Abstract
Organisms modify and choose components of their local environments. This ‘niche construction’ can alter ecological processes, modify natural selection and contribute to inheritance through ecological legacies. Here, we propose that niche construction initiates and modifies the selection directly affecting the constructor, and on other species, in an orderly, directed and sustained manner. By dependably generating specific environmental states, niche construction co-directs adaptive evolution by imposing a consistent statistical bias on selection. We illustrate how niche construction can generate this evolutionary bias by comparing it with artificial selection. We suggest that it occupies the middle ground between artificial and natural selection. We show how the perspective leads to testable predictions related to: (i) reduced variance in measures of responses to natural selection in the wild; (ii) multiple trait coevolution, including the evolution of sequences of traits and patterns of parallel evolution; and (iii) a positive association between niche construction and biodiversity. More generally, we submit that evolutionary biology would benefit from greater attention to the diverse properties of all sources of selection.
Keywords: niche construction, trait coevolution, adaptive evolution
1. Introduction
Organisms modify and choose components of their local environments, a phenomenon known as ‘niche construction’ [1,2]. Animals construct nests, burrows, webs, dams, pupil cases; select habitats, microhabitats, mates, foods, oviposition and nesting sites; and build and provision nursery environments for their offspring. Plants modify the temperature, moisture level, cycling of nutrients and chemicals in the soil, alter atmospheric gasses, create shade, induce condensation from fog, alter wind speed and manufacture allelochemicals. Fungi, protists and bacteria play diverse roles in the decomposition of vegetative and animal matter, weathering, soil production and/or photosynthesis, while bacteria and protists also show microhabitat choice. Niche construction is a universal feature of living organisms [1].
That niche construction occurs, and that when it does it can both alter ecological processes and modify natural selection, is now widely accepted [1–5]. Many organismal traits modify environmental conditions in a manner that is adaptive to the organism, and these characters, sometimes called ‘extended phenotypes’ [6], are thought to have been fashioned by selection because they are adaptive. Other organismal traits modify environmental conditions in a manner that is not adaptive to the organism, and these characters are typically thought to have evolved as by-products of selection for some other character. Niche-constructing traits can modify selection, both on the constructor and on other organisms, and hence the causal link between niche-constructing activity and evolutionary responses to niche construction is, to a degree, appreciated (see, for instance, recent literatures on eco-evolutionary dynamics, [7,8]). In this limited sense, niche construction is recognized as a cause of evolution. However, from this traditional standpoint, the role that niche construction plays in evolution is no different from any other form of environmental change: it may elicit or modify selection, by setting the conditions that determine which alleles or genotypes will possess highest fitness. From that viewpoint, natural selection is typically construed as the evolutionary process, with niche construction (like environmental change more generally) treated as a background condition to selection [5].
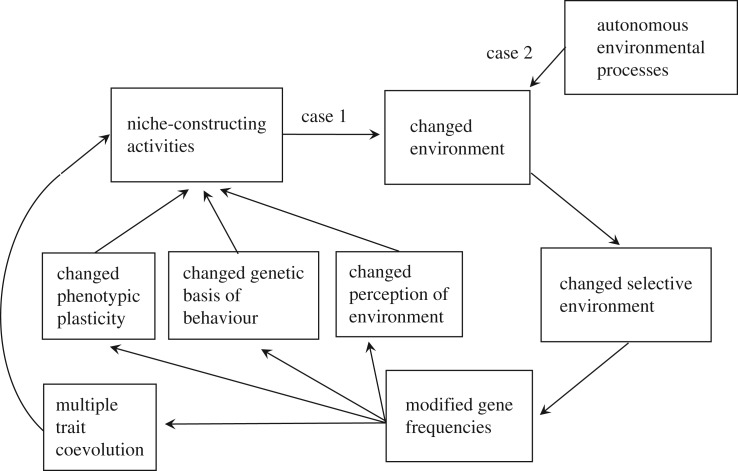
Traditionally in population and quantitative genetics, we tend to restrict evolutionary processes to those processes that directly change gene frequencies. This makes sense if evolution is regarded, as it commonly has been since Dobzhansky [9], as comprising or requiring change in gene frequencies. Natural selection, along with drift, mutation, gene flow, spatial sorting and some other population genetic phenomena, satisfies this definition of an evolutionary process. While niche construction can directly change gene frequencies (e.g. when a predator's consistent prey-choice decisions distort the prey distribution), it does not inherently do so: more frequently, niche construction becomes evolutionarily significant through modifying environmental conditions and thereby indirectly influencing selection, which is perhaps why it is not typically recognized as an evolutionary process. A clear logical distinction can be made between standard natural selection (direct environmental influence on genotype fitnesses) and organism-induced changes in environments (niche construction) which lead to differential survival and reproduction (natural selection). Below, we emphasize that niche construction has important consequences that indirectly result in changed gene frequencies through a self-reinforcing cycle of cause–effect relationships (figure 1).
Figure 1.
The cycle of cause–effect relationships associated with niche construction. The self-reinforcing nature of this cycle generates much less variation in the source of selection than where there is no feedback from organisms' activities to the environment. Here, ‘autonomous’ refers to environmental processes that are not affected, or only weakly regulated, by the activities of organisms. Consequences of niche-constructed aspects of the environment (case 1) may be qualitatively different from environmental changes resulting from autonomous environmental processes (case 2), leading to experimentally detectable differences in selective environments, gene-frequency changes and patterns of trait coevolution in the two cases.
