In addition to having a reputation as the causative agent of several types of hospital-acquired infections, Klebsiella pneumoniae has gained widespread attention as a pathogen with a propensity for acquiring antibiotic resistance. It is capable of causing a range of infections, including urinary tract infections, pneumonia, and sepsis. Because of the rapid emergence of carbapenem resistance among Klebsiella strains, there is a dire need for a better understanding of virulence mechanisms and identification of new drug targets. Here, we identify the periplasmic transporter FepB as one such potential target.
KEYWORDS: Klebsiella, RamA, enterobactin, pneumonia, siderophore, yersiniabactin
ABSTRACT
Klebsiella pneumoniae is considered a significant public health threat because of the emergence of multidrug-resistant strains and the challenge associated with treating life-threatening infections. Capsule, siderophores, and adhesins have been implicated as virulence determinants of K. pneumoniae, yet we lack a clear understanding of how this pathogen causes disease. In a previous screen for virulence genes, we identified a potential new virulence locus and constructed a mutant (smr) with this locus deleted. In this study, we characterize the smr mutant and show that this mutation renders K. pneumoniae avirulent in a pneumonia model of infection. The smr mutant was expected to have a deletion of three genes, but subsequent genome sequencing indicated that a much larger deletion had occurred. Further analysis of the deleted region indicated that the virulence defect of the smr mutant could be attributed to the loss of FepB, a periplasmic protein required for import of the siderophore enterobactin. Interestingly, a ΔfepB mutant was more attenuated than a mutant unable to synthesize enterobactin, suggesting that additional processes are affected. As FepB is highly conserved among the members of the family Enterobacteriaceae, therapeutic targeting of FepB may be useful for the treatment of Klebsiella and other bacterial infections.
IMPORTANCE In addition to having a reputation as the causative agent of several types of hospital-acquired infections, Klebsiella pneumoniae has gained widespread attention as a pathogen with a propensity for acquiring antibiotic resistance. It is capable of causing a range of infections, including urinary tract infections, pneumonia, and sepsis. Because of the rapid emergence of carbapenem resistance among Klebsiella strains, there is a dire need for a better understanding of virulence mechanisms and identification of new drug targets. Here, we identify the periplasmic transporter FepB as one such potential target.
INTRODUCTION
Klebsiella pneumoniae is a Gram-negative bacterium commonly classified as an opportunistic nosocomial pathogen capable of causing a variety of infections, including urinary tract infections, pneumonia, and sepsis (1–5). It is often found as a commensal resident of the gastrointestinal tract, and this is believed to be a primary source of infection (2, 6–8). Recently, K. pneumoniae also has been shown to be capable of causing community-acquired infections such as pyogenic liver abscesses, meningitis, and endophthalmitis (9–11). The increasing prevalence of antibiotic-resistant strains only serves to compound the clinical importance of K. pneumoniae and the difficulty of treating those infected with extended-spectrum β-lactamase-resistant or carbapenem-resistant strains (12–16). Resistance to carbapenems is of particular concern, as they are used as drugs of last resort to treat Gram-negative infections (12, 17).
During infection, sequestration of iron by the host limits the availability of free iron, and as a result, bacteria produce their own chelators to scavenge iron. Iron acquisition is an essential component of most bacterial pathogens, as iron is required for cellular and metabolic activities (18). Siderophores are small secreted molecules with a high affinity for ferric iron; these are classified on the basis of the chemical nature of the Fe3+ coordination (19). The catecholate-type siderophore enterobactin is produced by most K. pneumoniae strains (20, 21). However, community-acquired isolates and those that cause invasive disease typically encode additional siderophore systems (salmochelin, yersiniabactin, aerobactin) (22). Salmochelin is a C-glucosylated enterobactin produced by some isolates of Salmonella, Escherichia coli, and Klebsiella, and its synthesis is dependent on enterobactin. Mutants unable to produce enterobactin are also unable to produce salmochelin (23, 24). The iroA locus encodes enzymes necessary to modify enterobactin, as well as proteins required for salmochelin transport (25). The yersiniabactin locus is found in many invasive K. pneumoniae isolates and encodes a phenolate-type siderophore that was first identified as part of a pathogenicity island in Yersinia (26). Interestingly, in a genome-wide association study of a broad range of K. pneumoniae isolates, yersiniabactin was found to be the most prevalent virulence-associated locus and was found to be a predictor of infection versus carriage (22). Aerobactin is yet another siderophore produced by a smaller fraction of K. pneumoniae strains than either enterobactin or yersiniabactin (22). Although aerobactin has a lower affinity for Fe3+ than enterobactin or yersiniabactin, it is frequently produced by isolates from pyogenic liver abscesses (27).
To date, the identified virulence factors of K. pneumoniae primarily include capsule, lipopolysaccharide (LPS), fimbriae, and siderophores, and these factors also have been identified as virulence factors in the strain used for the studies presented here (4, 28–34). Several high-throughput studies have been done with mouse models to identify additional bacterial virulence factors (34–40). Two of these screens were signature-tagged mutagenesis (STM) screens for factors affecting gastrointestinal colonization and/or infection of the urinary tract (36, 37). These studies identified adhesins, LPS, and capsule. Another screen for gain of function when Klebsiella genes were expressed in E. coli identified a response regulator, AcrA, and LPS (40). A screen for genes expressed in vivo during septicemia identified genes involved in the use of siderophores (aerobactin and enterobactin) (39), and an STM screen in a model of liver abscess formation identified adhesins and regulators (38). Two of these studies focused on the identification of bacterial genes needed for survival in the lung; one approach used STM, and the other used transposon insertion site sequencing (34, 35). These screens identified capsule, LPS, siderophores, and transcriptional regulators. All of these screens also identified genes predicted to contribute generally to growth, as well as genes of unknown function.
Overall, there has been a lack of overlap in identified genes among the different screens conducted with lung, urinary tract, liver infection, and gastrointestinal colonization models. This may be due to the fact that none of the screens were saturating, or it could be indicative of mechanisms that compensate for the loss of individual genes. These findings are further complicated by the use of different infection models and different pathogen and host strain backgrounds. While typically focused on the goal of identifying previously unknown bacterial factors contributing to disease, these screens primarily identified known virulence factors of K. pneumoniae, as well as metabolic functions generally contributing to growth.
We previously conducted an STM screen of K. pneumoniae in an intranasal model of pneumonia to identify virulence genes (34). From this screen, yersiniabactin was identified as important for the abilities of our strain to colonize the lungs and to cause disseminated infection (33). In addition, a number of mutants with insertions in or near ramA were identified (34). RamA has been implicated in virulence and multidrug resistance in other pathogenic bacteria, and mutations in ramA have been associated with fluoroquinolone resistance in K. pneumoniae (41–44). Furthermore, a recent study reported that overexpression of RamA affects virulence and results in modified LPS (45). Thus, we sought to determine if RamA is a virulence determinant for a highly virulent K. pneumoniae strain. These studies found no role for ramA or nearby genes for virulence in a pneumonia model of infection. However, a serendipitous secondary mutation was identified, and further analysis of this mutation indicates that FepB, a periplasmic protein required for transport of enterobactin and salmochelin, is essential for virulence. Surprisingly, there were interesting differences in virulence between enterobactin synthesis mutants and the ΔfepB mutant.
RESULTS
The smr mutant is severely attenuated in a mouse model of pneumonia.
A previous screen of strain KPPR1 transposon mutants identified genes required for colonization and survival in the lungs of infected mice (34). Thirteen mutants containing disruptions within ramA or an adjacent gene, orf82, failed to be recovered from the lungs and spleens of infected mice. RamA is a transcriptional regulator linked to Salmonella survival in RAW 264.7 macrophages and virulence in BALB/c ByJ mice (41, 42). This led us to hypothesize that the ramA locus is important for the ability of K. pneumoniae to infect the lungs. To test this, we constructed the smr (spontaneous multidrug resistance) mutant, where ramA and the two flanking genes (orf82 and romA) were targeted for deletion, and tested this strain in a mouse model of pneumonia (Fig. 1). The smr mutant caused slightly lower bacterial burdens at 24 h postinoculation (hpi) than KPPR1 (wild type [WT]). At 72 hpi, nearly 5 logs fewer CFU were recovered from mice infected with the smr mutant than from WT-infected mice. The spleens of mice infected with the WT strain had nearly 107 CFU/g of tissue, while the smr mutant was rarely detectable in the spleen at 72 hpi, reflecting a dissemination or systemic survival defect. Together, these data indicate that the smr mutant is essentially avirulent in this infection model.
FIG 1 .
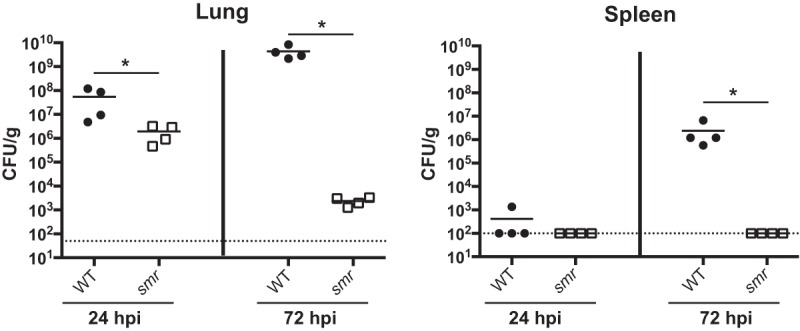
The smr mutant is attenuated in a mouse model of pneumonia. Mice were inoculated i.n. with 2 × 104 CFU of either the WT strain (KPPR1S; black circles) or the Δsmr mutant (VK82; white squares). At 24 or 72 hpi, mice were euthanized and their lungs and spleens were homogenized and plated for bacterial enumeration. Each symbol represents one mouse. The dotted line indicates the limit of detection, and symbols on the dotted line indicate that CFU counts were below the limit of detection. Data are from an individual representative experiment. Mann-Whitney tests were performed for statistical analysis. *, P < 0.05.
Deletions of individual genes in the targeted smr locus do not recapitulate the phenotype of the smr mutant.
To identify the gene(s) responsible for the phenotype of the smr mutant, we made in-frame deletions of each of the three genes (ΔramA, ΔromA, and Δorf82) in the smr locus and tested them in our pneumonia model (Fig. 2A). The phenotype of all three mutant strains resembled that of the WT, suggesting that the loss of a single gene was not sufficient to affect virulence (Fig. 2B). We concluded that neither ramA, orf82, nor romA, individually contributed to virulence in this model or was responsible for the phenotype of the smr mutant.
FIG 2 .
