Abstract
Background
The prevention and slowing of chronic kidney disease still represent major challenges in nephrology. To this end, a major contribution may come from the extensive knowledge on the molecular pathways involved in the pathogenesis of rare kidney diseases, since it is now possible to shed light on several aspects of these pathologies thanks to the introduction of new technologies, including next-generation sequencing.
Summary
In steroid-resistant nephrotic patients, a genetic background has been demonstrated in both children and adults; individualized mutations have been correlated with glomerular filtration barrier alterations. In addition, studies on genetic tubulopathies expressing hypertensive phenotypes can provide useful information for a correct diagnostic and therapeutic approach in patients with essential hypertension and a poor responsiveness to therapy.
Key Message
This review deals with the pathogenesis of rare glomerular diseases and tubulopathies associated with hypertension, highlighting the importance of the study of rare diseases to better understand the molecular basis of more common and complex disorders leading to end-stage renal disease.
Keywords: Genetic kidney diseases, Hypertension, Salt sensitivity, Steroid-resistant nephrotic syndrome, Tubulopathies
Introduction
Rare diseases are considered a health problem since they are characterized by a high social impact due to the limited knowledge, clinical severity, and restricted therapeutic options associated with them. In particular, rare kidney diseases are associated with the development of chronic kidney disease (CKD) and its progression to end-stage renal disease, requiring renal function substitution with dialysis or kidney transplantation. The early diagnosis and knowledge of the primary cause of a rare disorder are essential for improving the prognosis, especially if therapeutic options are available. In addition, an understanding of the molecular basis of the defect is important for the development of new targeted therapeutic tools. In most of the cases, however, the diagnosis of a rare kidney disease is still quite difficult and requires considerable experience, and an underestimation of the prevalence of these pathologies might be supposed [1]. Nevertheless, the advent of next-generation sequencing technologies, in recent years, has improved the timing and the quality of the diagnosis of rare kidney diseases, also providing the opportunity to better understand molecular defects shared by both rare and common renal diseases.
The achievement of proteinuria reduction and an optimal blood pressure control are recognized therapeutic goals able to slow CKD progression [2]. Despite significant therapeutic advances, it is conceivable that there is still much to learn about the pathogenesis of major kidney diseases in order to find more effective and useful therapies in nephrology [3, 4]. Furthermore, the heterogeneous phenotypic manifestations of these diseases suggest an influence of several genetic and environmental determinants [5]. In fact, unlike monogenic diseases, complex diseases do not show a classic Mendelian inheritance pattern. Generally, each gene involved in a complex disease is responsible only in part for its phenotype and defines simply a “susceptibility” to develop the disorder. The use of high-throughput DNA sequencing technologies appears to be a promising strategy since it permits to obtain a rapid and synchronized analysis of thousands of genes, thus increasing the chance to discover precise disease markers [6]. Furthermore, this quick and efficient diagnostic tool applied to different renal genetic diseases has recently provided a more accurate characterization of specific genetic and molecular patterns that appear also to be involved in the study of nongenetic and very common renal disorders.
This review aims at providing an updated description of the pathogenesis, diagnosis, and available therapeutic options for some rare glomerular and tubular kidney diseases, whose molecular basis might be useful in revealing unrecognized and/or unclear causes of steroid-resistant nephrotic syndrome and primary hypertension.
Steroid-Resistant Nephrotic Syndrome: Has It a Genetic Basis?
