaR was PCR-amplified from pCS-PesaR-gfp using the primers 5′-PesaR-XhoI and 3′-PesaR-KpnI-SMotA. The amplified PesaR and motA were assembled using assembly PCR with the primers 5′-PesaR-XhoI and 3′-MotA-BamHI. gfp was PCR-amplified from pCS- Pσ70-gfp using the primers 5′-NotI-SGFP and 3′-BglII. To construct pCS-PesaS -motA-gfp, the PesaS promoter was PCR-amplified from pCS-PesaS-lux 55 using the primers ZEO5 and 3′-KpnI-PesaS. The amplified product was digested with XhoI and KpnI and ligated into XhoI and KpnI-digested pCS-PesaR -motA-gfp. pAC- Pσ70-esaR and pAC- Pσ70-esaR-D91G 43 were used as esaR-expression plasmids. In these plasmids, a Pσ70-dependent promoter and either esaR or esaR-D91G genes were cloned between the XbaI and BamHI sites of the medium-copy, pACYC184 plasmid. To construct the CoMot and CoMot+ strains, ∆motA competent cells were transformed with pCS-PesaR -motA-gfp and pAC- Pσ70-esaR or pAC- Pσ70-esaR-D91G respectively. Similarly, to construct the CoMot-S and CoMot-S+ strains, pCS-PesaS -motA-gfp and pAC- Pσ70-esaR or pAC- Pσ70-esaR-D91G were transformed into ∆motA cells.
For construction of the sender strains, two esaI expression plasmids, pAC-Plac-(RBSweak)esaI or pAC-Plac-(RBSstrong)esaI, were constructed. To construct pAC-Plac-(RBSstrong)esaI, esaI with the strong-RBS was amplified from pAC-Plac-esaR-esaI 55 using the primers 5′-KpnI-esaI and 3′-BamHI-esaI. The amplified product was digested with KpnI and BamHI and ligated into KpnI and BamHI-digested pAC-Plac-esaR 43. To construct pAC-Plac-(RBSweak)esaI, the Plac promoter was amplified from pAC-Plac-esaR-esaI 55 using the primers 5′-pAC-promseq and 3′-Plac-BamHI. The amplified product was digested with XbaI and BamHI and ligated into XbaI and BamHI-digested pAC-Pσ70-esaR-esaI 56. For construction of the sender strains, ∆motA competent cells were transformed with pCS26 and an esaI expression plasmids. For construction of control strains, ∆motA competent cells were transformed with pCS26 and pACYC184. E. coli DH5α strain was used in all cloning procedures.
Overnight cultures
Overnight cultures were made by inoculating single colonies of strains picked from Luria Broth (LB) agar plates in 5 mL of LB media with chloramphenicol (50 μg/mL) and kanamycin (50 μg/mL). The cultures were incubated overnight at 37 °C with shaking (225 rpm).
Motility assays
Semi-solid media consisted of 1% tryptone, 0.5% NaCl and 0.25% agar with chloramphenicol (25 μg/mL) and kanamycin (25 μg/mL). For assays requiring a uniform 3OC6HSL concentration across the plate, 3OC6HSL was directly added to the media before pouring into petri dishes. To establish a 3OC6HSL gradient, 0.002 to 0.2 µmoles of 3OC6HSL was added to a Whatmann membrane (Grade 3–6 µm, diameter: 1.2 cm). 10 μM of 3OC6HSL would be the final concentration if 0.2 µmoles of 3OC6HSL diffused uniformly through the plate. The centre of the membrane was placed 1.25 cm from the edge of the plate and 3OC6HSL was allowed to diffuse from the membrane into the media at room temperature for 8 h prior to inoculation unless otherwise indicated. In the sender-receiver motility assays, overnight cultures of the sender strains were concentrated 10-fold by centrifuging the cultures and re-suspending them in LB. 10 μL of the concentrated cultures were added to a Whatmann membrane, which was placed 1.25 cm from the edge of the plate and incubated at 30 °C for 8 h prior to inoculation with the receiver cells. For all motility assays, receiver cells were inoculated using a pipette tip containing 1 μL of overnight culture. The tip was inserted at the centre of the plate, approximately 3 mm below the surface of the media, and the cells were ejected as the tip was pulled up through the media. To assess migration, images were obtained up to 48 h after incubation at 30 °C.