2. Niche construction as evolutionary bias
The niche-construction perspective within evolutionary biology focuses on the causal relations underlying adaptation through natural selection. Elaborating on Waddington [10] and Lewontin [11], niche-construction theory summarizes the impact organisms have on their own and other species selective environment with multiple effects on evolution ([1,12]; see also [13,14]). This perspective has motivated researchers to document the scale and extent of niche construction, to investigate its ecological consequences and to develop mathematical models to explore its evolutionary ramifications ([1,15–23]; summarized in [3]). This body of theory has led to the widespread acceptance of at least two general insights. First, niche construction can generate ecological legacies, in the form of modified conditions experienced by descendants, and this ‘ecological inheritance’ not only affects evolutionary dynamics but is an important component of broadened conceptions of inheritance [24,25]. Second, niche construction arising from acquired characters (such as learned and socially transmitted knowledge) can play an evolutionary role by modifying patterns of natural selection (for instance, experiments show that blue tits and great tits learn many aspects of their foraging niche from parents, [26]). In the case of humans, this process is known to trigger gene–culture coevolution [17,27–30].
Here, we wish to concentrate on a further insight that follows from the niche-construction perspective. Niche construction initiates and modifies the patterns of natural selection directly affecting the constructor (and other species that share its environment) in an orderly, directed and sustained manner, in part because feedback (figure 1) leads to a self-reinforcing process. As a consequence, niche construction directs adaptive evolution. Niche construction should be recognized as an evolutionary process because it imposes a statistical bias on the direction and mode of selection that ensue, and hence on the speed and direction of evolution. By systematically creating and reinforcing specific environmental states, niche construction directs evolution along particular trajectories. For niche construction to be recognized as an evolutionary process, a broadening of current conceptions of evolutionary process would be required. Endler [31,32] develops such a broader classification scheme, which specifies a number of categories of evolutionary process, including ‘adaptive processes’, ‘rate-determining processes’ and ‘direction-determining processes’. It is in these roles that niche construction has significant explanatory value.
We illustrate the potential of niche construction to generate an evolutionary bias by comparing it with artificial selection. We suggest that niche construction occupies the middle ground between artificial and natural selection: like artificial selection, niche construction reliably generates relatively consistent features in selective environments. During artificial selection, breeders and experimentalists deliberately select for particular characteristics (high yields, pretty flowers and attractive plumage); the breeder/experimentalist imposes direction on evolution by determining which individuals reproduce. There is a predictability and consistency to the pattern of evolution that ensues—the breeder/experimentalist can anticipate with confidence that a specific favoured trait will reliably increase in frequency until genetic variation is significantly depleted and can predict with some accuracy the direction of evolution. Selective breeding increases the frequency of the selected trait, frequently evoking characteristic and strong responses to selection.
The predictability and generality of artificial selection can be contrasted with the frequent unpredictability and local contingency of natural selection in natural populations without niche construction. Given knowledge of environmental conditions, researchers can, and do, still make predictions as to what natural selection may occur and which traits might be favoured on average, but researchers' confidence in these predictions, and their specificity, are typically less than for artificial selection because all natural environments fluctuate; the researcher has no control over or direct knowledge of the selective environment. As a result, it is difficult to be confident that the selective response observed in the current generation will continue in a repeatable, reliable and sustained manner in subsequent generations.
We now have extensive data on natural selection in the wild [33–39], which typically shows that strong directional selection that is consistent from one generation to the next is relatively uncommon. Peter and Rosemary Grant ([40], p. 707) in describing their classic long-term study of the evolution of Darwin's finches (now a 40-year-long study, [41]) state: ‘in the long-term evolution is unpredictable because environments, which determine the directions and magnitudes of selection coefficients, fluctuate unpredictably’. Their study of two populations of Darwin's finches (cactus finches and medium ground finches) on the Galapagos island of Daphne Major found that patterns of selection on body size and beak shape changed several times in the period of investigation. Natural selection occurred frequently in both species but varied from unidirectional to oscillating, and episodic to gradual. They conclude that ‘the phenotypic states of both species at the end of the … study could not have been predicted at the beginning’. While there are now many thousands of measured responses to selection in natural systems, which vary widely in their rates and predictability, it is probably a reasonable generalization to suggest that responses to natural selection in the wild are typically weaker, less consistent and less predictable than responses to artificial selection, although to our knowledge a detailed meta-analysis analysing this comparison has not yet been conducted.
We suggest that the relevant difference between artificial and natural selection relates to the properties of the source of selection [42], which can be viewed as differences in earlier events in a selection cycle (figure 1). In the case of artificial selection, the breeder/experimentalist's activities are more consistent, directed and sustained relative to the environmental change associated with natural selection in the wild, which results from independent and frequently erratic processes. The breeder/experimentalist is imposing a reliability and direction on natural selection through consistent and sustained activities that determine and control the fitness of individuals in the selected population. It is this activity on the part of the breeder/experimentalist that ensures that particular traits are consistently over-represented in subsequent generations. What is relevant about the breeder/experimentalists' activities here is not any conscious or deliberate attempt to achieve a particular phenotype but rather the consistent, reliable and sustained nature of their activities.
Like artificial selection, niche construction makes the selective environment much more predictable than if it were absent. With niche construction there is feedback between the activities of organisms and the environment, such that the entire process can be self-reinforcing (figure 1, case 1). Like artificial selection, the direction of evolution is less subject to fluctuations than if the feedback were absent. Consequently, selection resulting from niche construction is likely to be qualitatively different from selection arising from autonomous (unaffected by organism's activities) environmental processes (figure 1, case 2), which lack the feedback and hence environmental regulation. Indeed, the breeder/experimentalist's activities could be viewed as an extreme form of niche construction (one that imposes a constructed niche on a domesticated species).