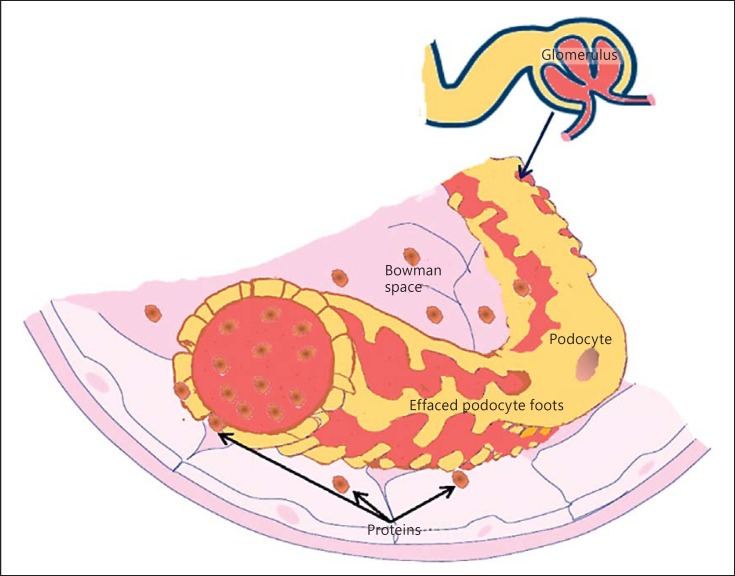
Proteinuria is recognized as a major and independent risk factor for the progression of renal damage [7, 8]. Consequently, the achievement of proteinuria reduction represents one of the most important challenges in nephrology [7, 8]. Urinary protein excretion exceeding the nephrotic range is associated with a high rate of progression to end-stage renal disease, quality of life worsening, hospitalization, and mortality [9, 10]. A genetic cause of nephrotic syndrome is always considered in children, whereas in adults, this hypothesis is explored mainly when conventional therapies are ineffective. In recent years, podocyte biology study has been one of the most exciting fields of investigation for an understanding of the physiopathology of nephrotic syndrome (Fig. 1).
Fig. 1.
Impairment of the glomerular filtration apparatus with effacement of podocyte foot and protein passage in the Bowman capsule.
Both genetic and molecular alterations associated with the development of massive proteinuria have been identified in experiments conducted in nephrotic syndrome animal models. Pathogenic mutations of genes encoding for filtration barrier proteins, such as podocin, nephrin, and collagen, have been discovered to be associated with nephrotic syndrome in focal and segmental glomerulosclerosis [11].
In 2014, Giglio et al. [12], in a retrospective study conducted by next-generation sequencing technology in 31 children with steroid-resistant nephrotic syndrome and 38 children with steroid-sensitive nephrotic syndrome, explored the possible genetic causes of steroid resistance. Genetic analysis was positive in 32.3% of children with the steroid-resistant variant of nephrotic syndrome, while no genetic alteration was reported in any of the children with steroid-sensitive disease. Compound heterozygosity or homozygosity of pathogenetic podocyte genes (NPHS2 and PLCE1) has been evidenced. Furthermore, a low podocin expression has been found in the biopsy specimens [12]. In the same year, Bullich et al. [13] reported a similar study conducted in adult patients with steroid-resistant nephrotic syndrome due to focal segmental glomerulosclerosis. The results confirmed the presence of 42 pathogenic mutations across NPHS1, NPHS2, WT1, TRPC6, and INF2 genes, all encoding for filtration barrier structural proteins. These findings have proven that steroid resistance has genetic causes with the main phenotypic response being located in the podocyte [13]. These results indicate that patients with steroid-resistant nephrotic proteinuria deserve a genetic counseling and possibly a genetic screening.
Studies on Tubulopathies Provide the Key to Understanding the Mechanisms of Renal Blood Pressure Control
Essential hypertension is a complex disorder associated with a high mortality risk for cardiovascular comorbidities and CKD development [14]. The pathogenetic basis of hypertension is only partially understood so far, and an association of environmental and genetic factors has been supposed [15, 16]. Indeed, a close relationship between salt dietary intake and blood pressure levels has been described in several studies. Weinberger et al. [17] were the first to define salt sensitivity based on the results of a study conducted on 378 healthy volunteers and 192 patients with essential hypertension. Blood pressure was measured after 0.9% NaCl infusion and after hydrosaline depletion induced by a low-sodium diet and furosemide administration. Compared to normal subjects, patients with hypertension showed higher blood pressure variations after NaCl administration and only a slight blood pressure reduction after hydrosaline depletion.
Salt sensitivity is, therefore, an individual response to an increased salt intake, and it is possible to divide patients into salt-sensitive and salt-resistant groups. Possible genetic causes of salt sensitivity, probably involving NaCl renal transporters, have been assumed [18]. In support, the central role exerted by the kidney in the development of salt intake-related hypertension has been confirmed in cross-kidney transplantation experiments [19].
Renal Na+ and Cl— tubular transport is the major mechanism of salt homeostasis maintenance [20]. Several gene mutations encoding different transporters involved in NaCl tubular handling have been identified and associated with monogenic or syndromic electrolytes and acid-base disorders with an impact on salt-water balance and blood pressure regulation [21, 22]. The proximal tubule is committed to recover most of the salt freely filtered by the glomerulus, whereas downstream tubular segments are the fine tuning of definitive urinary NaCl excretion. As a consequence, the regulation of body water content and blood pressure is mainly operated by the distal part of the nephron.