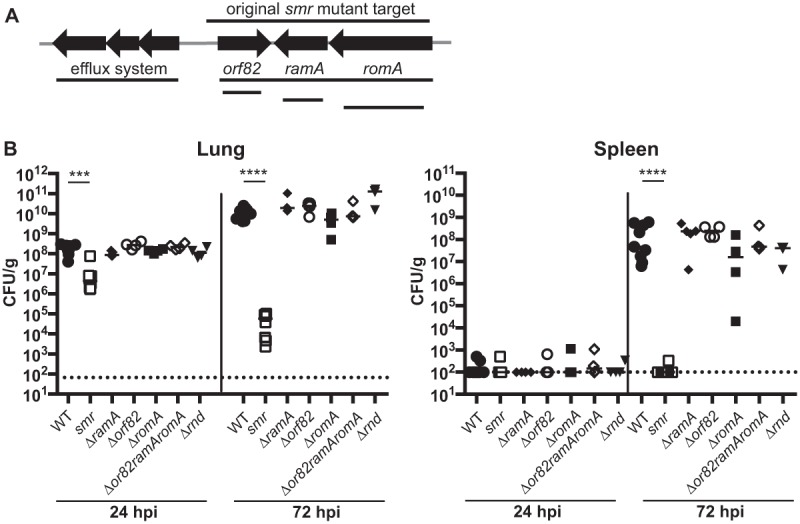
Schematic of smr targeted region and in vivo phenotypes of mutants. (A) Schematic depicting open reading frames within or adjacent to the smr target region (not to scale). Lines indicate the regions deleted in the mutants indicated. (B) Mice were inoculated i.n. with 2 × 104 CFU of the WT strain (KPPR1S; black circles) or the Δsmr (VK082; white squares), ΔramA (VK174; black diamonds), Δorf82 (VK270; white circles), ΔromA (VK131; black squares), Δorf82 ramA romA (VK266; white diamonds), or Δrnd (VK269; inverted triangles) mutant. At 24 or 72 hpi, mice were sacrificed and their lungs and spleens were homogenized and plated for bacterial enumeration. Each symbol represents one mouse. The dotted line indicates the limit of detection, and symbols on the dotted line indicate that CFU counts were below the limit of detection. These data were compiled from several independent experiments. Mann-Whitney tests were performed for statistical analysis. ***, P < 0.001; ****, P < 0.0001.
In examining the region more closely, we noted that an RND (resistance-nodulation-division superfamily) efflux pump system was encoded just upstream of orf82 and that the smr deletion could have impacted the promoter driving the expression of this locus (Fig. 2A). RND efflux systems have been shown to play roles ranging from resistance to human antimicrobial peptides in Pseudomonas to flagellar motility in Burkholderia (46). Thus, we constructed two additional mutants, one with the rnd genes and the other with orf82, ramA, and romA deleted but with the putative rnd promoter intact (Δrnd and Δorf82 ramA romA). The Δrnd mutant colonized mice as efficiently as the WT strain (Fig. 2B). Intriguingly, the second mutant lacking the same three genes as the smr mutant (Δorf82 ramA romA) also had no virulence defect.
Sequencing of the smr mutant reveals a large deletion.
As targeted genetic mutations in the smr locus failed to recapitulate the smr phenotype, we hypothesized that the smr mutant contained a secondary mutation. Whole-genome sequencing revealed that the deletion in the smr mutant was larger than intended. Instead of the targeted deletion of orf82, ramA, and romA, a single segment of 87,290 bp spanning 78 putative open reading frames was deleted.
A component of the enterobactin transport system contributes to virulence.
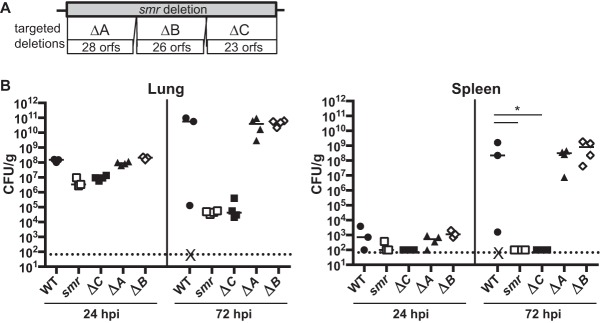
To identify the factor(s) responsible for the virulence defect of the smr mutant, we constructed three mutants (Δsmr_A, Δsmr_B, and Δsmr_C) each with a deletion of approximately one-third of the genes deleted in the smr mutant (Fig. 3A). The putative orf genes in each mutant are listed in Table 1. In our pneumonia model at 24 and 72 hpi, both Δsmr_A and Δsmr_B mutant-infected mice had bacterial burdens comparable to those of mice infected with the WT (Fig. 3B). However, the mice infected with Δsmr_C mutant had >1 log fewer CFU/g at 24 hpi and nearly 6 logs fewer CFU/g at 72 hpi than mice infected with the WT. Thus, the Δsmr_C mutant recapitulated the phenotype of the smr mutant, whereas the Δsmr_A and Δsmr_B mutants behaved like the WT strain.
FIG 3 .
The smr mutant phenotype is recapitulated by a smaller targeted deletion. (A) Schematic depicting targeted subregions of the smr mutant (not to scale). (B) Mice were inoculated i.n. with 2 × 104 CFU of the WT (KPPR1S; black circles) or the Δsmr (VK082; open squares), Δsmr_A (VK274; black triangles), Δsmr_B (VK275; open diamonds), or Δsmr_C (VK276; black squares) mutant. At 24 or 72 hpi, mice were sacrificed and their lungs and spleens were homogenized and plated for bacterial enumeration. Each symbol represents one mouse. The dotted line indicates the limit of detection, and symbols on the dotted line indicate that CFU counts were below the limit of detection. X indicates a mouse that succumbed to infection prior to 72 hpi. These data are from an individual representative experiment. Mann-Whitney tests were performed for statistical analysis. *, P < 0.05.
TABLE 1 .
Genes deleted in breakdown mutants
| Strain | Locus tag | Annotated gene product |
|---|---|---|
| Δsmr_A mutant | VK055_1987 | Oxygen-insensitive NADPH nitroreductase |
| VK055_1986 | Hypothetical protein | |
| VK055_1985 | Bacterial transcriptional regulator, TetR family | |
| VK055_1984 | Metallo-beta-lactamase superfamily protein (RomA) | |
| VK055_1983 | Bacterial regulatory helix-turn-helix, AraC family protein (RamA) | |
| VK055_1982 | Hypothetical protein (Orf82) | |
| VK055_1981 | Putative aldo/keto reductase | |
| VK055_1980 | HADa ATPase, P type | |
| VK055_1979 | Efflux transporter, RND family, MFP subunit | |
| VK055_1978 | Efflux pump membrane transporter, BepE | |
| VK055_1977 | Hypothetical protein | |
| VK055_1976 | Gamma-glutamyl cysteine ligase YbdK | |
| VK055_1975 | Hypothetical protein | |
| VK055_1974 | Bacterial extracellular solute-binding protein | |
| VK055_1973 | Binding-protein-dependent transport system inner membrane component | |
| VK055_1972 | Binding-protein-dependent transport system inner membrane component | |
| VK055_1971 | Oligopeptide/dipeptide ABC transporter, ATP binding | |
| VK055_1970 | Oligopeptide/dipeptide ABC transporter, ATP binding | |
| VK055_1969 | Amidase. Hydatoinase/carbamoylase family protein | |
| VK055_1968 | EamA-like transporter family protein | |
| VK055_1967 | Bacterial transcriptional regulator, GntR family protein | |
| VK055_1966 | Bacterial transcriptional regulator, GntR family protein | |
| VK055_1965 | Bacterial extracellular solute-binding | |
| VK055_1964 | ABC transporter, permease | |
| VK055_1963 | ABC-type amino acid transport system, permease | |
| VK055_1962 | ABC transporter family protein | |
| VK055_1961 | Serine 3-dehydrogenase | |
| VK055_1960 | Aminotransferase class III family protein | |
| Δsmr_B mutant | VK055_1959 | ABC transporter family protein |
| VK055_1958 | ABC transporter family protein | |
| VK055_1957 | Oligopeptide transport permease family protein | |
| VK055_1956 | Binding protein-dependent transport system inner membrane component family protein | |
| VK055_1955 | Bacterial extracellular solute-binding protein | |
| VK055_1954 | Acetyltransferase family protein | |
| VK055_1953 | Choline dehydrogenase | |
| VK055_1952 | Betaine aldehyde dehydrogenase | |
| VK055_1951 | Transcriptional repressor BetI | |
| VK055_1950 | Transporter, betaine/carnitine/choline transporter family protein | |
| VK055_1949 | ykfE, inhibitor of vertebrate C-type lysozyme | |
| VK055_1948 | Bacterial regulatory helix-turn-helix, LysR family protein | |
| VK055_1947 | Mechanosensitive ion channel family protein | |
| VK055_1946 | Hypothetical kinase | |
| VK055_1945 | Glycerol kinase | |
| VK055_1944 | l-Fucose isomerase, C-terminal domain protein | |
| VK055_1943 | Transketolase, pyrimidine binding domain protein | |
| VK055_1942 | Thiamine pyrophosphate enzyme, C-terminal TPPb binding domain protein | |
| VK055_1941 | Hypothetical protein | |
| VK055_1940 | Putative transcriptional regulator | |
| VK055_1939 | Branched-chain amino acid transport system/permease component family protein | |
| VK055_1938 | Heme ABC exporter, ATP-binding protein CcmA | |
| VK055_1937 | Hypothetical protein | |
| VK055_1936 | Periplasmic binding and sugar binding domain of LacI family protein | |
| VK055_1935 | 4′-Phosphopantetheinyl transferase superfamily protein, EntD | |
| VK055_1934 | TonB-dependent siderophore receptor family protein, FepA | |
| Δsmr_C mutant | VK055_1933 | Fes |
| VK055_1932 | MbtH-like family protein | |
| VK055_1931 | EntF | |
| VK055_1930 | FepC | |
| VK055_1929 | FepG | |
| VK055_1928 | FepD | |
| VK055_1927 | EntS | |
| VK055_1926 | FepB | |
| VK055_1925 | EntC | |
| VK055_1924 | EntE | |
| VK055_1923 | EntB | |
| VK055_1922 | EntA | |
| VK055_1921 | Proofreading thioesterase in enterobactin biosynthesis, YbdB2 | |
| VK055_1920 | Carbon starvation CstA family protein | |
| VK055_1919 | Helix-turn-helix family protein | |
| VK055_1918 | Hypothetical protein | |
| VK055_1917 | Plasmid stabilization system family protein | |
| VK055_1916 | Short-chain dehydrogenase family protein | |
| VK055_1915 | Iron-containing alcohol dehydrogenase family protein | |
| VK055_1914 | ABC transporter family protein | |
| VK055_1913 | Branched-chain amino acid transport system/permease component family protein | |
| VK055_1912 | Periplasmic binding and sugar binding domain of LacI family protein | |
| VK055_1911 | LVIVD repeat family protein |
HAD, haloacid dehalogenase.