We propose that niche construction in general is likely to generate relatively stable and repeated selection, leading to predictable consequences. An animal builds a nest and it immediately creates or modifies selection for ecophysiological traits affected by the improved egg and hatchling micro-environment. In addition, the nest needs to be defended, maintained, regulated and improved upon in design. Nest building also creates the opportunity for other animals to steal the nest material, destroy it, squat in it or dump eggs in it. These are all robust selective responses that can be anticipated irrespective of whether the builder is a bird, a fish, a wasp or a cockroach [1,43,44]. Likewise, a spider spins a web, reliably generating selection that favours sticky webs, web-site selection, anti-predator behaviour on webs and more. Nest building, web spinning, burrow digging and countless other niche-constructing activities generate consistent, reliable, sustained changes in environmental conditions, often regulating those conditions within precise bounds that are adaptive for the constructor [1,2,43,44]. Some desert insects spend their entire lives within bunch grasses which provide selective environments with much less temperature fluctuation and higher humidity than a few centimetres outside the clumps, and consequently completely different and more consistent selective environments than if they moved at random in the desert. Animals control certain elements in their environment, often pushing them into states that they would not otherwise occupy, thereby imposing an order or regularity on a subset of the selection that they encounter, and reliably triggering adaptive responses, or buffering such responses, depending on circumstances. Comparative evidence suggests that such adaptive responses have evolved time and time again [1].
Like the artificial selection elicited by the practices of the breeder/experimentalist, we propose that the selection pressures that niche construction generates will be reliable, directed, orderly and often highly consistent across diverse organisms performing similar niche-constructing activities. More than that, we propose that the selection generated by niche construction will be predictable, or at least more predictable than responses to environmental elements with little or no niche construction. This arises from the self-reinforcing nature of the process (figure 1). As both control of, and scientific knowledge of, the selective environment is never perfect, and as selective responses also depend on the availability of relevant genetic variation, predicting exactly what will happen in a given selective scenario may be difficult. Nonetheless, we suggest that across multiple populations researchers should be able to make predictions of particular expected patterns with more success than if there were no niche construction. If we are right, then this reasoning will potentially help evolutionary biologists to (i) identify traits in which evolutionary responses will be more predictable, (ii) predict longer-term evolutionary trends across multiple traits if they are involved in niche construction, (iii) predict patterns of parallel evolution in isolated populations and species, (iv) predict some qualitative properties of measured responses to selection in the wild, and (v) account for biodiversity.
The anticipated predictive qualities of the selection resulting from niche construction in large part follow from the fact that niche construction is guided by genetic and/or learned information. Other factors, such as the ecological legacies frequently generated by niche construction, known as ecological inheritance [45], also contribute to the stability of niche-construction-generated selection. The expectation that niche construction will generate reliable, consistent and sustained selection in predictable directions is not restricted to the individual's artefacts, but applies equally to by-product niche construction and negative niche construction (e.g. dumping detritus). By-products and the fitness-depreciating activities of organisms are also typically directed, consistent and sustained, largely because they too are guided by information accrued through earlier natural selection [46]. For instance, seabirds engage in very powerful niche construction through feeding at sea and defecating on the land, where their guano is a major source of nutrients. Croll et al. [47] describe how the introduction of artic foxes to the Aleutian Islands, which reduced seabird numbers through predation, transformed these subarctic islands from grassland to tundra, dramatically affecting community structure. However, the regularity of the natural grassland environment is a direct consequence of seabird niche construction, which illustrates the repeated, directional effects of by-product niche construction, with multiple species involved.
We expect that organisms will disproportionately generate environmental states in a primary or key dimension that are likely to match—that is, be coherent and integrated with—the constructing organism's phenotype and requirements and those of its descendants [1,48]. Constructed environments are therefore typically expected to be adaptive for the constructor or its descendants, at least in the short term, and with respect to this key dimension [1]. However, any process of niche construction will probably simultaneously modify numerous ecological factors. While each bout of niche construction part solves an adaptive ‘problem’ through creating a new ‘feature–factor match’ [49] in one or more key dimensions (e.g. spinning a web enhances spider foraging), in the process it creates new adaptive ‘problems’ through generating new feature–factor mismatches (e.g. vulnerability to avian predation on the web) that can trigger evolutionary episodes in other secondary dimensions, leading to the evolution of other traits (e.g. marking the web, construction of dummy spiders, defensive behaviour on the web) [11]. This means that niche construction can simultaneously dampen selection in a key dimension while potentially imposing novel and strong selection in other dimensions.
Is the focus on niche construction misplaced? As niche-constructing traits have themselves evolved, couldn't the bias imposed on selection by niche construction be regarded as no more than the legacy of history, that is, as a phylogenetic constraint, with past evolutionary events shaping future possibilities? After all, if a (non-niche-constructing) morphological trait evolves, it immediately modifies selection acting on other aspects of the phenotype. Is the selective feedback from niche-constructing traits any different from the selective feedback of other traits? Yes, and no. Certainly, organisms do not start over each bout of selection from scratch, but have characteristics that are built upon already existing ones that were inherited from their ancestors. When researchers speak of phylogenetic constraints, they recognize that existing characteristics limit the amount or pattern of evolution subsequently seen in that taxa. All new traits must be coherent and integrated with existing aspects of the organism's phenotype if they are to be adaptive.
Schwenk & Wagner [50] address this with their proposal that natural selection can be resolved into ‘external’ and ‘internal’ components. We certainly do not wish to suggest that niche construction is the only source of evolutionary bias. On the other hand, we are open to the possibility that adaptive responses to niche-constructing traits may be less constrained than adaptive responses to other (e.g. morphological) traits, at least in some dimensions, because they are physically located in the environment rather than in the organism. While niche-constructing traits are often environment buffering in a primary dimension, by modifying ecological resources in ecosystems, niche construction affects the flows of energy, matter and information to other individuals, including neighbours, descendants (some quite distant in time) and other species that share the constructor's environment, and in this manner generate eco-evolutionary feedbacks (figure 1; [1,3,4,8]). The environmental context of niche construction creates opportunities for inter-individual and inter-species interactions, including diverse indirect ecological and coevolutionary feedbacks in engineering webs [51,52], and the accumulation of ecological resources over periods of time that extend beyond the lifespan of the constructor and that drive the coevolution of recipient traits in the constructor's descendants [15,16,19]. This reasoning has a number of practical implications and allows for the specification of testable hypotheses.