Quantitative characterization of 3OC6HSL produced by sender strains
Overnight cultures of sender strains were diluted 100-fold in 5 mL LB with appropriate antibiotics and grown at 37 °C with shaking (225 rpm) for 8 h. 1 mL of the culture was centrifuged at 16,000 rcf for 3 minutes. The supernatant was filter sterilized using a 0.2 μM polyether sulfone filer. Two E. coli reporter strains (DH5α cells transformed with (i) pCS-PesaR-lux and pAC-Pσ70-esaR-I70V/D91G (ii) pCS-PesaR-lux and pAC- Pσ70-esaR 43) that luminesces in a 3OC6HSL concentration-dependent manner were used for quantification of 3OC6HSL produced by the sender cells, by comparing luminescence in response to 3OC6HSL in the supernatants to the response to known amounts of 3OC6HSL. The quantitative characterization of 3OC6HSL using the reporter strain was performed as described by Shong et al.43.
Statistical analysis
Two-tailed paired t-tests were applied to evaluate significance when required. p values are reported in the text for each statistical test.
Modelling of signal molecule-guided bacterial motility
All simulations were run in COMSOL Multiphysics version 5.1. A 2-dimensional version of the experimental set up was simulated in the model. Circular, 8 cm-diameter plates were used in all simulations except in pattern-formation simulations. In pattern-formation simulations, either circular 15 cm-diameter plates or 13 × 13 cm square plates were used. Neumann boundary conditions are imposed on Equations (1) and (3) to ensure that motile cells and the signal molecule do not diffuse beyond the boundaries. All simulations were started with 3.5*107 static cells/m2 (equivalent of 1000 cells) as the inoculum and 170 μmoles/m2 of the signal as the signal source and run for a simulation time of 24 h unless otherwise noted. The location of the cells (inoculation point) and signal source were similar to the experimental set up. When reported, forward and reverse migration distances were measured as distances from the inoculation point towards and away from the signal source where a total cell concentration (m + s) ≥ 108 cells/m2 was observed. In all simulated plate images log (total cell concentration) is shown. To simulate the migration response of CoMot and CoMot+ cells K 2 values of 2.5 and 1 nmoles/cm2 were used respectively. In the studies where simulations were run varying the value of each parameter, the gradient was established using 85 μmoles/m2 of the signal near edge of the plate (migration distance = 4 cm).
Electronic supplementary material
Acknowledgements
This work was supported by the Army Research Office (W911NF1110490).
Author Contributions
J.D.R., A.G.B. and C.H.C. conceived the ideas for this work and planned experiments. J.D.R. and A.G.B. executed experiments and analysed data. J.D.R., A.A.J. and C.H.C. developed the mathematical model. J.D.R., A.A.J. and C.H.C. wrote and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08870-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sourjik V, Wingreen NS. Responding to chemical gradients: bacterial chemotaxis. Curr. Opin. Cell Biol. 2012;24:262–268. doi: 10.1016/j.ceb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guttenplan SB, Kearns DB. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013;37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr B, Riley MA, Feldman MW, Bohannan BJM. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- 4.Salis, H., Tamsir, A. & Voigt, C. In Bacterial Sensing and Signaling16, 194–225 (KARGER, 2009). [DOI] [PubMed]
- 5.Tecon R, van der Meer JR. Bacterial biosensors for measuring availability of environmental pollutants. Sensors. 2008;8:4062–4080. doi: 10.3390/s8074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voigt CA. Genetic parts to program bacteria. Curr. Opin. Biotechnol. 2006;17:548–557. doi: 10.1016/j.copbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Sinha J, Reyes SJ, Gallivan JP. Reprogramming bacteria to seek and destroy an herbicide. Nat. Chem. Biol. 2010;6:464–470. doi: 10.1038/nchembio.369. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Tien S-M, et al. Engineering bacteria to search for specific concentrations of molecules by a systematic synthetic biology design method. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weibel DB, et al. Microoxen: microorganisms to move microscale loads. Proc. Natl. Acad. Sci. USA. 2005;102:11963–11967. doi: 10.1073/pnas.0505481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steager EB, Julius AA, Kim M, Kumar V, Pappas GJ. Modeling, control and experimental characterization of microbiorobots. Int. J. Rob. Res. 2011;30:647–658. doi: 10.1177/0278364910394227. [DOI] [Google Scholar]