TPP, thiamine pyrophosphate.
Located within the region deleted in the Δsmr_C mutant are genes necessary for the synthesis, export, and import of the siderophore enterobactin. We therefore hypothesized that a component of the enterobactin transport system was responsible for the virulence defect of the smr mutant. We did not believe that the siderophore itself was responsible, as an ΔentB mutant, which is unable to synthesize enterobactin and salmochelin, is only modestly attenuated in this mouse pneumonia model (33). The enterobactin receptor FepA also was not implicated, as FepA is encoded within the region deleted in the Δsmr_B mutant.
Siderophore transport involves several membrane proteins. For enterobactin, EntS and TolC are required for export, whereas FepA, FepDGC, and Fes are required for import. In addition, the periplasmic protein FepB is required for the import of both enterobactin and salmochelin. Because previous studies had implicated siderophore transport components in virulence (47), we targeted specific components of the enterobactin siderophore transport system and tested loss-of-function (ΔentS, Δfes, ΔfepB, and fepD::pKAS46) mutants in our pneumonia model (Fig. 4A). We included a different enterobactin synthesis (ΔybdB2 entABEC [referred to as Δentsyn]) mutant to confirm our previous findings obtained with the ΔentB mutant (33). We found that only the ΔfepB mutant recapitulated the phenotype of the smr mutant, as demonstrated by the attenuation in the lungs and the lack of dissemination at 24 and 72 hpi (Fig. 4B). Consistent with previously studies, neither the Δentsyn mutant (Fig. 4B) nor the ΔentB mutant (Fig. 5) recapitulated the smr phenotype (33). In addition, loss of fepB did not affect the expression of the yersiniabactin system (Fig. 6), consistent with results previously obtained with an enterobactin synthesis mutant (33). Thus, the periplasmic transport protein FepB contributes to virulence in a manner distinct from that of enterobactin and salmochelin uptake alone.
FIG 4 .
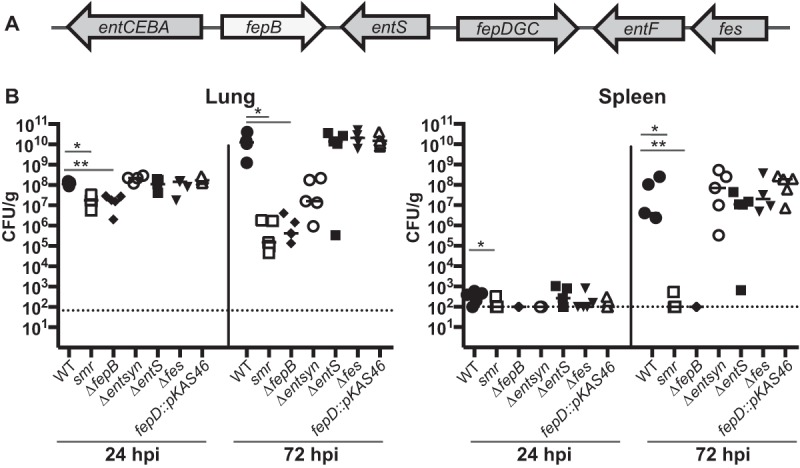
FepB is responsible for the smr mutant’s phenotype. (A) Schematic of the enterobactin genes located in the Δsmr_C region. (B) Mice were inoculated i.n. with 2 × 104 CFU of the WT (KPPR1S; black circles) or the Δsmr (VK082; open squares), ΔfepB (VK412; black diamonds), Δent syn (VK321; open circles), ΔentS (VK411; black squares), Δfes (VK320; black inverted triangles), or fepD::kan (VK413; open triangles) mutant. At 24 or 72 hpi, mice were sacrificed and their lungs and spleens were homogenized and plated for bacterial enumeration. Each symbol represents one mouse. The dotted line indicates the limit of detection, and symbols on the dotted line indicate that CFU counts were below the limit of detection. The data are from an individual representative experiment. Mann-Whitney tests were performed for statistical analysis. *, P < 0.05. **, P < 0.01.
FIG 5 .
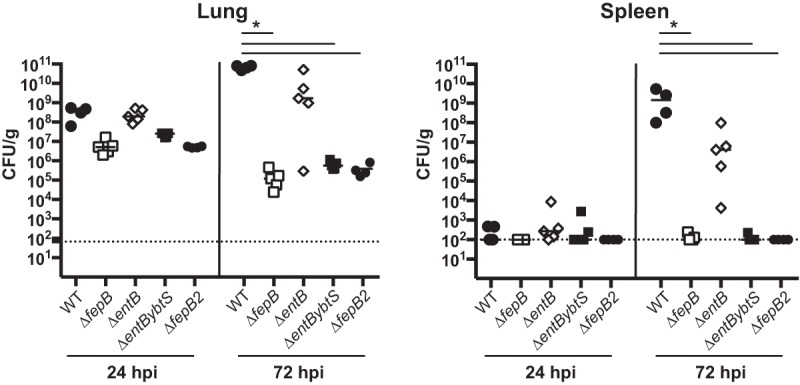
A ΔfepB mutant resembles a triple siderophore mutant in vivo. Mice were inoculated i.n. with 2 × 104 CFU of the WT (KPPR1S; black circles) or the ΔfepB (VK412; open squares, small closed circles), ΔentB mutant (VK087; open diamonds), or ΔentBybtS (VK089; black squares) mutant. At 24 or 72 hpi, mice were sacrificed and their lungs and spleens were homogenized and plated for bacterial enumeration. Each symbol represents one mouse. The dotted line indicates the limit of detection, and symbols on the dotted line indicate that CFU counts were below the limit of detection. The data are from an individual representative experiment. Mann-Whitney tests were performed for statistical analysis. *, P < 0.05.
FIG 6 .
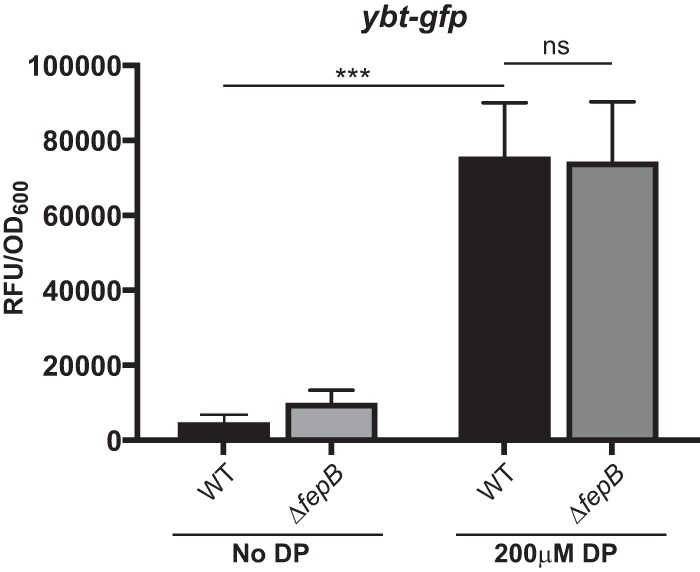
ybtA expression is unchanged in the ΔfepB mutant. The WT strain and a ΔfepB mutant containing the yersiniabactin synthesis gene, ybtA, promoter cloned into the pPROBE gfp reporter plasmid were grown overnight, subcultured to an OD600 of 0.2, and grown in LB medium for 6 h with or without 200 µM DP. These data are from strains grown in triplicate in an individual experiment. Student t tests were performed for statistical analysis. ***, P < 0.0001; ns, not significant. RFU, relative fluorescence units.
A variety of different approaches were used to complement the ΔfepB mutant, but all were unsuccessful. Plasmid-based approaches failed to complement growth under iron-depleted conditions, despite the constitutive expression of fepB (data not shown). We also attempted to repair the deletion, but this strain could not be obtained, for reasons we do not understand. Problems with fepB complementation are not unprecedented and were also reported for a Salmonella fepB mutant (47). To ensure that the observed phenotype of the ΔfepB mutant was not a consequence of secondary mutations, a second fepB mutant (fepB2) was constructed and found to recapitulate the virulence and growth phenotypes of the original fepB mutant (Fig. 5). Additionally, we sequenced across the deletion junction of both of the ΔfepB mutants and obtained the expected sequence, suggesting that a larger deletion of the region surrounding fepB had not occurred (data not shown). Expression of the genes adjacent to fepB, entC and entS, was assessed by quantitative reverse transcription-PCR. Expression of entC and entS was not detected in the ΔfepB mutant but was in the WT (data not shown). EntC and EntS may be needed for growth under low-iron conditions, and their lack of expression provides a possible explanation for failed complementation in trans. However, this alone cannot explain the attenuation in vivo, as a ΔentS mutant was not attenuated and a ΔentC mutant (enterobactin synthesis) had a more modest attenuation level than the ΔfepB mutant (Fig. 4A) (33). Thus, we conclude that deletion of fepB results in a phenotype distinct from that of other enterobactin system mutants.
A fepB mutant resembles a ΔentB ΔybtS double mutant.