3. Practical implications and predictions
We have suggested that niche construction occupies the middle ground between artificial and natural selection. Like artificial selection, niche construction reliably generates consistent features in selective environments, whereas there is frequent unpredictability and local contingency of natural selection in other natural populations. Unlike artificial selection, diverse living organisms rather than humans produce the evolutionary bias, but unlike natural selection stemming from non-constructed environments, here there is feedback from the organisms' niche-constructing activities and the environment, which stabilizes environmental states, and hence stabilizes the strength and direction of natural selection.
While the aforementioned differences between artificial selection and natural selection in the wild are widely accepted, to our knowledge they have not yet been confirmed through rigorous meta-analysis on actual experimental data. Hence, as a starting point, we make the following baseline prediction (table 1, prediction 1): Artificial selection will typically be associated with stronger, more consistent and more unidirectional responses to selection than natural selection (with or without niche construction). This expectation follows directly from the consistent, directed and repeated manner in which breeders/experimentalists control the selective environment or context. A preliminary analysis for the research programme that we outline will be to confirm this expectation drawing on meta-analyses of data on natural selection in wild and in domesticated species.
Table 1.
Predictions.
| Prediction 1: Artificial selection will typically be associated with stronger, more consistent and more directional responses to selection than natural selection in the wild, an expectation that follows directly from the consistent, directed and repeated manner in which breeders/experimentalists control the selective environment or context (the source of selection). |
| Prediction 2: Selection arising from niche-constructed aspects of the environment will have similar (if weaker) regularities and consequences to that observed in artificial selection, but significantly more regularity than natural selection arising from autonomous environmental factors. Responses to niche construction are likely to be qualitatively (or at least quantitatively) different from selection arising from autonomous environmental processes, leading to qualitatively different genetic responses and patterns of trait coevolution. |
| Prediction 3: Niche construction will typically generate more consistent selection, both in time and space, manifest as reduced temporal and spatial variance in selection differentials, relative to non-constructed environments. |
| Prediction 4: Innovations in niche construction will commonly lead to the rapid evolution of functionally coordinated and eventually genetically correlated suites of traits. |
| Prediction 5: Well-established environment buffering (counteractive) niche construction will typically reduce the rate of response to selection relative to autonomous sources of selection, as manifest in reduced directional, stabilizing and correlational selection magnitudes. |
| Prediction 6: Novel (inceptive) niche construction activities will initially on average generate unusually strong selection, as manifest in larger selection gradients/differentials, but this should typically be followed by a weakening in the directional response to selection as a result of strong selection rapidly depleting genetic variation, followed by stabilizing selection once the species becomes adapted. |
| Prediction 7: Consideration of the properties of the sources of selection, and specifically the feedback between organisms' activities and the selective environment, will help to account for variation in responses to natural selection in the wild. |
| Prediction 8: It should be possible to predict sequences of trait evolution and trait coevolution across multiple traits in instances where these result from niche construction, with the predictability of responses to constructed environmental factors enhanced relative to autonomous factors. |
| Prediction 9: Niche construction will frequently generate parallel patterns in selective responses among independent lineages. |
| Prediction 10: With caveats, diversity patterns are likely to covary with the prevalence of niche construction. |
With this established, we foresee considerable potential for researchers to test a series of predictions specifically concerned with the responses to selection arising from niche construction. Here, we anticipate that niche construction parameters are likely to be intermediate between artificial and natural selection (table 1, prediction 2), for example, exhibiting less regularity than artificial selection but more than natural selection emanating from autonomous (non-constructed) environmental components. In general, responses to niche construction are likely to be qualitatively (or at least quantitatively) different from selection arising from autonomous environmental processes, leading to qualitatively different genetic responses and patterns of trait coevolution.
Caveats arise because, while self-constructed features of the environment clearly meet case 1 (figure 1) as examples of niche construction, and while autonomous abiotic environmental sources clearly meet case 2 (figure 1) as examples of autonomous environmental processes, some biotic sources of environmental change require more careful consideration. For example, the source of selection may be a member of the same species, as in cases of sexual selection. Here, as mate-choice technically meets broad definitions of niche construction (i.e. mates are environmental resources chosen by animals) [1], we would categorize trait evolution in response to mating preferences as case 1 (niche construction), and the resulting sexual selection is also a self-reinforcing process [53]. Moreover, the niche construction of other species can be an important source of selection. Where one species evolves specifically in response to an environmental factor constructed by members of another species (e.g. egg dumpers or inquilines in birds’ nests), we would again categorize the evolution of such features as case 1 (niche construction). In other diffuse coevolutionary scenarios, however, it may not be possible to discern a clear constructed feature of the environment that serves as the source of selection, in which case we would categorize the example is case 2 (autonomous environmental effects). Some other forms of coevolution, notably predator–prey interactions, are less easily categorized. Further discussion can be found in the ‘Implementation Guidelines' section below.
Our general expectation is that the properties of constructed environments will differ from those of non-constructed environments with sufficient frequency, in sufficiently predictable ways (e.g. reduced variation in time and space) that knowledge of niche construction can enhance predictions concerning the patterns of response to selection. Elliot Sober [42] distinguished between ‘source laws’ (concerned with the properties of processes) and ‘consequence laws’ (concerned with their outcomes). A deeper understanding of ecology can potentially provide source laws for natural selection, which will complement those consequence laws currently studied through population genetics, enhancing the predictive power of evolutionary analyses [48]. Specifically, there are opportunities to use niche-construction theory to derive ‘source laws’ for natural selection by focusing on the properties of the source; niche construction can be a source of selection. As detailed below, even where the direction of the response cannot be predicted, the fact that there is a directing bias may change some statistical properties of the response to selection.
