Abstract
Previously, we characterized a Shaker-related family of voltage-gated potassium channels (RCK) in rat brain. Now, we describe a second family of voltage-gated potassium channels in the rat nervous system. This family is related to the Drosophila Shaw gene and has been dubbed Raw. In contrast to the RCK potassium channel family the Raw family utilizes extensive alternative splicing for expressing potassium channel subunits with variant C-termini. These alternative C-termini do not appear to influence the electrophysiological and pharmacological properties as studied in the Xenopus oocyte expression system. In situ hybridizations to sections of rat brain indicate that members of the Raw family are expressed in distinct areas of the central nervous system. Probably, Raw channels are expressed predominantly as homomultimers. Immunocytochemical experiments with antibodies against Raw3 and RCK4 proteins which form two distinct A-type potassium channels indicate that in hippocampus the two channels are expressed both in different neurons and in the same ones. In general, properties of Raw potassium channels appeared to be similar to RCK channels. However, Raw outward currents, in contrast to RCK currents, exhibit an intense rectification at test potentials higher than +20 to +40 mV. RCK and Raw channel subunits did not measurably coassemble into RCK/Raw heteromultimers after coinjecting RCK and Raw cRNA into Xenopus oocytes. These results suggest that members of the RCK and the Raw potassium channel families express potassium channels which form independent outward current systems. Combining the results of in situ hybridizations, immunocytochemical staining and expression of the cloned potassium channels in Xenopus oocytes demonstrates that unrestrained mixing of potassium channel subunits to form hybrid channels does not occur in the rat central nervous system. A single neuron is able to express multiple, independently assembled potassium channels.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanilla F., Perozo E., Papazian D. M., Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 1991 Nov 1;254(5032):679–683. doi: 10.1126/science.1948047. [DOI] [PubMed] [Google Scholar]
- Butler A., Wei A. G., Baker K., Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989 Feb 17;243(4893):943–947. doi: 10.1126/science.2493160. [DOI] [PubMed] [Google Scholar]
- Chandy K. G. Simplified gene nomenclature. Nature. 1991 Jul 4;352(6330):26–26. doi: 10.1038/352026b0. [DOI] [PubMed] [Google Scholar]
- Christie M. J., North R. A., Osborne P. B., Douglass J., Adelman J. P. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990 Mar;4(3):405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Wei A. A., Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991 Nov;7(5):763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Gautam M., Tanouye M. A. Alteration of potassium channel gating: molecular analysis of the Drosophila Sh5 mutation. Neuron. 1990 Jul;5(1):67–73. doi: 10.1016/0896-6273(90)90034-d. [DOI] [PubMed] [Google Scholar]
- Grupe A., Schröter K. H., Ruppersberg J. P., Stocker M., Drewes T., Beckh S., Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO J. 1990 Jun;9(6):1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Hardie R. C., Voss D., Pongs O., Laughlin S. B. Novel potassium channels encoded by the Shaker locus in Drosophila photoreceptors. Neuron. 1991 Mar;6(3):477–486. doi: 10.1016/0896-6273(91)90255-x. [DOI] [PubMed] [Google Scholar]
