Abstract
The ability of Listeria monocytogenes to move within the cytosol of infected cells and their ability to infect adjacent cells is important in the development of infection foci leading to systemic disease. Interaction with the host cell microfilament system, particularly actin, appears to be the basis for propelling the bacteria through the host cell cytoplasm to generate the membraneous protrusions whereby cell-to-cell spread occurs. The actA locus of L.monocytogenes encodes a 90 kDa polypeptide that is a key component of bacterium-host cell microfilament interactions. Cloning of the actA gene allowed the identification of its gene product and permitted construction of an isogenic mutant strain defective in the production of the ActA polypeptide. Sequencing of the region encoding the actA gene revealed that it was located region encoding the actA gene revealed that it was located between the metalloprotease (mpl) and phosphatidylcholine-specific phospholipase C (plcB) genes. Within the cytoplasm of the infected cells, the mutant strain grew as microcolonies, was unable to accumulate actin following escape from the phagocytic compartment and was incapable of infecting adjacent cells. It was also dramatically less virulent, demonstrating that the capacity to move intracellularly and spread intercellularly is a key determinant of L.monocytogenes virulence. Like all other virulence factors described for this microorganism, expression of the ActA polypeptide is controlled by the PrfA regulator protein. The primary sequence of this protein appeared to be unique with no extended homology to known protein sequences. However, an internal repeat sequence showed strong regional homology to a sequence from within the hinge region of the cytoskeletal protein vinculin.
Full text
PDF




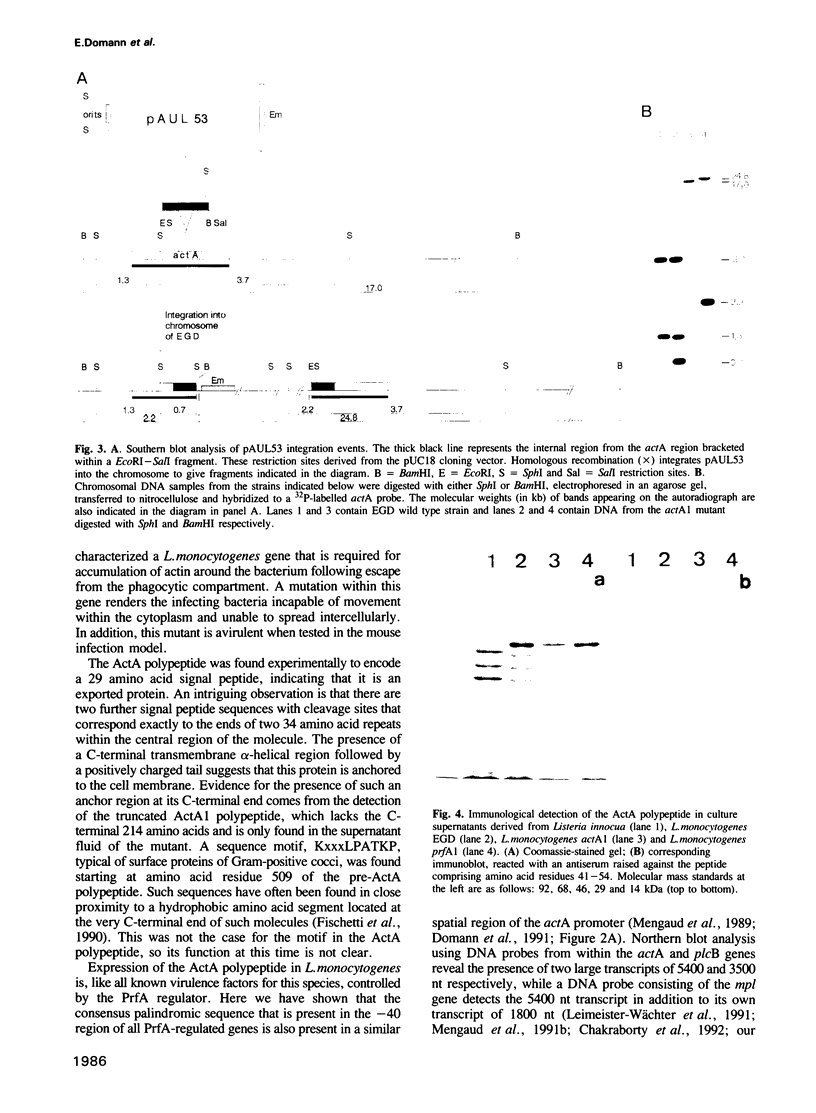