We recognize that, in its general form, prediction 2 is likely to be of limited use. Nonetheless, it is possible to refine this expectation into a series of more specific predictions, which we anticipate can readily be put to the test. These predictions span three general domains: (i) measuring natural selection in the wild, (ii) predicting patterns of trait coevolution, and (iii) predicting patterns of biodiversity.
3.1. Measuring natural selection in the wild
Through generating biases in environmental conditions, niche construction is expected to affect the presence, direction, rate and consistency of evolution through natural selection among genotypes and phenotypes in the wild. The selection resulting from niche-constructing traits should often be more predictable than other forms of natural selection because constructing organisms partly control their environment, and act to ensure that key environmental variables remain within suitable tolerance ranges. In principle, this predictability should be detectable through meta-analysis. We predict that niche construction will typically generate more consistent selection, both across generations (i.e. sustained over significant periods of time) and in space (i.e. the same, or closely related, species should construct consistent niches over most of their geographical range), which will be manifest as reduced temporal and spatial variance in selection differentials relative to non-constructed environments (table 1, prediction 3). It may also be manifest as reduced phenotypic variation compared to traits not involved in niche construction. We expect constructed environments to be associated with reduced variance in selection gradients and selection differentials relative to non-constructed environments, and that this expectation will be manifest both within and between samples.
Innovations in niche construction are expressed in the environment, and hence are both less vulnerable to disrupting the internal functionality of the phenotype, and are more likely to instigate indirect forms of selective feedback. In addition, specific environments favour specific combinations of traits. Consequently we expect innovations in niche construction commonly to lead to the rapid evolution of functionally coordinated and eventually genetically correlated suites of traits (table 1, prediction 4). This should occur with greater frequency than innovations in non-niche-constructing traits. These coordinated adaptive responses to inceptive niche construction arise as a result of the secondary dimensions of selection that the niche-constructing trait generates (e.g. ‘correlational selection’, [31,33]), including through diffuse and direct coevolutionary interactions.
We expect that well-established environment buffering (counteractive) niche construction will typically reduce the rate of response to selection relative to autonomous sources of selection, as manifest in smaller linear and quadratic selection differentials (table 1, prediction 5) once the adaptation to the constructed environment has occurred. Conversely, novel and inceptive acts of niche construction will on average initially generate unusually strong selection, as manifest in larger directional, stabilizing and correlational selection differentials, but this should typically be followed by a weakening in the response to selection (table 1, prediction 6), as genetic variation is eroded under strong and consistent selection, and the constructed environment becomes more stable. Thus, both the positive and negative effects on rates arise because niche construction typically generates consistent environmental conditions. Consistent with prediction 6, Alberti et al. [54] describe more rapid evolutionary changes in diverse species exposed to urban compared to natural environments.
The fact that niche construction can have an omnidirectional impact on rates should not greatly reduce the predictability of the response, because the circumstances under which niche construction will accelerate and decelerate responses remain a priori predictable. For illustration, we expect bugs that live as inquilines in bird's nests will on initial occupancy evolve more rapidly than bugs living in non-constructed environments, but thereafter evolve more slowly than other bugs, because their constructed environment is more stable. More generally, we anticipate that diffuse coevolution mediated by niche-constructed environmental resources will often initially be a source of strong selection. One example is the rapid evolved responses of diverse organisms to anthropogenic change (e.g. heavy metal tolerance in plants, moth colouration in response to air pollution). In addition, other species' niche construction can leave ecological legacies in the environment (ecological inheritance) that may persist in the absence of the constructor, and hence remain as consistent and persistent sources of selection.
A further general prediction is that consideration of the properties of the source of selection will help to account for variation in responses to natural selection in the wild (table 1, prediction 7). As described above, while niche construction will not always generate unusually weak or unusually strong responses to selection, it will frequently generate a priori predictable environmental conditions, and there is potential to use this knowledge to make predictions about where responses to selection will arise and to specify some of their properties, including direction and strength. A caveat to prediction 3 is the possibility (e.g. in humans) that niche construction arises from short-lived culturally transmitted activities that do not lead to sustained environmental change. Yet, such caveats aside, niche construction should usually be to some degree predictable, and other factors held equal should generally be more predictable in its properties than independent aspects of environmental change. Such considerations have the potential to set some established findings in a wider explanatory context. Meta-analyses of responses to selection in the wild have detected broad patterns in the properties of the evolving species, including that selection on life-history traits is typically weaker than that on morphology, and that selection on mating success is stronger than selection on survival [34–37]. A consideration of the properties of the source of selection may help to explain why these findings arise (prediction 7) if, for instance, life-history traits are more likely than morphological traits to be regulated by environment buffering forms of niche construction, and/or if selection on mating success is more likely than selection on survival to result from inceptive niche construction.
Naturally, any such analyses would need to control for additional factors that potentially affect the rates and consistency of responses to selection. For instance, certain environments (e.g. semi-arid, arid environments) are known to have extreme fluctuations, while the duration of the measured response is known to affect its magnitude.
3.2. Predicting trait coevolution
We anticipate that it should be possible to predict sequences of trait evolution and trait coevolution across multiple traits in instances where these result from niche construction, and that this predictability will be enhanced by the regularity with which niche construction occurs relative to autonomous features of the environment traits (table 1, prediction 8). This leads us to the expectation that niche construction will often result in correlational selection and therefore create long-term multivariate trends, including across multiple characters, in ways that are potentially predictable [1,34,55,56] and testable [57,58], with longer and/or more reliable sequences being associated with constructed compared to non-constructed environments. These predictions can be tested using comparative phylogenetic methods applied to animal artefact construction and associated behaviour. Researchers can specify predictions as sequence information related to traits (i.e. character B will tend to evolve following the evolution of character A, or A→B). By combining predictions concerning pairs of discrete traits (A→B, B→C), and considering traits with multiple levels (i.e. A→A’→A’’), we expect that researchers will be able to predict more extended sequences (such as that spider web building led to the evolution of refined or larger web structure, which in turn favoured subsociality, and then reduced aggression).