In normal conditions, in the thick ascending limb (TAL) of the loop of Henle, luminal NaCl enters the cell through the Na+-2Cl—-K+ cotransporter (NKCC2) [23], while potassium (K+) recycles into the lumen via an adenosine triphosphate (ATP)-sensitive K+ channel (ROMK), assuring the maintenance of NKCC2 activity [24]. Na+ ions gain the bloodstream pumped through the basolateral membrane by the Na+/K+-ATPase system. Cl-, instead, leaves the cell through a basolateral Cl— channel and/or is cotransported with K+. The paracellular reabsorption of Na+, K+, Ca2+, and Mg2+ is driven by a lumen-positive transepithelial voltage gradient generated by both the K+ recirculation to the lumen and the Cl— basolateral reabsorption [25]. In the distal convoluted tubule, NaCl cotransporter (NCC) mediates NaCl reabsorption [26]. Even in this portion of the tubule, Cl— leaves the cell through a specific channel, whereas Na+ is pumped in the blood by the Na+/K+-ATPase system. A specific luminal channel provides the internalization of Ca2+ into epithelial distal tubular cells. Subsequently, Ca2+ is shuttled by Calbindin 28k to the basolateral membrane and pumped [27] into the blood by a Na+/Ca2+ exchange and a Ca2+ ATPase [28].
In the collecting duct, Na+ ions are reabsorbed by the principal cell via the luminal Na+ channel (ENaC) and exit the basolateral membrane by the Na+/K+-ATPase system [28]. Na+ reabsorption is accomplished with K+ extrusion by ROMK and large K+ channels. Through stimulation of its receptor, aldosterone enhances Na+ transport in the collecting duct and, consequently, induces K+ loss in the urine [29].
The renin-angiotensin-aldosterone system (RAAS) is the major hormonal regulator of NaCl tubular transport and provides blood pressure modulation in response to extracellular volume variations [30, 31]. Furthermore, RAAS is described as a pleiotropic system able to exert numerous adjunctive renal and cardiac profibrotic effects, and its upregulation, in hypertensive and proteinuric patients, is associated with the progression of renal and cardiovascular damage [30, 31]. Several RAAS genetic polymorphisms have also been demonstrated in hypertensive patients with congenital kidney anomalies and progressive CKD [32].
The key role of the RAAS system in blood pressure regulation is prominent in some inherited tubular disorders characterized by reduced or enhanced Na+ reabsorption, leading to volume depletion or expansion and consequent activation or suppression of the RAAS system, respectively. On this basis, the study of inherited tubulopathies characterized by an altered tubular salt handling appears useful to extend the knowledge on mechanisms of body volume regulation and to identify possible genetic factors involved in the development of hypertension and salt sensitivity [33].
Hypotensive Syndromes: Bartter and Gitelman Syndromes Mimic Diuretic Activity
Diuretics are first-choice drugs in the treatment of naïve hypertensive patients in which NaCl retention and extracellular expansion represent the key mechanisms for high blood pressure onset [34]. The study of altered NaCl mechanisms underlying hypotensive genetic tubulopathy development aids in understanding the mechanisms of diuretic-related blood pressure reduction, abnormal responses to diuretics, and the development of side effects [35]. Furthermore, the knowledge of genetically impaired NaCl transport mechanisms might contribute to the identification of possible inherited pathways associated with so-called “essential hypertension.”