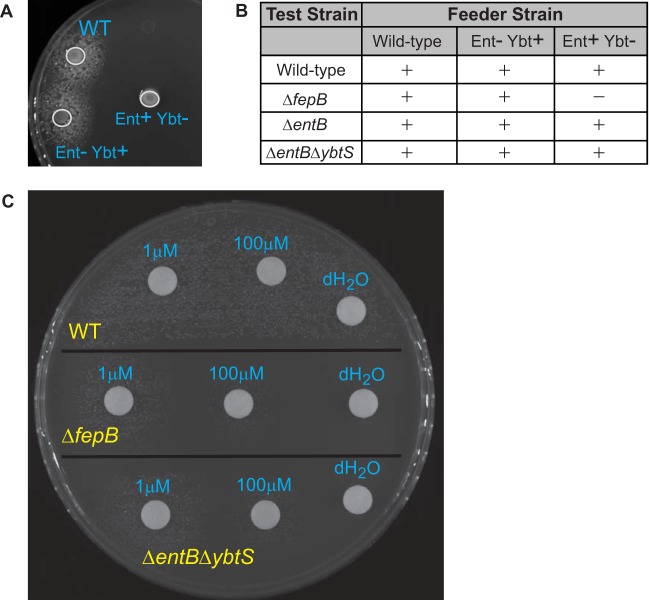
We previously showed that a ΔentB ΔybtS mutant that is deficient in all siderophore production was severely attenuated (33). In comparing the defect of the ΔfepB mutant strain to those of other siderophore mutants, we noticed that the phenotype of the ΔfepB mutant was similar to that of the ΔentB ΔybtS mutant. Because the attenuation of the ΔfepB mutant was much greater than that of the ΔentB mutant, we hypothesized that the role of FepB is not limited to enterobactin import and that it might be involved in an additional iron acquisition system. To gain a better understanding of the relationship between the phenotypes of these mutants, we tested the ΔfepB mutant together with the ΔentB ΔybtS mutant to determine if its virulence defect resembles that of a ΔentB ΔybtS mutant in vivo and included a ΔentB mutant as a control (Fig. 5). The ΔfepB and ΔentB ΔybtS mutants had similar attenuation levels, which were more severe than that of the ΔentB mutant. This finding raises the question of whether FepB may be required for iron acquisition via systems other than enterobactin and salmochelin.
To address the role of FepB in iron uptake and to determine if the virulence defect could be due to reduced iron acquisition, we used an in vitro growth model. The ΔfepB, ΔentB, and ΔentB ΔybtS mutants were grown in defined medium with or without the iron-chelating agent 2,2′-dipyridyl (DP). All of the strains had similar growth rates in the absence of DP, indicating that the mutants grow normally when iron levels are sufficient (Fig. 7A). However, in the presence of DP, the growth of the ΔfepB and ΔentB ΔybtS mutants was severely restricted (Fig. 7B). Interestingly, the growth of the ΔentB mutant was restricted compared to that of the WT strain, but the triple siderophore (ΔentB ΔybtS) mutant and the ΔfepB mutant grew even more slowly than the ΔentB mutant. These data suggest that FepB contributes to growth in an iron-dependent manner that is distinct from its known role in enterobactin and salmochelin uptake.
FIG 7 .
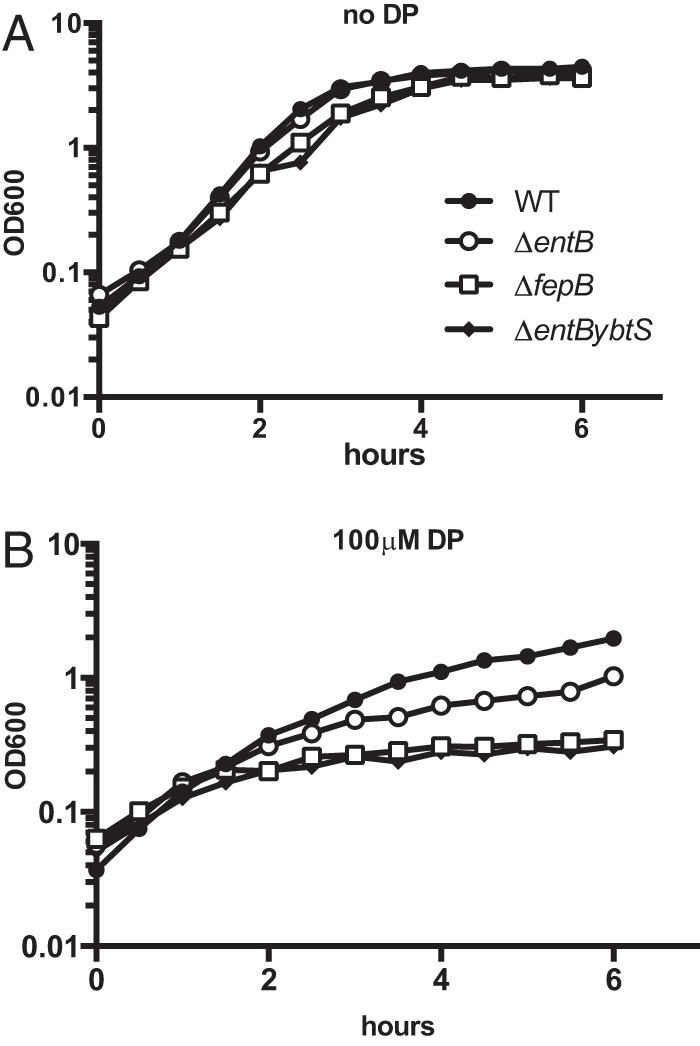
The ΔfepB mutant has a growth defect under iron-limited conditions. The WT strain (KPPR1S; black circles) and the ΔfepB (VK412; open squares), ΔentB (VK087; open circles), and ΔentBybtS (VK089; black diamonds) mutants were grown in M9-CAA (A) or in M9-CAA supplemented with 100 µM DP (B). The OD600 was monitored for 6 h. The data shown are from an individual representative experiment.
Yersiniabactin import is unaffected in a ΔfepB mutant.
The ΔfepB mutant had a stronger phenotype than an enterobactin/salmochelin synthesis mutant, and it resembled that of a triple siderophore mutant in both virulence and growth under iron limitation. Yersiniabactin is the only known siderophore produced by the ΔentB mutant but not the ΔentB ΔybtS mutant. Thus, we wanted to assess if the ΔfepB mutant is defective in yersiniabactin uptake. To do this, we performed a cross-feeding experiment to determine if the growth defect of the ΔfepB mutant under iron-limited conditions could be restored in the presence of yersiniabactin by coculturing the ΔfepB mutant with a yersiniabactin-producing strain. We predicted that if FepB is required for yersiniabactin import, a feeder strain producing yersiniabactin would be unable to restore the growth of the ΔfepB mutant. In this assay, test strains were spread onto M9 medium supplemented with 0.4% glucose and 0.2% Casamino acids (M9-CAA) agar containing DP and feeder strains were then spotted onto the surface of the plates. The WT and ΔentB, ΔentB ΔybtS, and ΔfepB mutant strains were used as test strains, and the WT and the ΔentB (capable of producing yersiniabactin) and ΔybtS (does not produce yersiniabactin) mutants were used as feeder strains. As expected, the ΔybtS mutant was not able to complement the growth defect of the ΔfepB mutant, as the ΔfepB mutant should not be able to use the enterobactin produced by this strain (Fig. 8A). The WT and the ΔybtS mutant were able to complement the growth of the ΔentB ΔybtS mutant, as expected (Fig. 8B). Importantly, the ΔentB mutant and the WT were able to restore the growth of the ΔentB mutant (as expected), as well as the ΔfepB mutant. This finding suggests that yersiniabactin can still be imported by a ΔfepB mutant.
FIG 8 .
Addition of yersiniabactin restores the growth defect of the ΔfepB mutant under iron-limited conditions. Test strains were grown in M9-CAA and spread plated onto M9-CAA agar containing 100 µM DP. (A) Plate testing of the ΔfepB mutant (spread plated). Feeder (WT and ΔentB and ΔybtS mutant) strains were then spot plated to test for complementation (growth restoration around the feeder spot). (B) Summary of results represented as + for growth and – for no growth of the WT strain, the ΔfepB mutant, the ΔentB mutant, or the ΔentB ΔybtS double mutant. (C) Addition of purified yersiniabactin (1 mM or 100 µM) or the dH2O vehicle to the WT strain, the ΔfepB mutant, or the ΔentB ΔybtS double mutant. Shown are data from an individual experiment that are representative of data obtained from several independent experiments.
To determine if the complementation of the ΔfepB mutant’s growth defect by a yersiniabactin-producing strain in the cross-feeding experiment was due to yersiniabactin production rather than the production of other secreted bacterial products, we performed a similar experiment by spotting purified apo-yersiniabactin instead of feeder strains. As described above, test strains (WT strain and ΔfepB and ΔentB ΔybtS mutants) were spread onto M9-CAA agar containing DP. Various concentrations of apo-yersiniabactin were applied to paper discs that were placed on the agar plate to test for growth restoration and thus the ability to utilize yersiniabactin (Fig. 8C). The WT strain was able to grow even without yersiniabactin supplementation. The ΔentB ΔybtS and ΔfepB mutants did not grow around the vehicle control (distilled H2O [dH2O]) disc. However, upon the addition of yersiniabactin, the growth defect of the ΔentB ΔybtS mutant was restored in a concentration-dependent manner; this is an expected result because this strain is still able to import exogenous yersiniabactin. Addition of apo-yersiniabactin also restored the growth of the ΔfepB mutant (Fig. 8C). Together, these data suggest that FepB is not required for yersiniabactin import in vitro and that the virulence defect of the ΔfepB mutant is due to a mechanism unrelated to yersiniabactin import.
Capsule production is not responsible for the ΔfepB mutant’s phenotype.
Capsule is considered a primary virulence factor of K. pneumoniae (reviewed in reference 4). Therefore, to test if there was a change in capsule production that could contribute to the ΔfepB mutant’s phenotype, we measured its uronic acid content. When the ΔfepB mutant and the WT strain were grown in Luria-Bertani (LB) medium at 37°C, the same conditions used for the inoculum used in mouse experiments, there was no difference in capsule production (Fig. 9A). Similarly, when mucoviscosity was measured (another assay for capsule phenotypes), we saw no measureable difference between the WT and the ΔfepB mutant (Fig. 9B).
FIG 9 .