- 11.Berg HC. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Sourjik V. Assembly and stability of flagellar motor in Escherichia coli. Mol. Microbiol. 2011;80:886–899. doi: 10.1111/j.1365-2958.2011.07557.x. [DOI] [PubMed] [Google Scholar]
- 13.Fahrner KA, Block SM, Krishnaswamy S, Parkinson JS, Berg HC. A mutant hook-associated protein (HAP3) facilitates torsionally induced transformations of the flagellar filament of Escherichia coli. J. Mol. Biol. 1994;238:173–186. doi: 10.1006/jmbi.1994.1279. [DOI] [PubMed] [Google Scholar]
- 14.Kojima S, Blair DF. Conformational Change in the Stator of the Bacterial Flagellar Motor. Biochemistry. 2001;40:13041–13050. doi: 10.1021/bi011263o. [DOI] [PubMed] [Google Scholar]
- 15.Sourjik V, Berg HC. Functional interactions between receptors in bacterial chemotaxis. Nature. 2004;428:437–441. doi: 10.1038/nature02406. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson J. S. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J. Bacteriol. 1976;126:758–770. doi: 10.1128/jb.126.2.758-770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker MD, Wolanin PM, Stock JB. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28:9–22. doi: 10.1002/bies.20343. [DOI] [PubMed] [Google Scholar]
- 18.Kuo SC, Koshland DE. Roles of cheY and cheZ gene products in controlling flagellar rotation in bacterial chemotaxis of Escherichia coli. J. Bacteriol. 1987;169:1307–1314. doi: 10.1128/jb.169.3.1307-1314.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishler DM, Topp S, Reynoso CMK, Gallivan JP. Engineering bacteria to recognize and follow small molecules. Curr. Opin. Biotechnol. 2010;21:653–656. doi: 10.1016/j.copbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, et al. Sequential establishment of stripe patterns in an expanding cell population. Science. 2011;334:238–241. doi: 10.1126/science.1209042. [DOI] [PubMed] [Google Scholar]
- 21.Weiss LE, et al. Engineering motility as a phenotypic response to LuxI/R-dependent quorum sensing in Escherichia coli. Biotechnol. Bioeng. 2008;100:1251–1255. doi: 10.1002/bit.21862. [DOI] [PubMed] [Google Scholar]
- 22.Topp S, Gallivan JP. Guiding bacteria with small molecules and RNA. J. Am. Chem. Soc. 2007;129:6807–6811. doi: 10.1021/ja0692480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derr P, Boder E, Goulian M. Changing the specificity of a bacterial chemoreceptor. J. Mol. Biol. 2006;355:923–932. doi: 10.1016/j.jmb.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Bi S, et al. Discovery of novel chemoeffectors and rational design of Escherichia coli chemoreceptor specificity. Proc. Natl. Acad. Sci. USA. 2013;110:16814–16819. doi: 10.1073/pnas.1306811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi S, Pollard AM, Yang Y, Jin F, Sourjik V. Engineering hybrid chemotaxis receptors in bacteria. ACS Synth. Biol. 2016;5:989–1001. doi: 10.1021/acssynbio.6b00053. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Hortelão AC, Patiño T, Sánchez S. Enzyme catalysis to power micro/nanomachines. ACS Nano. 2016;10:9111–9122. doi: 10.1021/acsnano.6b04108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muddana HS, Sengupta S, Mallouk TE, Sen A, Butler PJ. Substrate catalysis enhances single-enzyme diffusion. J. Am. Chem. Soc. 2010;132:2110–2111. doi: 10.1021/ja908773a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta S, et al. Enzyme molecules as nanomotors. J. Am. Chem. Soc. 2013;135:1406–1414. doi: 10.1021/ja3091615. [DOI] [PubMed] [Google Scholar]
- 29.Dey KK, et al. Micromotors powered by enzyme catalysis. Nano Lett. 2015;15:8311–8315. doi: 10.1021/acs.nanolett.5b03935. [DOI] [PubMed] [Google Scholar]
- 30.Ng W-L, Bassler BL. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papenfort K, Bassler BL. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhary S, Schmidt-Dannert C. Applications of quorum sensing in biotechnology. Appl. Microbiol. Biotechnol. 2010;86:1267–1279. doi: 10.1007/s00253-010-2521-7. [DOI] [PubMed] [Google Scholar]
- 33.Garg N, Manchanda G, Kumar A. Bacterial quorum sensing: circuits and applications. Antonie Van Leeuwenhoek. 2014;105:289–305. doi: 10.1007/s10482-013-0082-3. [DOI] [PubMed] [Google Scholar]