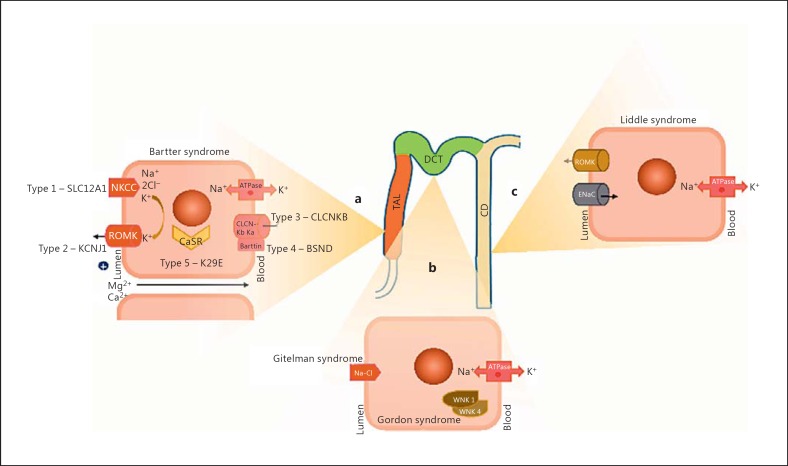
In 1962, Bartter et al. [36] first reported a genetic syndrome characterized by altered NaCl handling in the TAL in 2 homozygous twin sisters. Bartter syndrome is characterized by a normotensive/hypotensive phenotype associated with hypokalemia, hypochloremic alkalosis, hypocalcemia, and hyperplasia of the juxtaglomerular apparatus. So far, 5 genetic Bartter syndrome variants have been found to be associated with different loss-of-function mutations of genes encoding electrolyte transporters in the TAL (Fig. 2a). In particular, Bartter syndrome is characterized by NaCl urinary waste, extracellular fluid volume reduction, and hypotension, despite the coexistence of hyperaldosteronism. Type 1 Bartter syndrome is induced by loss-of function mutations of the SLC12A1 gene inhibiting NKCC2 activity and mimicking furosemide pharmacodynamics [37]. Instead, type 2 Bartter syndrome is associated with mutations in the KCNJ1 gene encoding the apical ROMK in the TAL. The decrease in ROMK activity induces a reduction of K+ back-filtration in the lumen and, again, inhibits NKCC2 activity. CLCNKB gene mutations are responsible for chloride voltage-gated channel Kb (CLCNKB) blockage and are associated with type 3 Bartter syndrome. Mutations of the CLCNKB and CLCNKA genes encoding for basolateral Cl— channel or mutations of the BSND gene encoding the Barttin subunit of the channel induce type 4 Bartter syndrome, which is characterized by deafness associated with Bartter syndrome. The impairment of Cl— channels, in fact, leads to reduced renal salt reabsorption and potassium recycling in the inner ear [38].
Fig. 2.
Along the tubular portion of the nephron, the mutated malfunctioning cell sites in Bartter syndrome (a), in Gitelman and Gordon syndrome (b), and in Liddle syndrome (c) are depicted.
Type 5 Bartter syndrome has recently been discovered and described to be associated with a gain-of-function mutation of the K29E gene encoding the extracellular Ca2+-sensing receptor (CaSR) [39]. This Bartter-like syndrome is characterized by a mild phenotype due to the enhanced inhibition exerted by CaSR activity on sodium transport in the TAL [40].
Another hypotensive tubulopathy is Gitelman syndrome, whose pathogenetic basis is due to altered salt handling in the distal convoluted tubule, which mimics thiazide diuretics pharmacodynamics (Fig. 2b). In fact, linkage studies have demonstrated that Gitelman syndrome is induced by loss-of-function mutations of the locus encoding the apical NCC. In Gitelman syndrome, in fact, conversely to Bartter syndrome, hypotension, hypokalemia, and hypochloremic alkalosis are associated with hypomagnesemia and hypocalciuria [41].
Genetic mutations mirroring those identified in the hypotensive tubulopathies could explain cases of hypertension with variable response to conventional diuretic therapy [42]. Thus, a careful electrolytes and acid-base study and, eventually, a genetic counseling might be indicated in naïve patients with “essential” hypertension showing an abnormal response to salt dietary restriction and to diuretic stimulation.
Mendelian Hypertensive Tubulopathies: Mechanisms of Blood Pressure Increase
Advanced genomic techniques have permitted the identification of rare monogenic forms of hypertension characterized by either disorders of distal NaCl transport or dysregulation of mineralocorticoid hormone secretion/activity. Liddle syndrome is an autosomal dominant form of hypertension in which gain-of-function mutations of a single gene encoding β or γ subunits of the amiloride-sensitive epithelial ENaC of the principal cells in the collecting duct are associated with early onset of severe hypertension, hypokalemia, metabolic alkalosis, and hypoaldosteronism (Fig. 2c). Hypokalemia and metabolic alkalosis depend on the absence of a lumen-positive gradient in the collecting duct. This luminal electronegativity is due to the excessive Na+ reabsorption by ENaC that inhibits both K+ and H+ transepithelial transport and increases the urinary loss of the 2 cations. Treatment of Liddle syndrome with amiloride or triamterene is effective in blocking ENaC hyperactivity in the collecting tubule. Instead, spironolactone does not correct electrolyte and acid-base disorders in Liddle syndrome because the increased activity of the ENaC is aldosterone independent [43]. Thus, in patients with essential hypertension, a diversified response to different K+-sparing diuretics might deserve a careful diagnostic study, possibly including genetic insights.