We conjecture that adaptive niche-constructing responses evolve time and time again, generating signatures of environmental change that are quite distinct from processes independent of the organism, to produce parallel evolution in independent lineages [56]. Accordingly, another key expectation is that niche construction will frequently generate parallel patterns in selective responses among independent lineages (table 1, prediction 9). For illustration, we predict that burrow digging creates vulnerability to fungal infections in burrowing insects, spiders, caecilians and mammals and hence favours the evolution of common traits which mitigate these problems. We expect this process of similar niche construction leading to convergent selection in independent lineages to account for a significant number of cases of parallel evolution. This expectation, as well as prediction 4 (Innovations in niche construction will commonly lead to the rapid evolution of coordinated suites of traits), can also be tested using established comparative phylogenetic tools.
3.3. Predicting biodiversity
Finally, it is also well established that, by creating habitat and resources that can be exploited by other species that share its ecosystem, niche-constructing organisms potentially create new niches for other species. Classic studies of plant and animal community succession document niche changes and new species occurring as niches develop during succession. Classic niche-constructing species like beavers, coral or kelp are known to create habitat for countless other organisms [1,51,59]. Niche construction through bioturbation is thought to be partly responsible for the Cambrian explosion [60]. Likewise, both nest building in birds and the evolution of orb webs in spiders have been suggested to allow for expansion into novel habitats, driving increased evolutionary and ecological diversification [61–63]. It follows that patterns of biodiversity should covary positively with the prevalence of niche construction (table 1, prediction 10). Once again, a caveat is required here, because this expectation may not apply in cases where the niche construction is insufficiently longstanding for adaptive responses from other species to have evolved, nor where the niche construction destroys habitat and resources (as in many cases of anthropogenic change). These expectations can again be tested through comparative phylogenetic methods applied to niche-constructing and recipient traits.
4. Implementation guidelines
In practice, categorizing real-life examples according to whether the source of selection is constructed or not will require careful consideration, as well as at least some basic knowledge of the study system. Here, we summarize guidelines for the researcher to aid experimental testing, on which we elaborate in the electronic supplementary material, including through illustrative examples and a training set. We have found that most published studies presenting data on selective responses in the wild can be reliably categorized using these guidelines, and hence can be used to test our predictions. However, in the longer term, we encourage researchers to conduct experimental studies specifically designed to test our predictions, and anticipate that such studies will offer greater resolution and reliability.
(i) We make a distinction between the focal trait and source of selection, emphasizing that our predictions concern differential evolutionary responses of focal traits to constructed versus non-constructed source environments; we make no predictions about the evolution of niche-constructing versus non-niche-constructing focal traits here.
(ii) Our predictions are premised on the assumption that the source of selection acting on a focal trait can be identified. In practice, this will not always be the case, in which case these data cannot be used to test our predictions.
(iii) In some cases, rather than relying on a binary (constructed versus non-constructed) categorization, it may be useful for the researcher to deploy a third category of ‘mixed’ source of selection comprising both constructed and non-constructed elements, with the expectation that the relevant measures will be intermediate.
(iv) Our predictions should be implemented on a trait-by-trait basis, recognizing that, in a given study system, some traits may be responses to constructed elements of the environment and others not.
(v) In principal, our predictions concerning niche construction extend beyond the construction of physical artefacts, and should apply equally to the choices of animals, for mates, habitats (including flower sources among pollinators) and prey types.
(vi) Where the source of selection comprises multiple species, the key question is whether they collectively engage in niche construction in a consistent and coherent manner. Closely related species may engage in similar forms of niche construction, while conversely multiple species with different, and mutually inconsistent, activities, behaviour and preferences should be categorized as ‘not constructed’.
(vii) Our predictions concerning the rate of response of selection to constructed environments (4–6) can only be tested using data where the source of selection can also be categorized as novel (inceptive) or environment buffering (counteractive) forms of niche construction.
5. Concluding remarks
In his Presidential address to the American Society of Naturalists, Steven Arnold [64] characterized evolutionary biology as ‘in the midst of its greatest period of synthesis' (p. 729) and concluded ‘to synthesize, we need diverse perspectives and bridges between them’ (p. 744). Niche construction theory potentially offers evolutionary biologists a fresh perspective that brings with it a characteristic set of novel but testable predictions. These predictions derive from the assumption that niche construction co-directs adaptive evolution by imposing a statistical bias on selection, generating regularities in environmental states that create an externally expressed form of evolutionary bias. It remains to be seen whether any of these predictions will be confirmed. However, if, in time, considerations of niche construction do demonstrably enhance the predictability of patterns of selection, this would strengthen the argument that niche construction be regarded as an evolutionary process. Either way, we see considerable potential for a greater focus on the properties of the source of selection to open up fruitful new lines of enquiry for evolutionary biologists and evolutionary ecologists.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Andrew Clark, Hilton Japyassú, Sally Street, Tobias Uller and two anonymous referees for helpful comments on earlier drafts.
Data accessibility
Additional data has been uploaded as electronic supplementary material.
Authors' contributions
All authors contributed to the development of the ideas and writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The research was supported in part by a grant from John Templeton Foundation (60501) entitled ‘Putting the extended evolutionary synthesis to the test’.
