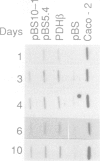
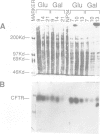
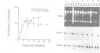Abstract
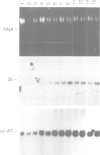
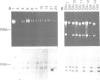
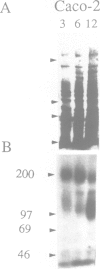
CFTR, the protein defective in cystic fibrosis is regulated during differentiation of intestinal epithelial cells. The undifferentiated cells (Caco-2 and HT-29) show a lower level of CFTR mRNA, while a 10-fold increase is seen in differentiated cells. These differences correlate well with those of other intestinal-specific genes, including sucrase-isomaltase, villin and alpha 1-antitrypsin, indicating that the regulation is cell specific. In Caco-2 cells the increase in CFTR mRNA cannot be accounted for by increased transcription of the gene. These data indicate that CFTR mRNA stabilizing factor(s) might be present in differentiated cells. The higher levels of CFTR mRNA in differentiated cells are accompanied by decreased protein levels, indicating, as well, involvement of translational control in the regulation of CFTR in these cells. Finally, fully differentiated cells show lowered levels of cyclic AMP-activated C1- transport, the characteristic function of CFTR. Thus, CFTR function in differentiated cells is modulated by a complex interaction of regulatory elements. Caco-2 and HT-29 cells provide a suitable in vitro system in which to study the mechanism of regulation of CFTR.
Full text
PDF


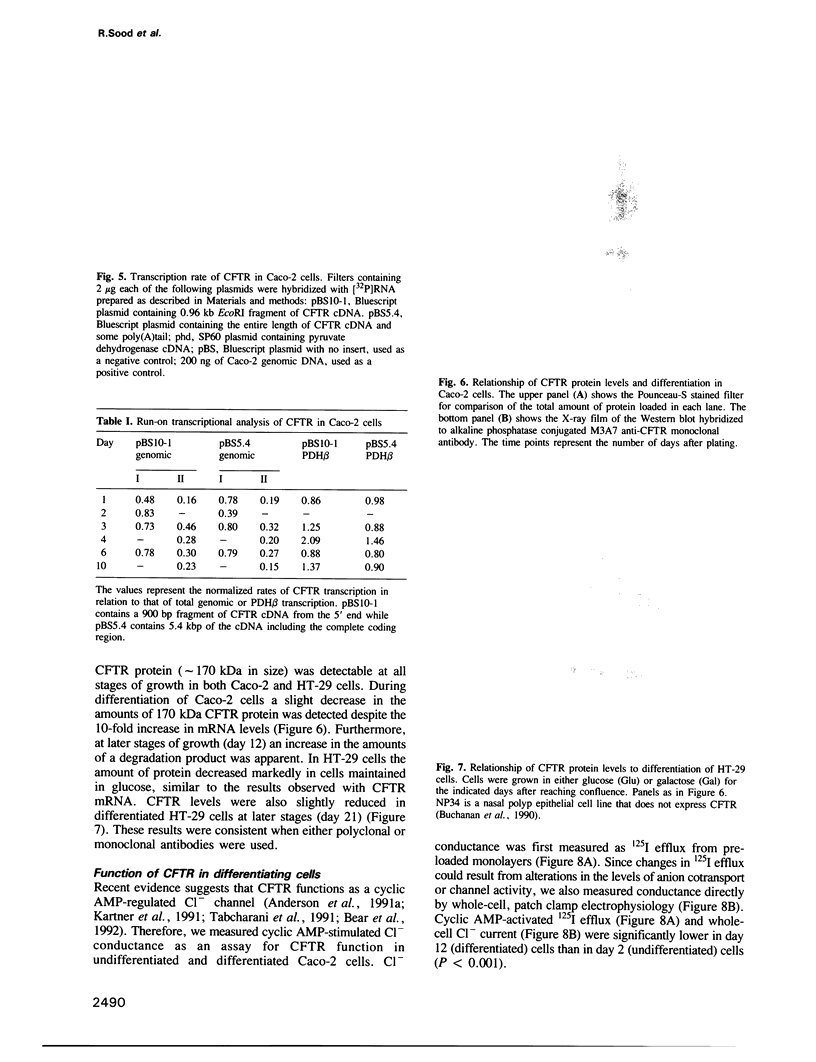
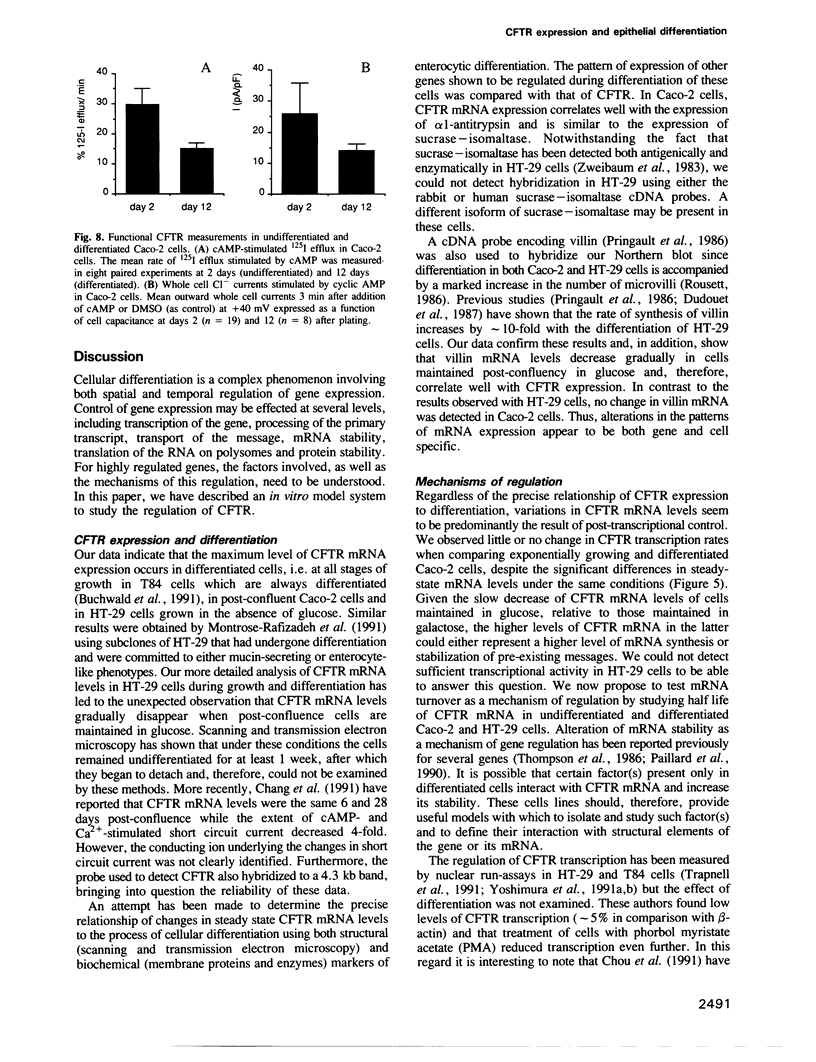



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 1992 Feb 21;68(4):809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Reyes E. F. cAMP-activated chloride conductance in the colonic cell line, Caco-2. Am J Physiol. 1992 Jan;262(1 Pt 1):C251–C256. doi: 10.1152/ajpcell.1992.262.1.C251. [DOI] [PubMed] [Google Scholar]
- Bell D. R., Gerlach J. H., Kartner N., Buick R. N., Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. J Clin Oncol. 1985 Mar;3(3):311–315. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- Buchanan J. A., Yeger H., Tabcharani J. A., Jensen T. J., Auerbach W., Hanrahan J. W., Riodan J. R., Buchwald M. Transformed sweat gland and nasal epithelial cell lines from control and cystic fibrosis individuals. J Cell Sci. 1990 Jan;95(Pt 1):109–123. doi: 10.1242/jcs.95.1.109. [DOI] [PubMed] [Google Scholar]