Gordon syndrome or pseudohypoaldosteronism type II is a rare familial autosomal dominant disease caused by mutations of WNK1 and WNK4, 2 proteins of a serine-threonine kinases family that have recently been identified [44] (Fig. 2b). In the distal tubule, wild-type WNK1 and WNK4 inhibit NCC, whereas gain-of-function mutations stimulate NCC activity, inducing excessive NaCl reabsorption and volume expansion. Consequently, the Gordon syndrome phenotype is characterized by tardive onset of severe hypertension, low fractional Na+ excretion, and hyperchloremic metabolic acidosis in patients showing short height, muscle weakness, dental abnormalities, and cognitive defects [45]. The therapeutic approach aims at decreasing NCC activity also by limiting the substrate for NaCl transport. As a consequence, thiazide diuretics and a low-salt diet are mandatory in the treatment of Gordon syndrome. Different mutations or milder phenotypes may also be supposed in hypertensive patients that show a reduced response to nonthiazide diuretics.
Conclusions
Rare kidney diseases are an interesting field of investigation, especially because different pathogenic molecular pathways are only partially characterized. However, causes of several genetic kidney diseases have recently been evidenced by the use of advanced technologies for gene sequencing. Undoubtedly, many similarities with the pathogenesis of the most common kidney disorders are evident. Apparently, the complex pathogenesis of common kidney diseases associated with the onset and progression of CKD may have a genetic background that could be investigated starting from the study of rare kidney diseases. A genetic background for steroid resistance in nephrotic syndrome could be supposed, and several pathogenetic mutations impairing the glomerular filtration barrier integrity have been found only in nephrotic patients not responding to corticosteroids. Similarly, the response to antihypertensive drugs and to salt intake modifications is individual in hypertensive patients, and genetic mutations interfering with NaCl tubular handling might be a valid explanation. The characterization of hypotensive and hypertensive tubulopathies might indicate new investigation areas for a better understanding of the mechanisms of hypertension development and maintenance, thus providing precision therapeutic approaches.
Conflict of Interest Statement
The authors declare no conflicts of interest.
References
- 1.Shayman JA. Thinking about rare kidney diseases. J Am Soc Nephrol. 2006;17:15–16. doi: 10.1681/ASN.2005101143. [DOI] [PubMed] [Google Scholar]
- 2.Renkema KY, Stokman MF, Giles RH, Knoers NV. Next-generation sequencing for research and diagnostics in kidney disease. Nat Rev Nephrol. 2014;10:433–444. doi: 10.1038/nrneph.2014.95. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Llevin A, Powe NR, Rossert J, Wheeler DC, Lameire R, Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: common, harmful and treatable - World Kidney Day 2007. Am J Kidney Dis. 2007;49:175–179. doi: 10.1053/j.ajkd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Coppolino G, Simeoni M, Summaria C, Postorino MC, Rivoli L, Strazzulla A, Torti C, Fuiano GJ. The case of chronic hepatitis B treatment with tenofovir: an update for nephrologists. Nephrol. 2015;28:393–402. doi: 10.1007/s40620-015-0214-0. [DOI] [PubMed] [Google Scholar]
- 6.Drmanac R. The advent of personal genome sequencing. Genet Med. 2011;13:188–190. doi: 10.1097/GIM.0b013e31820f16e6. [DOI] [PubMed] [Google Scholar]
- 7.Simeoni M, Cianfrone P, Comi N, Gentile I, Fabiano FF, Piraina V, Talarico R, Lucisano G, Rivoli L, Andreucci M, Fuiano L, Foti D, Gulletta E, Fuiano G. Is it feasible to improve the duration and the efficiency of Ramipril anti-proteinuric response? (in Italian) G Ital Nefrol. 2015;32 [PubMed] [Google Scholar]