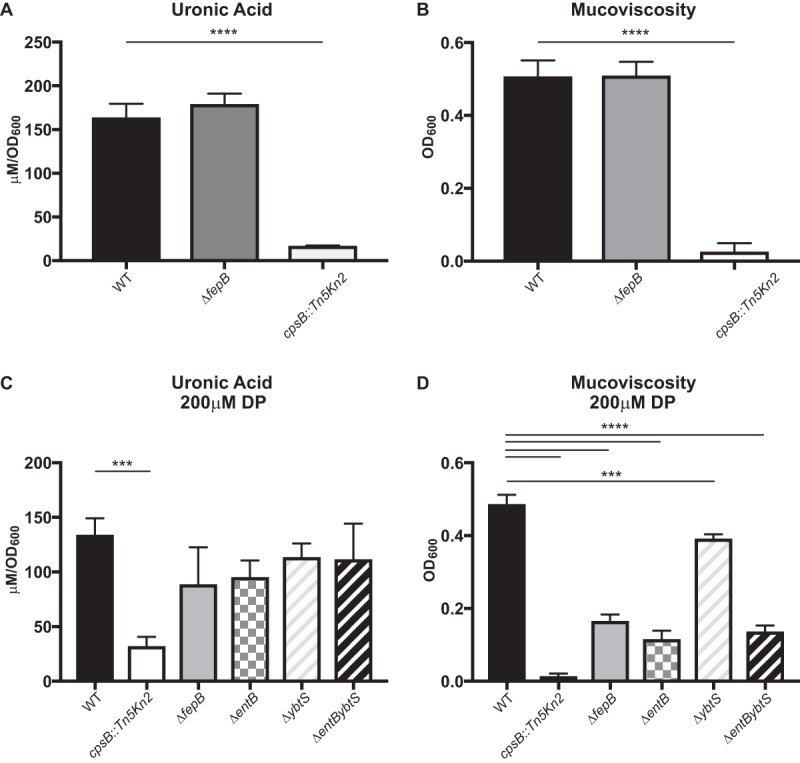
Capsule phenotype of the ΔfepB mutant. Overnight cultures of the WT strain, the ΔfepB mutant, and a capsule-deficient strain (cpsB::Tn5Kn2) were subcultured to an OD600 of 0.2 and grown in LB medium for 6 h, and total capsule production was measured with the uronic acid assay (A) and the low-speed centrifugation assay to measure mucoviscosity (B). These data are from strains grown in triplicate in an individual experiment. One-way analysis of variance, followed by Dunnett’s multiple-comparison test, was performed for statistical analysis. ***, P < 0.001; ****, P < 0.0001; ns, not significant.
Because iron levels can affect K. pneumoniae capsule production (48), we decided to test if capsule production is altered in the ΔfepB mutant under low-iron conditions. All four siderophore system (ΔfepB, ΔentB, ΔybtS, and ΔentB ΔybtS) mutants had a modest, nonsignificant reduction in capsule production (Fig. 9C). The mucoviscosity of the siderophore system mutants was also lower than that of the WT (Fig. 9D). Importantly, there was no difference between the capsule production levels of the ΔfepB and ΔentB mutants. How FepB affects virulence is not clear, but it does not appear to be related to the amount of capsule produced (Fig. 9A and C)) or the mucoviscosity of the capsule (Fig. 9B and D), as the uronic acid content and sedimentation of the ΔfepB mutant were comparable to those of the enterobactin synthesis mutant, which is only modestly attenuated.
DISCUSSION
The repertoire of confirmed K. pneumoniae virulence factors has changed little during the past 2 decades (2, 4). Although a number of large screens for K. pneumoniae virulence determinants have been performed (34–40), unfortunately, there have been few follow-up analyses of the results of these screens. In a screen of signature-tagged mutants in a pneumonia model of infection, we identified a locus that included ramA as potentially important for virulence (34), and a recent study suggested that overexpression of ramA affects virulence and leads to LPS modifications (45). In this study, we constructed a mutant (smr) with this locus deleted and found that it was cleared from the lungs following intranasal inoculation and that it was unable to spread systemically. Why deletion of ramA or the surrounding genes did not result in a virulence defect in the lungs and/or spleen when 11 insertions in this region were identified in the STM screen remains a mystery (34). One possibility is that in the STM screen, each insertion mutant was screened essentially in competition with 95 other mutants, most of which behave like the WT strain. Therefore, a ramA mutant may have a competitive disadvantage when at a ratio of ~1:100 with the WT but will not exhibit a defect when inoculated on its own. RamA has been implicated in the regulation of pathways important for multidrug resistance (43, 44), and thus, it may still be important in the context of antibiotic treatment or in a strain background that is not hypervirulent.
Subsequent analysis of the smr mutant indicated that the virulence defect was due not to deletion of the ramA locus but rather to the deletion of fepB, a gene encoding a protein required for enterobactin and salmochelin import (49–51). The fepB mutant had a more severe growth defect in iron-limited medium and a more severe in vivo defect than an enterobactin synthesis (ΔentB) mutant; the ΔentB mutant would also be deficient in salmochelin production. The contributions of the siderophores enterobactin, salmochelin, and yersiniabactin to Klebsiella virulence have been examined previously, and individually, they were found to contribute only minimally to infection (32, 33, 52). The data presented here reveal that while enterobactin/salmochelin may be dispensable for the virulence of a strain also able to produce yersiniabactin in a K. pneumoniae lung infection model, the enterobactin/salmochelin importer FepB is necessary to establish infection. Furthermore, under both in vitro and in vivo conditions, the ΔfepB mutant resembles a ΔentB ΔybtS mutant, which is unable to produce any of the three siderophores encoded by this strain (enterobactin, salmochelin, and yersiniabactin). Together, these observations suggest that FepB contributes to virulence and growth under iron limitation in an unanticipated way.
Siderophores are synthesized in the cytoplasm and require machinery for export and subsequent import following iron sequestration. Enterobactin is synthesized by EntABCDEF and is exported to the periplasm via the inner membrane protein EntS and subsequently through the outer membrane via the membrane channel protein TolC (53). Once bound to ferric iron, enterobactin (enterobactin-Fe3+) binds the outer membrane siderophore receptor FepA and is translocated into the periplasm by a TonB-dependent mechanism. In the periplasm, enterobactin-Fe3+ then binds the periplasmic chaperone FepB and is shuttled to the inner membrane, where it interacts with the inner membrane transport complex FepDGC and is ultimately released into the cytoplasm (50, 54, 55). Salmochelin utilizes a similar export apparatus but is imported via the bacterial outer membrane receptor IroN, and then FepB shuttles it to FepDGC (56). Export and yersiniabactin import appear to be similar, although several steps in yersiniabactin transport remain to be elucidated (53). Specifically, no periplasmic protein (FepB equivalent) has been identified in the yersiniabactin import system. Because of the similarities in the phenotypes of the ΔfepB and triple siderophore mutants and because no FepB equivalent has been identified in the yersiniabactin import system, we initially hypothesized that FepB may be involved in yersiniabactin import. However, our results show that a ΔfepB mutant can still utilize yersiniabactin for growth in vitro, and thus, the role of FepB in growth under iron limitation and virulence remains unclear. A recent crystal structure of FepB indicates that it can form a trimer (57) and thus possibly could coordinate a target other than enterobactin-Fe3+, but this has yet to be demonstrated.
A contribution of the periplasmic enterobactin transporter FepB to pathogenesis also was observed in Salmonella enterica (47). Salmonella produces both enterobactin and salmochelin, and both siderophores require FepB for import (25). However, Nagy et al. found that a fepB mutant had lower colonization levels in mice than a fepA-iroN double mutant (encoding the outer membrane receptors for enterobactin and salmochelin) in a gastric model of infection (47, 58). This is comparable to our results obtained with K. pneumoniae and suggests that the role of FepB in virulence extends beyond siderophore transport. The fact that this phenomenon has been reported in two Gram-negative pathogens hints that this may be a conserved mechanism in other bacterial species. One possible explanation for this observation is that in a ΔfepB mutant, enterobactin is not recycled properly and accumulates extracellularly and perhaps this is detrimental to the bacteria, given that enterobactin can enhance copper toxicity (59). However, in this scenario, the Δsmr_C mutant (which is a ΔentB ΔfepB double mutant and has other genes [listed in Table 1] deleted) should not have this phenotype, as it would be unable to produce enterobactin. However, the data presented here suggest that this is not the case, as the Δsmr_C mutant has a virulence defect comparable to that of a ΔfepB mutant.
Interestingly, recent studies have noted that the complement of siderophore systems produced by an individual strain of K. pneumoniae has a significant impact on its ability to colonize versus its ability to cause an infection or its ability to cause invasive disease associated with the hypervirulence phenotype (22). In an analysis of a broad sampling of over 300 strains, only 33% of an individual strain’s genome is part of the core Klebsiella genome, and the remaining 67% is composed of “accessory” genes that vary significantly from strain to strain (22). Until recently, the gene profiles necessary to cause the different types of infections associated with K. pneumoniae were not clear. However, recent bioinformatics analyses of large strain collections, combined with information on the type of infection, have revealed that some specific gene profiles are associated with colonization versus infection versus invasive disease. For example, the presence of rmpA (a regulator of capsule), as well as the genes required for the production and use of the siderophores aerobactin, salmochelin, and yersiniabactin, was highly associated with strains isolated from infections versus carriage alone (22). Interestingly, an additional five loci were associated with invasive infections (versus noninvasive infections or carriage), including fepB. This is consistent with the requirement we observed for fepB to cause disseminated infection in mice and what has been observed in Salmonella (47).
With antibiotic resistance on the rise, the development of new therapeutics to combat infection by multidrug-resistant bacteria is an urgent need (60). Siderophore systems present an attractive target for drug development because of the conservation of these systems among Gram-negative pathogens (61). Immunization with the yersiniabactin receptor FyuA or the siderophores themselves (yersiniabactin and aerobactin) was protective when tested in a murine model of E. coli urinary tract infection (62–64). FepB may be an especially attractive target to consider for drug development, as it is required for disseminated infections and is found in a wide variety of bacteria. In addition to being potential targets for drug development, siderophores represent an attractive system to exploit as a drug delivery mechanism to overcome the permeability barrier of the outer membrane. In essence, the siderophore can be used as a “Trojan horse” to target a siderophore-drug conjugate to the siderophore-iron transport systems (61). This would allow the delivery of drugs to the periplasm and potentially to the cytoplasm. From the work presented here and with Salmonella, one such periplasmic target could be FepB itself. Drug-siderophore conjugates have been developed, and a catechol-cephalosporin conjugate, cefiderocol (S-649266), was found to have lower MIC90s than the antibiotics cefepime, piperacillin-tazobactam, and meropenem when tested against several Gram-negative bacteria, including multidrug- and carbapenem-resistant strains (65–67). Cefiderocol displayed antibacterial properties when tested in vivo and is currently being tested in a phase 3 clinical trial against carbapenem-resistant Gram-negative infections in humans (66, 68). Thus, investigations probing the mechanisms of siderophore transport can provide the basis for promising new therapeutics.
MATERIALS AND METHODS
Ethics statement.
Mouse experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (69). All animal studies were approved by the Institutional Animal Care and Use Committee at the University of North Carolina (UNC) at Chapel Hill (protocols 11-127 and 14-110). All efforts were made to minimize suffering. Animals were monitored daily following inoculation and were euthanized upon exhibiting signs of morbidity.