References
- 1.Odling-Smee FJ, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Sultan SE. 2015. Organism and environment. Ecological development, niche construction, and adaptation. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Odling-Smee FJ, Erwin D, Palkovacs E, Feldman M, Laland KN. 2013. Niche construction theory: a practical guide for ecologists. Quart. Rev. Biol. 88, 3–28. ( 10.1086/669266) [DOI] [PubMed] [Google Scholar]
- 4.Matthews B, De Meester L, Jones CG, Ibelings BW, Bouma TJ, Nuutinen V, van de Koppel J, Odling-Smee J. 2014. Under niche construction: an operational bridge between ecology, evolution and ecosystem science. Ecol. Monogr. 84, 245–263. ( 10.1890/13-0953.1) [DOI] [Google Scholar]
- 5.Scott-Phillips TC, Laland KN, Shuker DM, Dickins TE, West SA. 2013. The niche construction perspective. A critical appraisal. Evolution 68, 1231–1243. ( 10.1111/evo.12332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawkins R. 1982. The extended phenotype. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Pelletier F, Garant D, Hendry AP. 2009. Eco-evolutionary dynamics. Phil. Trans. R. Soc. B 364, 1483–1489. ( 10.1098/rstb.2009.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640. ( 10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobzhansky T. 1937. Genetics and the origin of the species. Ney York, NY: Columbia University Press. [Google Scholar]
- 10.Waddington CH. 1959. Canalization of development and genetic assimilation of acquired characters. Nature 183, 1654–1655. ( 10.1038/1831654a0) [DOI] [PubMed] [Google Scholar]
- 11.Lewontin RC. 1983. Gene, organism and environment. In (ed. Bendall) Evolution from molecules to men. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Odling-Smee FJ, Laland KN, Feldman MW. 1996. Niche construction. Am. Nat. 147, 641–648. ( 10.1086/285870) [DOI] [Google Scholar]
- 13.Oyama S, Griffiths PE, Gray RD (eds). 2001. Cycles of contingency: developmental systems and evolution. Cambridge, MA: MIT Press. [Google Scholar]
- 14.Bateson P, Gluckman P. 2011. Plasticity, robustness, development and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Laland KN, Odling-Smee FJ, Feldman MW. 1996. On the evolutionary consequences of niche construction. J. Evol. Biol. 9, 293–316. ( 10.1046/j.1420-9101.1996.9030293.x) [DOI] [Google Scholar]
- 16.Laland KN, Odling-Smee FJ, Feldman MW. 1999. Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl Acad. Sci. USA 96, 10 242–10 247. ( 10.1073/pnas.96.18.10242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laland KN, Odling-Smee FJ, Feldman MW. 2001. Cultural niche construction and human evolution. J. Evol. Biol. 14, 22–33. ( 10.1046/j.1420-9101.2001.00262.x) [DOI] [PubMed] [Google Scholar]
- 18.Silver M, Di Paolo EA. 2006. Spatial effects favour the evolution of niche construction. Theor. Pop. Biol. 70, 387–400. ( 10.1016/j.tpb.2006.08.003) [DOI] [PubMed] [Google Scholar]
- 19.Lehmann L. 2008. The adaptive dynamics of niche constructing traits in spatially subdivided populations: evolving posthumous extended phenotypes. Evolution 62, 549–566. ( 10.1111/j.1558-5646.2007.00291.x) [DOI] [PubMed] [Google Scholar]
- 20.Kylafis G, Loreau M. 2008. Ecological and evolutionary consequences of niche construction for its agent. Ecol. Lett. 11, 1072–1081. ( 10.1111/j.1461-0248.2008.01220.x) [DOI] [PubMed] [Google Scholar]
- 21.Loreau M. 2010. From populations to ecosystems: theoretical foundations for a new ecological synthesis. Princeton, NJ: Princeton University Press. [Google Scholar]
- 22.van Dyken JD, Wade M. 2012. Origins of altruism diversity II: runaway coevolution of altruistic strategies via ‘reciprocal niche construction’. Evolution. 66, 2498–2513. ( 10.1111/j.1558-5646.2012.01629.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creanza N, Feldman MW. 2014. Complexity in models of cultural niche construction with selection and homophily. Proc. Natl Acad. Sci. USA 111, 10 830–10 837. ( 10.1073/pnas.1400824111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danchin E, Charmantier A, Champagne FA, Mesoudi A, Pujol B. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486. ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 25.Bonduriansky R. 2012. Rethinking heredity, again. Trends. Ecol. Evol. 27, 330–336. ( 10.1016/j.tree.2012.02.003) [DOI] [PubMed] [Google Scholar]
- 26.Slagsvold T, Weibe K. 2011. Social learning in birds and its role in shaping a foraging niche. Phil. Trans. R. Soc. B 366, 969–977. ( 10.1098/rstb.2010.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd R, Richerson PJ. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 28.Laland KN, Odling-Smee FJ, Myles S. 2010. How culture shaped the human genome: bringing genetics and the human sciences together. Nat. Rev. Genet. 11, 137–148. ( 10.1038/nrg2734) [DOI] [PubMed] [Google Scholar]
- 29.Richerson PJ, Boyd R, Henrich J. 2010. Gene-culture coevolution in the age of genomics. Proc. Natl Acad. Sci. USA 107, 8985–8992. ( 10.1073/pnas.0914631107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeder MA. 2016. Domestication as a model system for niche construction. Evol. Ecol. 30, 325–348. ( 10.1007/s10682-015-9801-8) [DOI] [Google Scholar]
- 31.Endler JA. 1986. The newer synthesis? Some conceptual problems in evolutionary biology. Oxford Surv. Evol. Biol. 3, 224–243. [Google Scholar]
- 32.Endler JA, McLellan T. 1988. The processes of evolution: towards a newer synthesis. Annu. Rev. Ecol. Systemat. 19, 395–421. ( 10.1146/annurev.es.19.110188.002143) [DOI] [Google Scholar]