- 8.Cianfrone P, Simeoni M, Comi N, Piraina V, Talarico R, Cerantonio A, Gentile I, Fabiano FF, Lucisano G, Foti D, Gulletta E, Fuiano G. How to improve duration and efficiency of the antiproteinuric response to Ramipril: RamiPROT - a prospective cohort study. J Nephrol. 2015;30:95–102. doi: 10.1007/s40620-015-0256-3. [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. Kidney Int. 1998;53:1209–1216. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Onofrio G, Simeoni M, Rizza P, Caroleo M, Capria M, Mazzitello G, Sacco T, Mazzuca E, Panzino MT, Cerantonio A, Segura-Garcia C, Andreucci M, De Fazio P, Fuiano G. Quality of life, clinical outcome, personality and coping in chronic hemodialysis patients. Ren Fail. 2017;39(1):45–53. doi: 10.1080/0886022X.2016.1244077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simic I, Tabatabaeifar M, Schaefer F. Animal models of nephrotic syndrome. Pediatr Nephrol. 2013;28:2079–2088. doi: 10.1007/s00467-012-2376-5. [DOI] [PubMed] [Google Scholar]
- 12.Giglio S, Provenzano A, Mazzinghi B, Becherucci F, Giunti L, Sansavini G, Ravaglia F, Roperto LM, Farsetti F, Benetti E, Rotondi M, Murer L, Lazzeri E, Lasagni L, Materassi M, Romagnani P. Heterogeneous genetic alterations in sporadic nephrotic syndrome associate with resistance to immunosuppression. J Am Soc Nephrol. 2015;26:230–236. doi: 10.1681/ASN.2013111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullich G, Trujillano D, Santìn S, Ossowski S, Medizàbal S, Fraga G, Madrid A, Ariceta G, Balarìn J, Torra R, Estivill X, Ars E. Targeted next-generation sequencing in steroid-resistant nephrotic syndrome: mutations in multiple glomerular genes may influence disease severity. Eur J Hum Genet. 2015;23:1192–1199. doi: 10.1038/ejhg.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carretero O, Oparil S. Essential hypertension. Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 15.Bullet MG. Genetics of hypertension. Current status. J Med Liban. 2010;58:175–178. [PMC free article] [PubMed] [Google Scholar]
- 16.Lang F, Capasso G, Schwab M, Waldegger S. Renal tubular transport and the genetic basis of hypertensive disease. Clin Exp Nephrol. 2005;9:91–99. doi: 10.1007/s10157-005-0355-x. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg LS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 18.Capasso G, Cantone A, Evangelista C, Zacchia M, Acone D, Trepiccione F, Rizzo M. Channels, carriers, and pumps in the pathogenesis of sodium-sensitive hypertension. Semin Nephrol. 2005;25:419–424. doi: 10.1016/j.semnephrol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep. 2011;13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry CA, Rector FC., Jr Mechanism of proximal NaCl reabsorption in the proximal tubule of the mammalian kidney. Semin Nephrol. 1991;11:86–97. [PubMed] [Google Scholar]
- 21.Lang F, Capasso G, Schwab M. Renal tubular transport and the genetic basis of hypertensive disease. Clin Exp Nephrol. 2005;9:91–99. doi: 10.1007/s10157-005-0355-x. [DOI] [PubMed] [Google Scholar]
- 22.Capasso G, Rizzo M, Evangelista C, Ferrari P, Geelen G, Lang F, Bianchi G. Altered expression of renal apical membrane Na+ transporters in the early phase of genetic hypertension. Am J Physiol Renal Physiol. 2005;288:F1173–F1182. doi: 10.1152/ajprenal.00228.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gamba G, Friedman PA. Thick ascending limb: the Na+:K+:2Cl- co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch. 2009;458:61–76. doi: 10.1007/s00424-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun A, Grossman EB, Lombardi M, Hebert SC. Vasopressin alters the mechanism of apical Cl- entry from Na+:Cl- to Na+:K+:2Cl- cotransport in mouse medullary thick ascending limb. J Membr Biol. 1991;120:83–94. doi: 10.1007/BF01868594. [DOI] [PubMed] [Google Scholar]
- 25.Subramanya AR, Hellison DH. Distal convoluted tube. Curr Hypertens Rep. 2011;13:55–66. [Google Scholar]
- 26.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10:676–687. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo M, Capasso G, Bleich M, Pica A, Grimaldi D, Bindels R, Greger R. The effect of chronic metabolic acidosis on calbindin expression along the rat distal tubule. J Am Soc Nephrol. 2000;11:203–210. doi: 10.1681/ASN.V112203. [DOI] [PubMed] [Google Scholar]