- Hartmann H. A., Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., Brown A. M. Exchange of conduction pathways between two related K+ channels. Science. 1991 Feb 22;251(4996):942–944. doi: 10.1126/science.2000495. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. A superfamily of ion channels. Nature. 1990 Jun 21;345(6277):672–672. doi: 10.1038/345672a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. How might the diversity of potassium channels be generated? Trends Neurosci. 1990 Oct;13(10):415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- Kamb A., Tseng-Crank J., Tanouye M. A. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988 Jul;1(5):421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Kanba S., Kanba K. S., McKinney M., Pfenning M., Abraham R., Nomura S., Enloes L., Mackey S., Richelson E. Desensitization of muscarinic M1 receptors of murine neuroblastoma cells (clone N1E-115) without receptor down-regulation and protein kinase C activity. Biochem Pharmacol. 1990 Sep 1;40(5):1005–1014. doi: 10.1016/0006-2952(90)90486-5. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leucine-zipper motif update. Nature. 1989 Jul 13;340(6229):103–104. doi: 10.1038/340103a0. [DOI] [PubMed] [Google Scholar]
- Lichtinghagen R., Stocker M., Wittka R., Boheim G., Stühmer W., Ferrus A., Pongs O. Molecular basis of altered excitability in Shaker mutants of Drosophila melanogaster. EMBO J. 1990 Dec;9(13):4399–4407. doi: 10.1002/j.1460-2075.1990.tb07890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Hess P., Weaver F., Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991 Oct 24;353(6346):752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
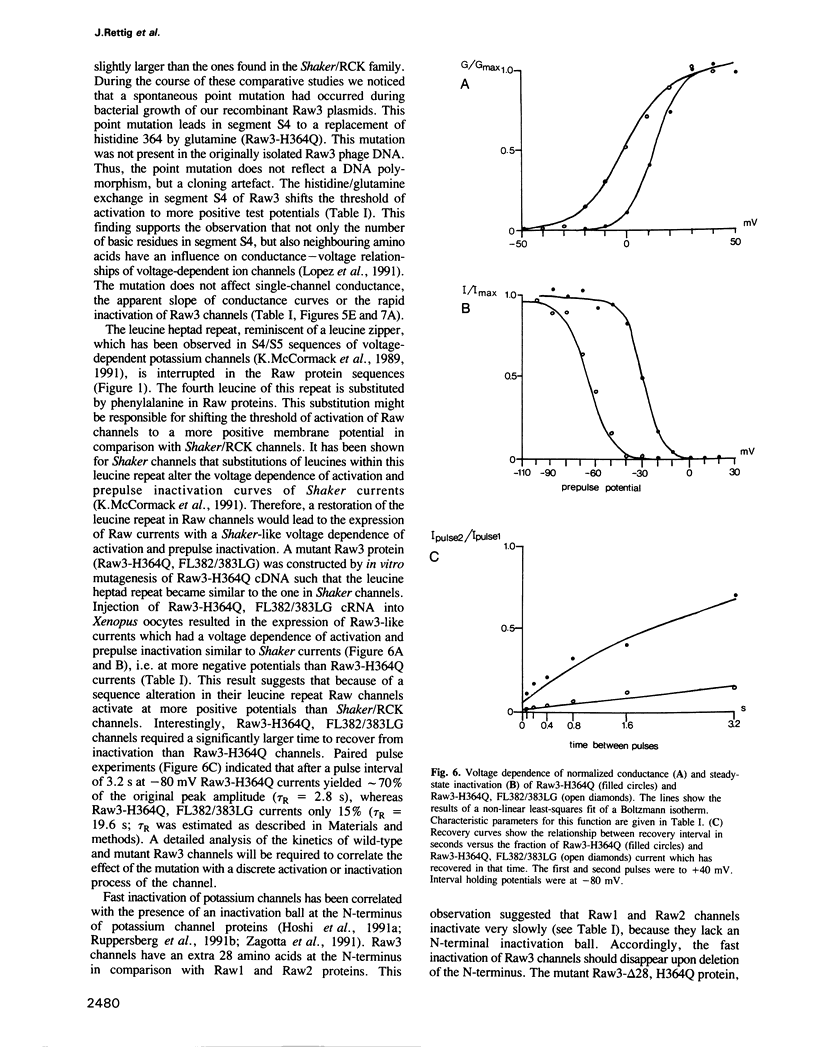
- Lopez G. A., Jan Y. N., Jan L. Y. Hydrophobic substitution mutations in the S4 sequence alter voltage-dependent gating in Shaker K+ channels. Neuron. 1991 Aug;7(2):327–336. doi: 10.1016/0896-6273(91)90271-z. [DOI] [PubMed] [Google Scholar]
- Luneau C. J., Williams J. B., Marshall J., Levitan E. S., Oliva C., Smith J. S., Antanavage J., Folander K., Stein R. B., Swanson R. Alternative splicing contributes to K+ channel diversity in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luneau C., Wiedmann R., Smith J. S., Williams J. B. Shaw-like rat brain potassium channel cDNA's with divergent 3' ends. FEBS Lett. 1991 Aug 19;288(1-2):163–167. doi: 10.1016/0014-5793(91)81026-5. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Heginbotham L., Abramson T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron. 1990 Dec;5(6):767–771. doi: 10.1016/0896-6273(90)90335-d. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989 Sep 22;245(4924):1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990 Oct 12;250(4978):276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- McCormack K., Tanouye M. A., Iverson L. E., Lin J. W., Ramaswami M., McCormack T., Campanelli J. T., Mathew M. K., Rudy B. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T., Vega-Saenz de Miera E. C., Rudy B. Molecular cloning of a member of a third class of Shaker-family K+ channel genes in mammals. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5227–5231. doi: 10.1073/pnas.87.13.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T., Vega-Saenz de Miera E. C., Rudy B. Molecular cloning of a member of a third class of Shaker-family K+ channel genes in mammals. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4060–4060. doi: 10.1073/pnas.88.9.4060-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. M., Long G. L. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase chain reaction. Anal Biochem. 1989 Jul;180(1):147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Pak M. D., Baker K., Covarrubias M., Butler A., Ratcliffe A., Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D. M., Timpe L. C., Jan Y. N., Jan L. Y. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991 Jan 24;349(6307):305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Pongs O., Kecskemethy N., Müller R., Krah-Jentgens I., Baumann A., Kiltz H. H., Canal I., Llamazares S., Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988 Apr;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Frank R., Pongs O., Stocker M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 1991 Oct 17;353(6345):657–660. doi: 10.1038/353657a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter K. H., Ruppersberg J. P., Wunder F., Rettig J., Stocker M., Pongs O. Cloning and functional expression of a TEA-sensitive A-type potassium channel from rat brain. FEBS Lett. 1991 Jan 28;278(2):211–216. doi: 10.1016/0014-5793(91)80119-n. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stocker M., Pongs O., Hoth M., Heinemann S. H., Stühmer W., Schröter K. H., Ruppersberg J. P. Swapping of functional domains in voltage-gated K+ channels. Proc Biol Sci. 1991 Aug 22;245(1313):101–107. doi: 10.1098/rspb.1991.0094. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Stocker M., Pongs O., Heinemann S. H. Gating currents of inactivating and non-inactivating potassium channels expressed in Xenopus oocytes. Pflugers Arch. 1991 May;418(4):423–429. doi: 10.1007/BF00550881. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Methfessel C., Sakmann B., Noda M., Numa S. Patch clamp characterization of sodium channels expressed from rat brain cDNA. Eur Biophys J. 1987;14(3):131–138. doi: 10.1007/BF00253837. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Marshall J., Smith J. S., Williams J. B., Boyle M. B., Folander K., Luneau C. J., Antanavage J., Oliva C., Buhrow S. A. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990 Jun;4(6):929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Papazian D. M., Schwarz T. L., Jan Y. N., Jan L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittka R., Stocker M., Boheim G., Pongs O. Molecular basis for different rates of recovery from inactivation in the Shaker potassium channel family. FEBS Lett. 1991 Jul 29;286(1-2):193–200. doi: 10.1016/0014-5793(91)80972-6. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Imoto K., Kawamura T., Higashida H., Iwabe N., Miyata T., Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Lett. 1989 Dec 18;259(1):37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Alterations in activation gating of single Shaker A-type potassium channels by the Sh5 mutation. J Neurosci. 1990 Jun;10(6):1799–1810. doi: 10.1523/JNEUROSCI.10-06-01799.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]