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are described in Table 2. The WT parental strains are KPPR1, a Rifr derivative of ATCC 43816 (34), and KPPR1S, a Strr derivative of KPPR1; they have identical growth characteristics in vitro and in vivo. K. pneumoniae strains were grown aerobically in LB medium or M9-CAA overnight at 37°C. Where indicated, 100 or 200 µM DP (Sigma-Aldrich, St. Louis, MO) was added to M9 or LB medium, respectively, to deplete the available iron. Antibiotics were added to the medium as appropriate at the following concentrations: kanamycin, 50 µg/ml (Kan50); rifampin, 30 µg/ml (Rif30); streptomycin, 500 µg/ml (Strep500). Bacterial growth was monitored by measuring the optical density at 600 nm (OD600).
TABLE 2 .
Bacterial strains and plasmids used in this work
| Strain or plasmid | Description | Reference |
|---|---|---|
| E. coli | ||
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoP recA1 endA1 hsdR17(rK− mK−) | Invitrogen |
| S17-1 λpir | Tpr Strr recA thi pro hsdR hsdM+ RP4-2-Tc::Mu Km Tn7 λpir (lysogen) | 72 |
| K. pneumoniae | ||
| KPPR1 | Rifr derivative of ATCC 43816 | 34 |
| KPPR1S | Strr derivative of KPPR1 | This work |
| VK060 | KPPR1 cpsB::Tn5Kn2 | 34 |
| VK082 | smr mutant | This work |
| VK087 | KPPR1 ΔentB | 33 |
| VK088 | KPPR1 ΔybtS | 33 |
| VK089 | KPPR1 ΔentB ΔybtS | 33 |
| VK131 | KPPR1 ΔromA | This work |
| VK174 | KPPR1S ΔramA | This work |
| VK266 | KPPR1S Δorf82 ΔramA ΔromA | This work |
| VK269 | KPPR1S Δrnd | This work |
| VK270 | KPPR1S Δorf82 | This work |
| VK274 | KPPR1S Δsmr_A | This work |
| VK275 | KPPR1S Δsmr_B | This work |
| VK276 | KPPR1S Δsmr_C | This work |
| VK320 | KPPR1S Δfes | This work |
| VK321 | KPPR1S ΔybdB2 entABEC (Δentsyn) | This work |
| VK411 | KPPR1S ΔentS | This work |
| VK412 | KPPR1S ΔfepB | This work |
| VK413 | KPPR1S fepD::pKAS46 | This work |
| VK555 | KPPR1S ΔfepB2 | This work |
| Plasmids | ||
| pKAS46 vector | Kanamycin resistance, suicide vector, rpsL+ | 70 |
| pK03 vector | sacB, temperature-sensitive origin of replication | 73 |
| pPROBE vector | Kmr, gfp expression vector | 77 |
| pKO3ΔromA | romA flanking region in pKO3 | This work |
| pKO3ΔramKO | smr flanking region in pKO3 | This work |
| pKAS46ΔramA | ramA flanking region in pKAS46 | This work |
| pKAS46Δorf82 | orf82 flanking region in pKAS46 | This work |
| pKAS46Δorf82ramAromA | orf82 ramA romA flanking region in pKAS46 | This work |
| pKAS46Δrnd | rnd flanking region in pKAS46 | This work |
| pKAS46ΔfepB | fepB flanking region in pKAS46 | This work |
| pKAS46Δsmr_A | smr_A flanking region in pKAS46 | This work |
| pKAS46Δsmr_B | smr_B flanking region in pKAS46 | This work |
| pKAS46Δsmr_C | smr_C flanking region in pKAS46 | This work |
| pKAS46Δfes | fes flanking region in pKAS46 | This work |
| pKAS46ΔentS | entS flanking region in pKAS46 | This work |
| pKAS46ΔybdB2entABEC | ybdB2 entABEC (Δentsyn) flanking region in pKAS46 | This work |
| pfepD::pKAS46 | Disruption of fepD | This work |
| pY4 | ybtA promoter in pPROBE | 33 |
Construction of bacterial mutants.
Mutations in KPPR1S (ΔramA Δorf82, Δorf82ΔramAΔromA, ΔentS, ΔybdB2 entABEC [referred to as Δentsyn], Δfes, ΔfepB, Δsmr_A Δsmr_B, and Δsmr_C) were generated by allelic exchange by using pKAS46, a suicide vector that allows the use of streptomycin for counterselection (70, 71). Sequences up- and downstream (~500 bp each) were generated by PCR with the primer sets indicated in Table 3, cloned into pKAS46, and confirmed by sequence analysis. Overnight cultures of KPPR1S and E. coli S17-1 λpir (72) carrying a derivative of pKAS46 were mixed, collected by centrifugation, plated on LB agar (no antibiotics), and grown overnight at 37°C. Transconjugants were selected by plating on LB agar with Rif30 and Kan50. Several Rifr Kanr colonies were grown for 5 to 6 h in LB medium (no antibiotics) and then plated on LB agar with Strep500 to select for transconjugants that had excised the plasmid. Kans clones were screened by PCR to verify the loss of the targeted gene(s).
TABLE 3 .
Primers used in this study
| Primer | Sequencea (5′ to 3′) | Description |
|---|---|---|
| MP66 | TGACTAGATATCGCTGATTACCGAAGCGGACTG | 5′ flank forward ΔramA |
| MP67 | TGCATATCTAGAGGAAATCGTCATATGCTCTCT | 5′ flank reverse ΔramA |
| MP68 | TGCATATCTAGACACTGAGGCGCGCCTCTCCTG | 3′ flank forward ΔramA |
| MP69 | TCGATAGCGGCCGCCGACTGGCGCTGTACATCGCG | 3′ flank reverse ΔramA |
| MP114 | TGACTAGATATCTCGCCCGAGGGCGTCGTAAAC | 5′ flank forward Δorf82 |
| MP71 | TGCATATCTAGACTCGAGCGGTAAACCAGGAGA | 5′ flank reverse Δorf82 |
| MP72 | TGCATATCTAGACAGTGGATGTTTCATGTCATG | 3′ flank forward Δorf82 |
| MP115 | TCGATAGCGGCCGCGGGATGAACCGTATCAACGGC | 3′ flank reverse Δorf82 |
| MP124 | TGACTAGATATCCGATTTTGCCTGCTATGCGCA | 5′ flank forward Δrnd |
| MP125 | TGCATATCTAGACATCGGCGGGGGTAAGCGCGG | 5′ flank reverse Δrnd |
| MP126 | TGCATATCTAGAGTTCACCCGGTCGCCCAGCGG | 3′ flank forward Δrnd |
| MP127 | TCGATAGCGGCCGCGCCACGGCAGGTCTGGCAGCA | 3′ flank reverse Δrnd |
| MP103 | TGACTAGATATCGGCGTCGTAAACTTTGGGTTA | 5′ for Δorf82 ramA romA |
| MP104 | TGCATATCTAGATTCCAGTGGATGTTTCATGTC | 5′ rev Δorf82 ramA romA |
| MP105 | TGCATATCTAGACTGACCAGACAAAAGCCCCCA | 3′ for Δorf82 ramA romA |
| MP106 | TCGATAGCGGCCGCCGACAGCTGGCACATTTCGTT | 3′ rev Δorf82 ramA romA |
| MP171 | TCGATAGCGGCCGCCTGTGCGCTCCCTGCGCCATG | 5′ flank forward smrΔA |
| MP172 | TGCATATCTAGACTGGCGAAGTAGGGGAGGGGG | 5′ flank reverse smrΔA |
| MP173 | TGCATATCTAGAACCGAGATTTAATCTCTCCAC | 3′ flank forward smrΔA |
| MP174 | TGACTAGATATCTCCAACTTTTGGGGTGCAGTC | 3′ flank reverse smrΔA |
| MP175 | TGACTAGATATCCCATGCGCTTGCGCGGGCCTA | 5′ flank forward smrΔB |
| MP176 | TGCATATCTAGAGCTTACGATATTTCCAATCCG | 5′ flank reverse smrΔB |
| MP177 | TGCATATCTAGATGCGCCTCATTAAGCGGGTCC | 3′ flank forward smrΔB |
| MP178 | TCGATAGCGGCCGCAATGACAGAATGTTAAGGACA | 3′ flank reverse smrΔB |
| MP179 | TGACTAGATATCTGCGCCTCATTAAGCGGGTCC | 5′ flank forward smrΔC |
| MP180 | TGCATATCTAGAAGTCACGCTATACATAGGGTT | 5′ flank reverse smrΔC |
| MP181 | TGCATATCTAGAGCGCACCCTGGCGGAGCCACT | 3′ flank forward smrΔC |
| MP182 | TCGATAGCGGCCGCATTAACGACAGGTTGCGCGAA | 3′ flank reverse smrΔC |
| MP282 | TGACTAGATATCAGAATTTAACAACACCGAAAC | 5′ flank forward ΔybdB2 entABEC |
| MP192 | TGCATATCTAGAACCGCGGTGCTGGGCTAAGAA | 5′ flank reverse ΔybdB2 entABEC |
| MP193 | TGCATATCTAGAAGCCAGTGACGTTTCCATATC | 3′ flank forward ΔybdB2 entABEC |
| MP194 | TCGATAGCGGCCGCGCAACCTCGCTCCACTGGCGC | 3′ flank reverse ΔybdB2 entABEC |
| MP195 | TGACTAGATATCGGATATAGAGCTCGGAAGGCT | 5′ flank forward ΔfepB |
| MP196 | TGCATATCTAGAGAAGTTCACGTCATCGCATCC | 5′ flank reverse ΔfepB |
| MP197 | TGCATATCTAGACTGTTCGGCTAACGCGGGCTG | 3′ flank forward ΔfepB |
| MP198 | TCGATAGCGGCCGCCGCTGGCGCTTGTCGGCGTGC | 3′ flank reverse ΔfepB |
| MP199 | TGACTAGATATCGCGCTCTGCTGGTGCTCCAGC | 5′ flank forward ΔentS |
| MP200 | TGCATATCTAGAATTGTCAACGAAAGTTAAGTA | 5′ flank reverse ΔentS |
| MP201 | TGCATATCTAGAGGATTGTCGGTTCATTACAGC | 3′ flank forward ΔentS |
| MP202 | TCGATAGCGGCCGCCGGGTGAGCGTCTGCATCAGC | 3′ flank reverse ΔentS |
| MP207 | TGACTAGATATCGCGCGGCAACCAGCGGTAAAC | 5′ flank forward Δfes |
| MP208 | TGCATATCTAGAGGCCAACGCGAACCGATTATT | 5′ flank reverse Δfes |
| MP244 | TGCATATCTAGATGCGCCTCATTAAGCGGGTCC | 3′ flank forward Δfes |
| MP231 | TCGATAGCGGCCGCAATGACAGAATGTTAAGGACA | 3′ flank reverse Δfes |
| MP313 | TGACTAGATATCCCTTAGCCGCCGCGCTTA | 5′ forward fepD::kan |
| MP314 | TGCATATCTAGATTGCGGGTGAGCGTCTGC | 3′ reverse fepD::kan |
| ramKOA5′INsmaI | TCCCCCGGGACCGCTTTGACGGTCAT | 5′ flank forward smr |
| ramKOA3′IN2 | CGCGGTAGATTCCAAACATA | 5′ flank reverse smr |
| ramKOB5′IN | ATCCTGACCAGACAAAAGCCCCATCC | 3′ flank forward smr |
| ramKOB3′INSma | TCCCCCGGGGACAGCTGGCACATTTC | 3′ flank reverse smr |
| romA5′inXba | GCTCTAGAGCCAGTCCGCTTCGGTAA | 5′ flank forward ΔromA |
| romA5′in | CGACTTTCATCGCTTTCCTAATA | 5′ flank reverse ΔromA |
| romA3′in | CGTCATATGCTCTCTCCTCTGAT | 3′ flank forward ΔromA |
| romA3′inXbaI | GCTCTAGAGCACAGCTTAGCCAGGTG | 3′ flank reverse ΔromA |
Restriction sites are in bold.