- 28.Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10:1257–1272. doi: 10.2215/CJN.09750913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suhail M. Na+, K+-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J Clin Med Res. 2010;2:1–17. doi: 10.4021/jocmr2010.02.263w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simeoni M, Cerantonio A, Pastore I, Liguori R, Greco M, Foti D, Gulletta E, Brunetti A, Fuiano G. The correct renal function evaluation in patients with thyroid dysfunction. J Endocrinol Invest. 2016;39:495–507. doi: 10.1007/s40618-015-0402-8. [DOI] [PubMed] [Google Scholar]
- 31.Simeoni M, Nicotera R, Colao M, Citraro ML, Pelagi E, Cerantonio A, Comi N, Coppolino G, Fuiano G. Direct inhibition of plasmatic renin activity with Aliskiren: a promising but under-investigated therapeutic option for non-diabetic glomerulonephritis. Int Urol Nephrol. 2016;48:229–237. doi: 10.1007/s11255-015-1128-4. [DOI] [PubMed] [Google Scholar]
- 32.Wong C, Kanetsky P, Raj D. Genetic polymorphisms of the RAS-cytokine pathway and chronic kidney disease. Pediatr Nephrol. 2008;23:1037–1051. doi: 10.1007/s00467-008-0816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelikovic I. Hypokalaemic salt-losing tubulopathies: an evolving story. Nephrol Dial Transplant. 2003;18:1696–1700. doi: 10.1093/ndt/gfg249. [DOI] [PubMed] [Google Scholar]
- 34.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman NH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 35.Unwin RJ, Capasso G. Bartter's and Gitelman's syndromes: their relationship to the actions of loop and thiazide diuretics. Curr Opin Pharmacol. 2006;6:208–213. doi: 10.1016/j.coph.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Bartter FC, Pronove P, Gill JR. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 37.Shibli AA, Narchi H. Bartter and Gitelman syndromes: spectrum of clinical manifestations caused by different mutations. World J Methodol. 2015;5:55–61. doi: 10.5662/wjm.v5.i2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amirlak I, Dawson KP. Bartter syndrome: an overview. QJM. 2000;93:207–215. doi: 10.1093/qjmed/93.4.207. [DOI] [PubMed] [Google Scholar]
- 39.Capasso G, Geibel PJ, Damiano S, Jaeger P, Richards WG, Geibel JP. The calcium sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney Int. 2013;84:277–284. doi: 10.1038/ki.2013.137. [DOI] [PubMed] [Google Scholar]
- 40.Vezzoli G, Arcidiacono T, Paloschi V, Terranegra A, Biasion R, Weber G, Mora S, Syren ML, Coviello D, Cusi D, Bianchi G, Soldati L. Autosomal dominant hypocalcemia with mild type 5 Bartter syndrome. J Nephrol. 2006;19:525–528. [PubMed] [Google Scholar]
- 41.Cotovio P, Silva C, Oliveira N, Costa F. Gitelman syndrome. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-009095. DOI: 10.1136/bcr-2013-009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capasso G, Rizzo M, Garavaglia ML, Trepiccione F, Zacchia M, Magione A, Ferrari P, Paulmichl M, Lang F, Loffing J, Carrel M, Damiano S, Wagner CA, Bianchi G, Meyer G. Up-regulation of apical sodium chloride cotransporter and basolateral chloride channels are responsible for the maintenance of salt sensitive hypertension. Am J Physiol Renal Physiol. 2008;295:F556–F567. doi: 10.1152/ajprenal.00340.2007. [DOI] [PubMed] [Google Scholar]
- 43.Cui Y, Tong A, Jiang J, Wang F, Li C. Liddle syndrome: clinical and genetic profiles. J Clin Hypertens (Greenwich) 2016 doi: 10.1111/jch.12949. DOI: 10.1111/jch.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruel A, Vargas-Poussou R, Jeunemaitre X, Labbe A, Merlin E, Bessenay L. Gordon syndrome: the importance of measuring blood pressure in children. Arch Pediatr. 2016;23:827–831. doi: 10.1016/j.arcped.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Cantone A, Wang T, Pica A, Simeoni M, Capasso G. Use of transgenic mice in acid-base balance studies. J Nephrol. 2006;((suppl 9)):S121–S127. [PubMed] [Google Scholar]