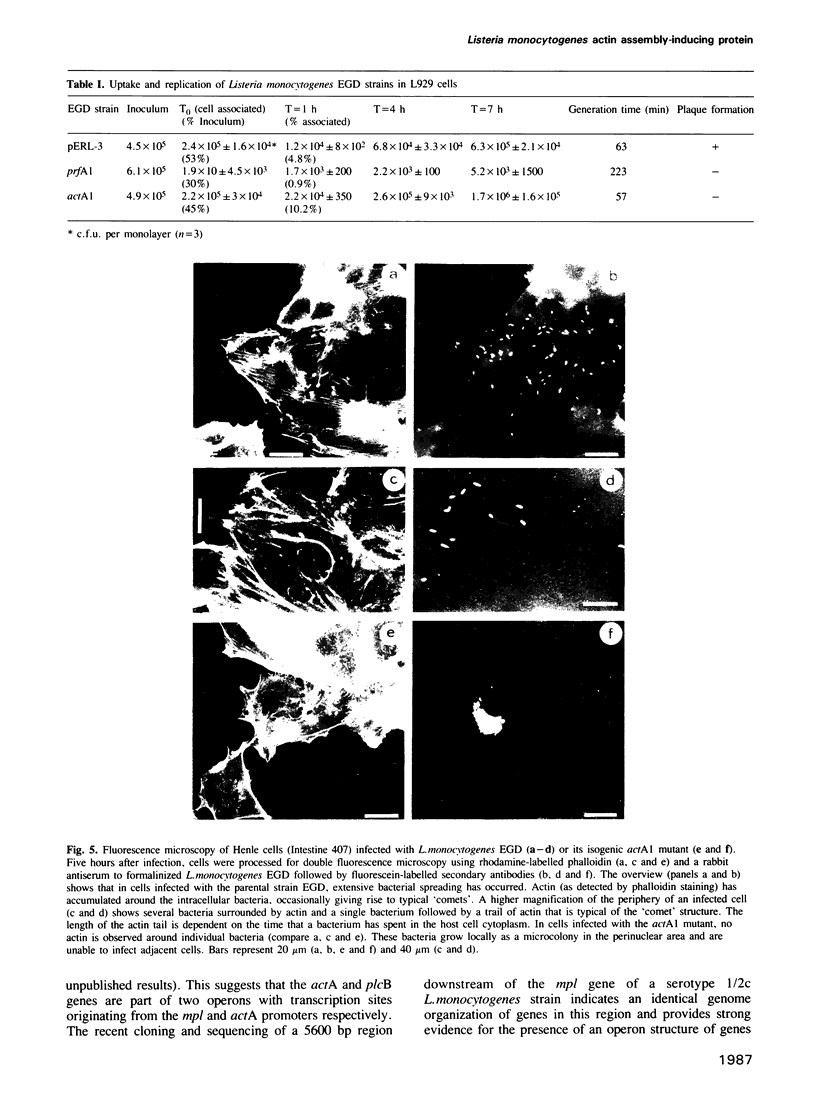
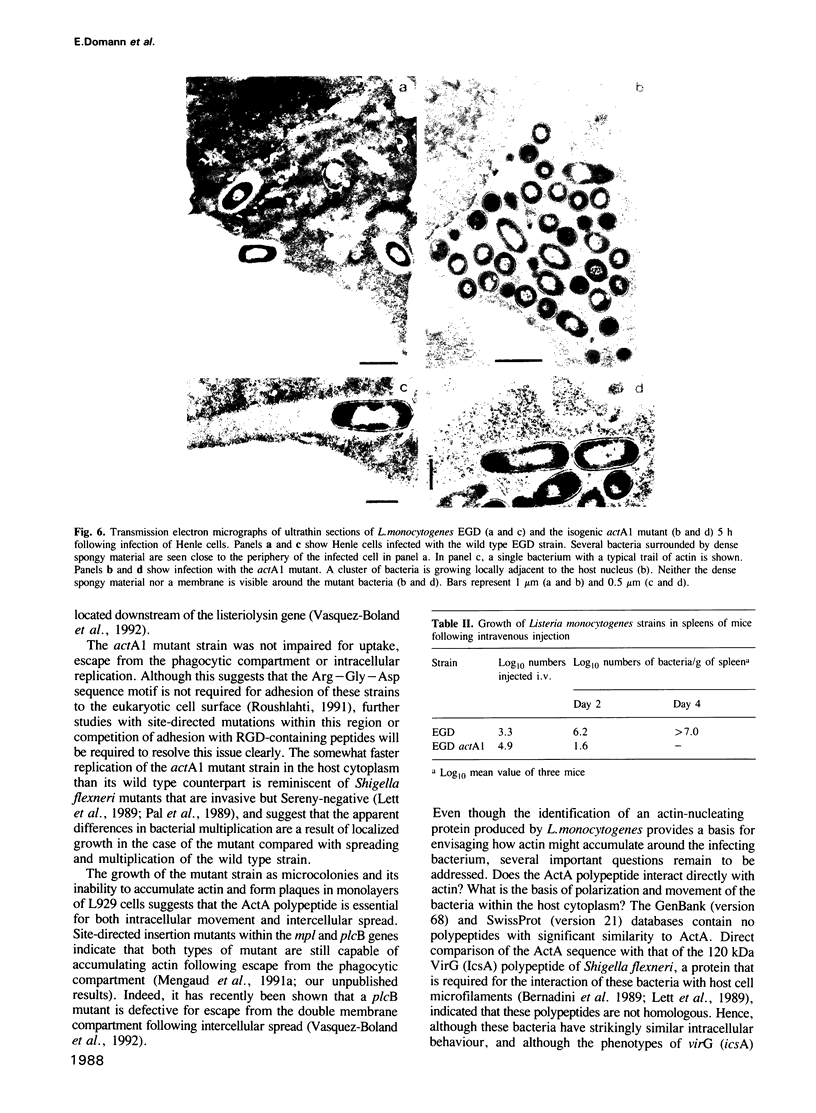


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Leimeister-Wächter M., Domann E., Hartl M., Goebel W., Nichterlein T., Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992 Jan;174(2):568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Leimeister-Wächter M., Goebel W., Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991 Jan;59(1):65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991 Jun 28;65(7):1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Cruz-Rodz A. L., Leimeister-Wächter M., Kreft J., Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989 Feb;171(2):744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T., Holm T., Guddal P. H., Sletten K., Haugli F. B., Little C. Cloning and sequencing of the gene encoding the phosphatidylcholine-preferring phospholipase C of Bacillus cereus. Gene. 1988 May 30;65(2):293–304. doi: 10.1016/0378-1119(88)90466-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Acquired resistance to facultative intracellular bacteria: relationship between persistence, cross-reactivity at the T-cell level, and capacity to stimulate cellular immunity of different Listeria strains. Infect Immun. 1984 Jul;45(1):234–241. doi: 10.1128/iai.45.1.234-241.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989 Aug;57(8):2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991 Feb;5(2):361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Haffner C., Domann E., Goebel W., Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of listeria monocytogenes. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett M. C., Sasakawa C., Okada N., Sakai T., Makino S., Yamada M., Komatsu K., Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989 Jan;171(1):353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Dramsi S., Gouin E., Vazquez-Boland J. A., Milon G., Cossart P. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol Microbiol. 1991 Sep;5(9):2273–2283. doi: 10.1111/j.1365-2958.1991.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991 Mar;59(3):1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect Immun. 1989 Dec;57(12):3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Newland J. W., Tall B. D., Formal S. B., Hale T. L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989 Feb;57(2):477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J. Genetic and molecular basis of epithelial cell invasion by Shigella species. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S285–S292. doi: 10.1093/clinids/13.supplement_4.s285. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sun A. N., Camilli A., Portnoy D. A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990 Nov;58(11):3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball R. W., Leslie D. L., Harvey S., Kelly D. Hemolytic and sphingomyelinase activities of Clostridium perfringens alpha-toxin are dependent on a domain homologous to that of an enzyme from the human arachidonic acid pathway. Infect Immun. 1991 May;59(5):1872–1874. doi: 10.1128/iai.59.5.1872-1874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland J. A., Kocks C., Dramsi S., Ohayon H., Geoffroy C., Mengaud J., Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992 Jan;60(1):219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. A., Ogryzko E. P., Corben E. B., Zhidkova N. I., Patel B., Price G. J., Spurr N. K., Koteliansky V. E., Critchley D. R. Complete sequence of human vinculin and assignment of the gene to chromosome 10. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5667–5671. doi: 10.1073/pnas.87.15.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]