- 34.Basu S, Gerchman Y, Collins CH, Arnold FH, Weiss R. A synthetic multicellular system for programmed pattern formation. Nature. 2005;434:1130–4. doi: 10.1038/nature03461. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JC, Clarke EJ, Arkin AP, Voigt CA. Environmentally controlled invasion of cancer cells by engineered bacteria. J. Mol. Biol. 2006;355:619–27. doi: 10.1016/j.jmb.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 36.Moon TS, Lou C, Tamsir A, Stanton BC, Voigt CA. Genetic programs constructed from layered logic gates in single cells. Nature. 2012;491:249–253. doi: 10.1038/nature11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennig S, et al. Artificial cell-cell communication as an emerging tool in synthetic biology applications. J. Biol. Eng. 2015;9 doi: 10.1186/s13036-015-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, et al. Artificially constructed quorum-sensing circuits are used for subtle control of bacterial population density. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garza AG, Bronstein PA, Valdez PA, Harris-Haller LW, Manson MD. Extragenic suppression of motA missense mutations of Escherichia coli. J. Bacteriol. 1996;178:6116–6122. doi: 10.1128/jb.178.21.6116-6122.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garza AG, Harris-Haller LW, Stoebner RA, Manson MD. Motility protein interactions in the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA. 1995;92:1970–1974. doi: 10.1073/pnas.92.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blair DF, Berg HC. Restoration of torque in defective flagellar motors. Science. 1988;242:1678–1681. doi: 10.1126/science.2849208. [DOI] [PubMed] [Google Scholar]
- 42.Minogue TD, Wehland-von Trebra M, Bernhard F, Von Bodman SB. The autoregulatory role of EsaR, a quorum-sensing regulator in Pantoea stewartii ssp. stewartii: evidence for a repressor function. Mol. Microbiol. 2002;44:1625–1635. doi: 10.1046/j.1365-2958.2002.02987.x. [DOI] [PubMed] [Google Scholar]
- 43.Shong J, Huang Y-M, Bystroff C, Collins CH. Directed evolution of the quorum-sensing regulator esar for increased signal sensitivity. ACS Chem. Biol. 2013;8:789–795. doi: 10.1021/cb3006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seila AC, Core LJ, Lis JT, Sharp PA. Divergent transcription: a new feature of active promoters. Cell Cycle. 2009;8:2557–2564. doi: 10.4161/cc.8.16.9305. [DOI] [PubMed] [Google Scholar]
- 45.Toews ML, Goy MF, Springer MS, Adler J. Attractants and repellents control demethylation of methylated chemotaxis proteins in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1979;76:5544–5548. doi: 10.1073/pnas.76.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bren A, Eisenbach M. How signals are heard during bacterial chemotaxis: protein-protein interactions in sensory signal propagation. J. Bacteriol. 2000;182:6865–6873. doi: 10.1128/JB.182.24.6865-6873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel U, Jensen KF. The RNA chain elongation rate in Escherichia coli depends on the growth rate. J. Bacteriol. 1994;176:2807–2813. doi: 10.1128/jb.176.10.2807-2813.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milo R, Jorgensen P, Moran U, Weber G, Springer M. BioNumbers–the database of key numbers in molecular and cell biology. Nucleic Acids Res. 2010;38:D750–3. doi: 10.1093/nar/gkp889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steager EB, et al. Electrokinetic and optical control of bacterial microrobots. J. Micromechanics Microengineering. 2011;21 doi: 10.1088/0960-1317/21/3/035001. [DOI] [Google Scholar]
- 50.Karig D, Weiss R. Signal-amplifying genetic circuit enables in vivo observation of weak promoter activation in the Rhl quorum sensing system. Biotechnol. Bioeng. 2005;89:709–718. doi: 10.1002/bit.20371. [DOI] [PubMed] [Google Scholar]
- 51.Tamsir A, Tabor JJ, Voigt CA. Robust multicellular computing using genetically encoded NOR gates and chemical ‘wires’. Nature. 2011;469:212–215. doi: 10.1038/nature09565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shong, J. & Collins, C. H. Quorum sensing-modulated AND-gate promoters control gene expression in response to a combination of endogenous and exogenous signals. (2013). [DOI] [PubMed]
- 53.Salis HM, Mirsky EA, Voigt CA. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espah Borujeni A, Channarasappa AS, Salis HM. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res. 2014;42:2646–2659. doi: 10.1093/nar/gkt1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shong J, Collins CH. Engineering the esaR promoter for tunable quorum sensing- dependent gene expression. ACS Synth. Biol. 2013;2:568–75. doi: 10.1021/sb4000433. [DOI] [PubMed] [Google Scholar]
- 56.Shong, J. Quorum-sensing repressor-based tools for cell-cell communication in synthetic biology. PhD thesis, Rensselaer Polytechnic Institute, Department of Chemical and Biological Engineering (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