- 33.Endler JA. 1986. Natural selection in the wild. Princeton, NJ: Princeton University Press. [Google Scholar]
- 34.Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 35.Kingsolver JG, Diamond SE. 2011. Phenotypic selection in natural populations: what limits directional selection? Am. Nat. 177, 346–357. ( 10.1086/658341) [DOI] [PubMed] [Google Scholar]
- 36.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. 2012. Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol. Ecol. 26, 1101–1118. ( 10.1007/s10682-012-9563-5) [DOI] [Google Scholar]
- 37.Linnen CR, Hoekstra HE. 2009. Measuring natural selection on genotypes and phenotypes in the wild. Cold Spring Harb. Symp. Quant. Biol. 74, 155–168. ( 10.1101/sqb.2009.74.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siepielski AM, Gotanda KM, Morrissey MB, Diamond SE, DiBattista JD, Carlson SM. 2013. The spatial patterns of directional phenotypic selection. Ecol. Lett. 16, 1382–1392. ( 10.1111/ele.12174) [DOI] [PubMed] [Google Scholar]
- 39.Morrissey MB. 2016. Meta-analysis of magnitudes, differences and variation in evolutionary parameters. J. Evol. Biol. 29, 1882–1904. ( 10.1111/jeb.12950) [DOI] [PubMed] [Google Scholar]
- 40.Grant PR, Grant BR. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711. ( 10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 41.Grant PR, Grant BR. 2014. 40 years of evolution: Darwin's finches on Daphne Major island. Princeton, NJ: Princeton University Press. [Google Scholar]
- 42.Sober E. 1984. The nature of selection. Cambridge, MA: MIT Press. [Google Scholar]
- 43.Hansell MH. 2005. Animal architecture. Oxford, UK: Oxford University Press. [Google Scholar]
- 44.Hansell MH. 2007. Built by animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 45.Odling-Smee FJ. 1988. Niche-constructing phenotypes. In The role of behavior in evolution (ed. Plotkin HC.), pp. 73–132. Cambridge, MA: MIT Press. [Google Scholar]
- 46.Connelly BD, Dickinson KJ, Hammarlund SP, Kerr B. 2016. Negative niche construction favors the evolution of cooperation. Evol. Ecol. 30, 267–283. ( 10.1007/s10682-015-9803-6) [DOI] [Google Scholar]
- 47.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. 2005. Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961. ( 10.1126/science.1108485) [DOI] [PubMed] [Google Scholar]
- 48.Laland KN, Uller T, Feldman MW. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Phil. Trans. R. Soc. B 282, 20151019 ( 10.1098/rspb.2015.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bock WJ. 1980. The definition and recognition of biological adaptation. Am. Zool. 20, 217–227. ( 10.1093/icb/20.1.217) [DOI] [Google Scholar]
- 50.Schwenk K, Wagner GP. 2004. The relativism of constraints on phenotypic evolution. In Phenotypic integration: studying the ecology and evolution of complex phenotypes (eds Pigliucci M, Preston K). Oxford, UK: Oxford University Press. [Google Scholar]
- 51.Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos 69, 373–386. ( 10.2307/3545850) [DOI] [Google Scholar]
- 52.Krakauer DC, Page KM, Erwin DH. 2009. Diversity, dilemmas, and monopolies of niche construction. Am. Nat. 173, 26–40. ( 10.1086/593707) [DOI] [PubMed] [Google Scholar]
- 53.Anderson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 54.Alberti M, Correa C, Mazluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y. 2017. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl Acad. Sci. USA. ( 10.1073/pnas.1606034114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laland KN, Odling-Smee FJ, Gilbert SF. 2008. EvoDevo and niche construction: building bridges. J. Exp. Zool. B 310, 549–566. ( 10.1002/jez.b.21232) [DOI] [PubMed] [Google Scholar]
- 56.Laland KN. 2014. On evolutionary causes and evolutionary processes. Behav. Process 117, 97–104. ( 10.1016/j.beproc.2014.05.008) [DOI] [PubMed] [Google Scholar]
- 57.Blows MW, Brooks R, Kraft PG. 2003. Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male guppies. Evolution 57, 1622–1630. ( 10.1111/j.0014-3820.2003.tb00369.x) [DOI] [PubMed] [Google Scholar]
- 58.Cole GL, Endler JA. 2015. Variable environmental effects on a multicomponent sexually selected trait. Am. Nat. 185, 452–468. ( 10.1086/680022) [DOI] [PubMed] [Google Scholar]
- 59.Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957. ( 10.1890/0012-9658(1997)078%5B1946:PANEOO%5D2.0.CO;2) [DOI] [Google Scholar]
- 60.Herringshaw LG, Callow RHT, McIlroy D. 2017. Engineering the Cambrian explosion; the earliest bioturbators as ecosystem engineers. In Earth system evolution and early life: a celebration of the work of Martin Brasier, vol. 448 (eds Brasier AT, McIlroy D, McLoughlin N). Geological Society, London, Special Publications. [Google Scholar]
- 61.Collias NE. 1997. On the origin and evolution of nest building by passerine birds. Condor 99, 253–270. ( 10.2307/1369932) [DOI] [Google Scholar]
- 62.Kawamoto TH, Japyassu HF. 2008. Tenacity and silk investment of two orb weavers: considerations about the Araneoidea diversification. J. Arachnol. 36, 418–424. ( 10.1636/CA07-129.1) [DOI] [Google Scholar]
- 63.Blackledge TA, Scharff N, Coddington JA, Szuts T, Wenzel JW, Hayashi CY, Agnarsson I. 2009. Reconstructing web evolution and spider diversitication in the molecular era. Proc. Natl Acad. Sci. USA 106, 5229–5234. ( 10.1073/pnas.0901377106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnold SJ. 2014. Phenotypic evolution: the ongoing synthesis. Am. Nat. 1836, 729–746. ( 10.1086/675304) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data has been uploaded as electronic supplementary material.