An insertional disruption of the fepDCG operon was constructed in KPPR1S (fepD::pKAS46) by plasmid integration into the fepD gene. A DNA fragment generated by PCR with primers MP313 and MP314 (Table 3) was cloned into pKAS46. The resulting plasmid, pKAS46fepD::kan, was conjugated into KPPR1S as described above. Colonies with integration of the plasmid on the chromosome were identified by plating on LB agar with Rif30 and Kan50.
Isogenic mutants of KPPR1 (ΔromA and Δsmr) were generated by allelic exchange with pKO3 as previously described (73). pKO3 is a vector that allows the use of sucrose as a positive selection for the loss of the vector. DNA fragments were amplified by PCR with the primer sets indicated in Table 3 and cloned into pKO3, generating plasmids pKO3ΔromA and pKO3Δsmr.
Whole-genome sequencing of the smr mutant.
Total DNA from the smr mutant (VK82) was isolated with a genomic DNA purification kit (Qiagen), and the sample was submitted to the UNC High-Throughput Sequencing Facility for sequencing. An Illumina HiSeq 2000 instrument generated 2 × 75-bp paired-end reads. A mapped genome assembly was produced with the “Map Reads to Reference” tool in CLC Genomics Workbench v7.5.1 by using the published KPPR1 genome as the template (74). The smr mutant and KPPR1 parent strain genomes were then compared with the “Basic Variant Detection” tool in CLC Genomics Workbench to identify mutations in the smr strain. Mutations were visualized by aligning these genomes with Mauve (75).
Murine model of pneumonia.
Five- to 8-week-old, female C57BL/6 mice (Jackson Laboratories) were anesthetized by intraperitoneal injection with 200 µl of a mixture of ketamine (6.66 mg/kg) and xylazine (10.6 mg/kg). Overnight bacterial cultures were diluted in phosphate-buffered saline (PBS), and 20 µl was inoculated intranasally (i.n.) in two 10-µl aliquots for a total of ~2 × 104 CFU/mouse as previously described (34). At 24, 48, or 72 hpi, mice were euthanized by a lethal injection of 200 µl of sodium pentobarbital (150 mg/kg). Organs were removed, homogenized in PBS, serially diluted, and plated to quantify the number of CFU/g of tissue.
Mucoviscosity assay.
Mucoviscosity was determined as previously described (35, 76). Briefly, overnight cultures were grown in LB medium, subcultured to an OD600 of 0.2 in fresh medium, and grown at 37°C. After 6 h, cultures were normalized to 1.0 U of OD/ml and centrifuged for 5 min at 1,000 × g and the OD600 of the supernatant was measured.
Extraction and quantification of capsule.
Uronic acid was extracted and quantified as previously described (28). Briefly, overnight cultures were grown in LB medium, subcultured to an OD600 of 0.2 in fresh medium, and grown at 37°C. After 6 h, 500 µl of culture was added to 100 µl of 1% Zwittergent–100 mM citric acid and incubated at 50°C for 20 min. Cells were pelleted, and 300 µl of the supernatant was added to 1.2 ml of absolute ethanol, incubated at 4°C for 20 min, and centrifuged for 5 min at maximum speed. The pellet was resuspended in 200 µl of dH2O, added to 1.2 ml of 12.5 mM sodium tetraborate in sulfuric acid, and incubated for 5 min at 100°C. A 20-µl volume of 0.15% 3-phenylphenol was added, and the absorbance at 520 nm was measured. The glucuronic acid content was determined from a standard curve of glucuronic acid (Sigma-Aldrich, St. Louis, MO) and expressed in micromoles per OD unit.
Measurement of promoter activity.
Expression of the yersiniabactin-encoding locus was assessed in vitro with a transcriptional gfp reporter containing the sequence 500 bp upstream of the ybtA promoter cloned into pPROBE (33, 77). The bacteria were grown overnight at 37°C in LB medium, subcultured to an OD600 of 0.2, and grown for 6 h with or without 200 μM DP. All strains were assayed in triplicate. Fluorescence was detected with a Synergy HT microplate reader (BioTek Instruments, Winooski, VT) and measured in relative fluorescence units per OD600 unit.
In vitro growth curves.
To monitor bacterial growth, bacterial strains were grown overnight in M9-CAA at 37°C, subcultured to an OD600 of 0.05 in fresh medium in 250-ml flasks, and grown with aeration for 6 h at 37°C. OD600 readings were recorded at the intervals indicated. Medium was supplemented with 100 μM DP to examine bacterial growth under iron-limiting conditions.
Cross-feeding assay.
To determine if secreted siderophores could restore the growth of siderophore mutants in iron-depleted medium, a cross-feeding assay was performed as previously described, with minor modifications (78). Bacteria were grown overnight at 37°C in M9-CAA. Approximately 1 × 105 CFU of each test strain was spread onto M9-CAA agar plates containing 100 μM DP. Feeder strains were then spotted (2.5 µl of overnight culture) onto the agar, and the plates were incubated at 37°C overnight.
To determine if purified yersiniabactin could restore the growth of siderophore mutants in iron-depleted medium, test strains were spread on M9-CAA agar as described above. Iron-free yersiniabactin (EMC Microcollections, Germany) was resuspended in ethanol, and 10 µl of either 1 mM or 100 µM yersiniabactin (diluted in dH2O) was spotted onto filter disks on the plate to assess yersiniabactin-dependent growth complementation.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism, version 6.0 (GraphPad, San Diego, CA).
ACKNOWLEDGMENTS
We thank Deborah Ramsey for construction of KPPR1S, Matt Lawlor for construction of the Δsmr mutant, and Chris O’Connor for construction of the ΔromA mutant.
This work was supported by UNC Infectious Disease Pathogenicity training grant 5T32AI007151 to C.A.B. M.P. was supported in part by UNC Initiative for Maximizing Student Diversity (IMSD) award 5R25GM055336 from the NIGMS and a Howard Hughes Medical Institute (HHMI) Med into Grad Scholar grant to the UNC at Chapel Hill.
REFERENCES
- 1.Clegg S, Murphy CN. 2016. Epidemiology and virulence of Klebsiella pneumoniae. Microbiol Spectr 4:1–17. doi: 10.1128/microbiolspec.UTI-0005-2012. [DOI] [PubMed] [Google Scholar]
- 2.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broberg CA, Palacios M, Miller VL. 2014. Klebsiella: a long way to go towards understanding this enigmatic jet-setter. F1000Prime Rep 6:64. doi: 10.12703/P6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shon AS, Bajwa RPS, Russo TA. 2013. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RM, Cao J, Brisse S, Passet V, Wu W, Zhao L, Malani PN, Rao K, Bachman MA. 2016. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 1:e00261-16. doi: 10.1128/mSphere.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerner A, Adler A, Abu-Hanna J, Cohen Percia S, Kazma Matalon M, Carmeli Y. 2015. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect 21:470.e1–470.e7. doi: 10.1016/j.cmi.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Montgomerie JZ. 1979. Epidemiology of Klebsiella and hospital-associated infections. Rev Infect Dis 1:736–753. doi: 10.1093/clinids/1.5.736. [DOI] [PubMed] [Google Scholar]
- 9.Pope JV, Teich DL, Clardy P, McGillicuddy DC. 2011. Klebsiella pneumoniae liver abscess: an emerging problem in North America. J Emerg Med 41:e103–e105. doi: 10.1016/j.jemermed.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 10.Kashani AH, Eliott D. 2013. The emergence of Klebsiella pneumoniae endogenous endophthalmitis in the USA: basic and clinical advances. J Ophthalmic Inflamm Infect 3:28 doi: 10.1186/1869-5760-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. 2012. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 12.Robilotti E, Deresinski S. 2014. Carbapenemase-producing Klebsiella pneumoniae. F1000Prime Rep 6:80. doi: 10.12703/P6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SN, Khan AU. 2016. Breaking the spell: combating multidrug resistant “superbugs.” Front Microbiol 7:174. doi: 10.3389/fmicb.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathers AJ, Peirano G, Pitout JDD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LF, Anderson DJ, Paterson DL. 2012. Overview of the epidemiology and the threat of Klebsiella pneumoniae carbapenemases (KPC) resistance. Infect Drug Resist 5:133–141. doi: 10.2147/IDR.S26613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha R, Saha N, Donofrio RS, Bestervelt LL. 2013. Microbial siderophores: a mini review. J Basic Microbiol 53:303–317. doi: 10.1002/jobm.201100552. [DOI] [PubMed] [Google Scholar]
- 19.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond KN, Dertz EA, Kim SS. 2003. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koczura R, Kaznowski A. 2003. Occurrence of the Yersinia high-pathogenicity island and iron uptake systems in clinical isolates of Klebsiella pneumoniae. Microb Pathog 35:197–202. doi: 10.1016/S0882-4010(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 22.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, Brisse S, Cao H, Wilksch J, Gorrie C, Schultz MB, Edwards DJ, Nguyen KV, Nguyen TV, Dao TT, Mensink M, Minh VL, Nhu NTK, Schultsz C, Kuntaman K, Newton PN, Moore CE, Strugnell RA, Thomson NR. 2015. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A 112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hantke K, Nicholson G, Rabsch W, Winkelmann G. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A 100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bister B, Bischoff D, Nicholson GJ, Valdebenito M, Schneider K, Winkelmann G, Hantke K, Süssmuth RD. 2004. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals 17:471–481. doi: 10.1023/B:BIOM.0000029432.69418.6a. [DOI] [PubMed] [Google Scholar]
- 25.Zhu M, Valdebenito M, Winkelmann G, Hantke K. 2005. Functions of the siderophore esterases IroD and IroE in iron-salmochelin utilization. Microbiology 151:2363–2372. doi: 10.1099/mic.0.27888-0. [DOI] [PubMed] [Google Scholar]
- 26.Perry RD, Balbo PB, Jones HA, Fetherston JD, DeMoll E. 1999. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 145:1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- 27.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. 2008. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis 62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Lawlor MS, Handley SA, Miller VL. 2006. Comparison of the host responses to wild-type and cpsB mutant Klebsiella pneumoniae infections. Infect Immun 74:5402–5407. doi: 10.1128/IAI.00244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy CN, Clegg S. 2012. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol 7:991–1002. doi: 10.2217/fmb.12.74. [DOI] [PubMed] [Google Scholar]
