Abstract
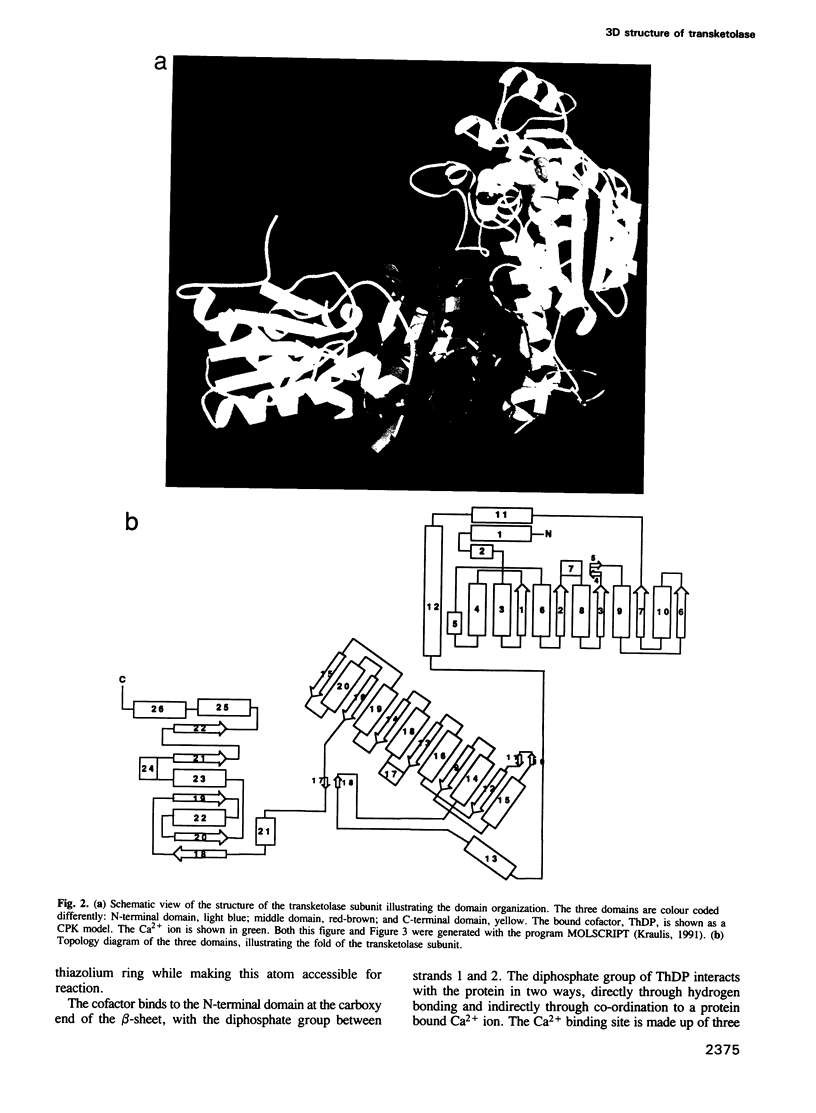
The crystal structure of Saccharomyces cerevisiae transketolase, a thiamine diphosphate dependent enzyme, has been determined to 2.5 A resolution. The enzyme is a dimer with the active sites located at the interface between the two identical subunits. The cofactor, vitamin B1 derived thiamine diphosphate, is bound at the interface between the two subunits. The enzyme subunit is built up of three domains of the alpha/beta type. The diphosphate moiety of thiamine diphosphate is bound to the enzyme at the carboxyl end of the parallel beta-sheet of the N-terminal domain and interacts with the protein through a Ca2+ ion. The thiazolium ring interacts with residues from both subunits, whereas the pyrimidine ring is buried in a hydrophobic pocket of the enzyme, formed by the loops at the carboxyl end of the beta-sheet in the middle domain in the second subunit. The structure analysis identifies amino acids critical for cofactor binding and provides mechanistic insights into thiamine catalysis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brändeén C. I. Relation between structure and function of alpha/beta-proteins. Q Rev Biophys. 1980 Aug;13(3):317–338. doi: 10.1017/s0033583500001712. [DOI] [PubMed] [Google Scholar]
- Cavaliere S. W., Neet K. E., Sable H. Z. Enzymes of pentose biosynthesis. The quaternary structure and reacting form of transketolase from baker's yeast. Arch Biochem Biophys. 1975 Dec;171(2):527–532. doi: 10.1016/0003-9861(75)90062-4. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Gibson J. L., McCue L. A., Tabita F. R. Identification, expression, and deduced primary structure of transketolase and other enzymes encoded within the form II CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Oct 25;266(30):20447–20452. [PubMed] [Google Scholar]
- Egan R. M., Sable H. Z. Transketolase kinetics. The slow reconstitution of the holoenzyme is due to rate-limiting dimerization of the subunits. J Biol Chem. 1981 May 25;256(10):4877–4883. [PubMed] [Google Scholar]
- Hawkins C. F., Borges A., Perham R. N. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989 Sep 11;255(1):77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Steffen H., Janser P., Wiss O. Studies on the reconstitution of apotransketolase with thiamine pyrophosphate and analogs of the coenzyme. Eur J Biochem. 1972 Nov 7;30(3):533–541. doi: 10.1111/j.1432-1033.1972.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Janowicz Z. A., Eckart M. R., Drewke C., Roggenkamp R. O., Hollenberg C. P., Maat J., Ledeboer A. M., Visser C., Verrips C. T. Cloning and characterization of the DAS gene encoding the major methanol assimilatory enzyme from the methylotrophic yeast Hansenula polymorpha. Nucleic Acids Res. 1985 May 10;13(9):3043–3062. doi: 10.1093/nar/13.9.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Jordan F., Chen G., Nishikawa S., Wu B. S. Potential roles of the aminopyrimidine ring in thiamin catalyzed reactions. Ann N Y Acad Sci. 1982;378:14–31. doi: 10.1111/j.1749-6632.1982.tb31183.x. [DOI] [PubMed] [Google Scholar]
- Kochetov G. A., Usmanov R. A. Charge transfer interactions in transketolase-thiamine pyrophosphate complex. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1134–1140. doi: 10.1016/0006-291x(70)90203-2. [DOI] [PubMed] [Google Scholar]
- Kochetov G. A., Usmanov R. A., Merzlov V. P. Thiaminepyrophosphate induced changes in the optical activity of baker's yeast transketolase. FEBS Lett. 1970 Aug 31;9(5):265–266. doi: 10.1016/0014-5793(70)80372-6. [DOI] [PubMed] [Google Scholar]
- Pletcher J., Sax M. Crystal and molecular structure of thiamine pyrophosphate hydrochloride. J Am Chem Soc. 1972 May 31;94(11):3998–4005. doi: 10.1021/ja00766a058. [DOI] [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Brändén C. I., Lorimer G. Three-dimensional structure of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum at 2.9 A resolution. EMBO J. 1986 Dec 20;5(13):3409–3415. doi: 10.1002/j.1460-2075.1986.tb04662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Sundström M., Lindqvist Y. Preliminary crystallographic data for transketolase from yeast. J Biol Chem. 1989 Dec 25;264(36):21619–21620. [PubMed] [Google Scholar]
- Shaw P. J., Muirhead H. Crystallographic structure analysis of glucose 6-phosphate isomerase at 3-5 A resolution. J Mol Biol. 1977 Jan 25;109(3):475–485. doi: 10.1016/s0022-2836(77)80025-9. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]