- 30.Clements A, Gaboriaud F, Duval JFL, Farn JL, Jenney AW, Lithgow T, Wijburg OLC, Hartland EL, Strugnell RA. 2008. The major surface-associated saccharides of Klebsiella pneumoniae contribute to host cell association. PLoS One 3:e3817 doi: 10.1371/journal.pone.0003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo TA, Olson R, MacDonald U, Metzger D, Maltese LM, Drake EJ, Gulick AM. 2014. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun 82:2356–2367. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA. 2015. Aerobactin, but not yersiniabactin, salmochelin and enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun 83:3325–3333. doi: 10.1128/IAI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawlor MS, O’Connor C, Miller VL. 2007. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect Immun 75:1463–1472. doi: 10.1128/IAI.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58:1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 35.Bachman MA, Breen P, Deornellas V, Mu Q, Zhao L, Wu W, Cavalcoli JD, Mobley HLT. 2015. Genome-wide identification of Klebsiella pneumoniae fitness genes during lung infection. mBio 6:e00775-15. doi: 10.1128/mBio.00775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroncle N, Balestrino D, Rich C, Forestier C. 2002. Identification of Klebsiella pneumoniae genes involved in intestinal colonization and adhesion using signature-tagged mutagenesis. Infect Immun 70:4729–4734. doi: 10.1128/IAI.70.8.4729-4734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Struve C, Forestier C, Krogfelt KA. 2003. Application of a novel multi-screening signature-tagged mutagenesis assay for identification of Klebsiella pneumoniae genes essential in colonization and infection. Microbiology 149:167–176. doi: 10.1099/mic.0.25833-0. [DOI] [PubMed] [Google Scholar]
- 38.Tu YC, Lu MC, Chiang MK, Huang SP, Peng HL, Chang HY, Jan MS, Lai YC. 2009. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infect Immun 77:2657–2671. doi: 10.1128/IAI.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai YC, Peng HL, Chang HY. 2001. Identification of genes induced in vivo during Klebsiella pneumoniae CG43 infection. Infect Immun 69:7140–7145. doi: 10.1128/IAI.69.11.7140-7145.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boll EJ, Nielsen LN, Krogfelt KA, Struve C. 2012. Novel screening assay for in vivo selection of Klebsiella pneumoniae genes promoting gastrointestinal colonisation. BMC Microbiol 12:201 doi: 10.1186/1471-2180-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey AM, Ivens A, Kingsley R, Cottell JL, Wain J, Piddock LJV. 2010. RamA, a member of the AraC/XylS family, influences both virulence and efflux in Salmonella enterica serovar Typhimurium. J Bacteriol 192:1607–1616. doi: 10.1128/JB.01517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Straaten T, Zulianello L, van Diepen A, Granger DL, Janssen R, van Dissel JT. 2004. Salmonella enterica serovar Typhimurium RamA, intracellular oxidative stress response, and bacterial virulence. Infect Immun 72:996–1003. doi: 10.1128/IAI.72.2.996-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George AM, Hall RM, Stokes HW. 1995. Multidrug resistance in Klebsiella pneumoniae: a novel gene, ramA, confers a multidrug resistance phenotype in Escherichia coli. Microbiology 141:1909–1920. doi: 10.1099/13500872-141-8-1909. [DOI] [PubMed] [Google Scholar]
- 44.Ruzin A, Visalli MA, Keeney D, Bradford PA. 2005. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother 49:1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Majumdar S, Yu J, Fookes M, McAteer SP, Llobet E, Finn S, Spence S, Monahan A, Monaghan A, Kissenpfennig A, Ingram RJ, Bengoechea J, Gally DL, Fanning S, Elborn JS, Schneiders T. 2015. Elucidation of the RamA regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLoS Pathog 11:e1004627. doi: 10.1371/journal.ppat.1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez-Ortega C, Olivares J, Martínez JL. 2013. RND multidrug efflux pumps: what are they good for? Front Microbiol 4:7. doi: 10.3389/fmicb.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagy TA, Moreland SM, Andrews-Polymenis H, Detweiler CS. 2013. The ferric enterobactin transporter Fep is required for persistent Salmonella enterica serovar Typhimurium infection. Infect Immun 81:4063–4070. doi: 10.1128/IAI.00412-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu CC, Wang CK, Chen YC, Lin TH, Jinn TR, Lin CT. 2014. IscR regulation of capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. PLoS One 9:e107812. doi: 10.1371/journal.pone.0107812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierce JR, Pickett CL, Earhart CF. 1983. Two fep genes are required for ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol 155:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens DL, Choe MD, Earhart CF. 1995. Escherichia coli periplasmic protein FepB binds ferrienterobactin. Microbiology 141:1647–1654. doi: 10.1099/13500872-141-7-1647. [DOI] [PubMed] [Google Scholar]
- 51.Sprencel C, Cao Z, Qi Z, Scott DC, Montague MA, Ivanoff N, Xu J, Raymond KM, Newton SM, Klebba PE. 2000. Binding of ferric enterobactin by the Escherichia coli periplasmic protein FepB. J Bacteriol 182:5359–5364. doi: 10.1128/JB.182.19.5359-5364.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachman MA, Miller VL, Weiser JN. 2009. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog 5:e1000622 doi: 10.1371/journal.ppat.1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garénaux A, Caza M, Dozois CM. 2011. The ins and outs of siderophore mediated iron uptake by extra-intestinal pathogenic Escherichia coli. Vet Microbiol 153:89–98. doi: 10.1016/j.vetmic.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Alipour M, Gargari SLM, Rasooli I. 2009. Cloning, expression and immunogenicity of ferric enterobactin binding protein FepB from Escherichia coli O157:H7. Indian J Microbiol 49:266–270. doi: 10.1007/s12088-009-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shea CM, McIntosh MA. 1991. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol 5:1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 56.Müller SI, Valdebenito M, Hantke K. 2009. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals 22:691–695. doi: 10.1007/s10534-009-9217-4. [DOI] [PubMed] [Google Scholar]
- 57.Li B, Li N, Yue Y, Liu X, Huang Y, Gu L, Xu S. 2016. An unusual crystal structure of ferric-enterobactin bound FepB suggests novel functions of FepB in microbial iron uptake. Biochem Biophys Res Commun 478:1049–1053. doi: 10.1016/j.bbrc.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 58.Rabsch W, Voigt W, Reissbrodt R, Tsolis RM, Bäumler AJ. 1999. Salmonella typhimurium IroN and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J Bacteriol 181:3610–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. 2012. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA. 2015. ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov 14:529–542. doi: 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- 61.Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P. 2016. Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res Int 23:3984–3999. doi: 10.1007/s11356-015-4294-0. [DOI] [PubMed] [Google Scholar]
- 62.Brumbaugh AR, Smith SN, Subashchandrabose S, Himpsl SD, Hazen TH, Rasko DA, Mobley HLT. 2015. Blocking yersiniabactin import attenuates extraintestinal pathogenic Escherichia coli in cystitis and pyelonephritis and represents a novel target to prevent urinary tract infection. Infect Immun 83:1443–1450. doi: 10.1128/IAI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brumbaugh AR, Smith SN, Mobley HLT. 2013. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect Immun 81:3309–3316. doi: 10.1128/IAI.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mike LA, Smith SN, Sumner CA, Eaton KA, Mobley HLT. 2016. Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. Proc Natl Acad Sci U S A 113:13468–13473. doi: 10.1073/pnas.1606324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, Rhomberg PR, Jones RN, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother 71:670–677. doi: 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 66.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore xephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito-Horiyama T, Ishii Y, Ito A, Sato T, Nakamura R, Fukuhara N, Tsuji M, Yamano Y, Yamaguchi K, Tateda K. 2016. Stability of novel siderophore cephalosporin S-649266 to clinically relevant carbapenemases. Antimicrob Agents Chemother 60:4384–4386. doi: 10.1128/AAC.03098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tillotson GS. 2016. Trojan horse antibiotics—a novel way to circumvent Gram-negative bacterial resistance? Infect Dis (Auckl) 9:45–52. doi: 10.4137/IDRT.S31567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC: https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf. [Google Scholar]
- 70.Skorupski K, Taylor RK. 1996. Positive selection vectors for allelic exchange. Gene 169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 71.Lai YC, Peng HL, Chang HY. 2003. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol 185:788–800. doi: 10.1128/JB.185.3.788-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Link AJ, Phillips D, Church GM. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol 179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broberg CA, Wu W, Cavalcoli JD, Miller VL, Bachman MA. 2014. Complete genome sequence of Klebsiella pneumoniae strain ATCC 43816 KPPR1, a rifampin-resistant mutant commonly used in animal, genetic, and molecular biology studies. Genome Announc 2:e00924-14. doi: 10.1128/genomeA.00924-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darling ACE, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ares MA, Fernández-Vázquez JL, Rosales-Reyes R, Jarillo-Quijada MD, von Bargen K, Torres J, González-y-Merchand JA, Alcántar-Curiel MD, De la Cruz MA. 2016. H-NS nucleoid protein controls virulence features of Klebsiella pneumoniae by regulating the expression of type 3 pili and the capsule polysaccharide. Front Cell Infect Microbiol 6:13. doi: 10.3389/fcimb.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller WG, Leveau JH, Lindow SE. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol Plant Microbe Interact 13:1243–1250. doi: 10.1094/MPMI.2000.13.11.1243. [DOI] [PubMed] [Google Scholar]
- 78.Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol 44:1225–1234. doi: 10.1046/j.1365-2958.2002.02885.x. [DOI] [PubMed] [Google Scholar]