- Chang E. B., Bookstein C., Vaandrager A., DeJonge H. R., Buse J., Musch M. W. Cystic fibrosis transmembrane regulator mRNA expression relative to ion-nutrient transport in spontaneously differentiating human intestinal CaCo-2 epithelial cells. J Lab Clin Med. 1991 Oct;118(4):377–381. [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991 Sep 6;66(5):1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou J. L., Rozmahel R., Tsui L. C. Characterization of the promoter region of the cystic fibrosis transmembrane conductance regulator gene. J Biol Chem. 1991 Dec 25;266(36):24471–24476. [PubMed] [Google Scholar]
- Clancy J. P., McCann J. D., Li M., Welsh M. J. Calcium-dependent regulation of airway epithelial chloride channels. Am J Physiol. 1990 Feb;258(2 Pt 1):L25–L32. doi: 10.1152/ajplung.1990.258.2.L25. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Billingsley G. D., Mansfield T. DNA restriction-site polymorphisms associated with the alpha 1-antitrypsin gene. Am J Hum Genet. 1987 Nov;41(5):891–906. [PMC free article] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Dudouet B., Robine S., Huet C., Sahuquillo-Merino C., Blair L., Coudrier E., Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones. J Cell Biol. 1987 Jul;105(1):359–369. doi: 10.1083/jcb.105.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Gowen C. W., Jr, Gowen M. A., Knowles M. R. Colonic transepithelial potential difference in infants with cystic fibrosis. J Pediatr. 1991 Mar;118(3):412–415. doi: 10.1016/s0022-3476(05)82158-4. [DOI] [PubMed] [Google Scholar]
- Green F., Edwards Y., Hauri H. P., Povey S., Ho M. W., Pinto M., Swallow D. Isolation of a cDNA probe for a human jejunal brush-border hydrolase, sucrase-isomaltase, and assignment of the gene locus to chromosome 3. Gene. 1987;57(1):101–110. doi: 10.1016/0378-1119(87)90181-8. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Koike K., Ohta S., Urata Y., Kagawa Y., Koike M. Cloning and sequencing of cDNAs encoding alpha and beta subunits of human pyruvate dehydrogenase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):41–45. doi: 10.1073/pnas.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Erickson R. H., Gum J. R., Yoshioka M., Gum E., Kim Y. S. Biosynthesis of alkaline phosphatase during differentiation of the human colon cancer cell line Caco-2. Gastroenterology. 1990 May;98(5 Pt 1):1199–1207. doi: 10.1016/0016-5085(90)90334-w. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C., Guggino W. B., Montrose M. H. Cellular differentiation regulates expression of Cl- transport and cystic fibrosis transmembrane conductance regulator mRNA in human intestinal cells. J Biol Chem. 1991 Mar 5;266(7):4495–4499. [PubMed] [Google Scholar]
- Paillard F., Sterkers G., Vaquero C. Transcriptional and post-transcriptional regulation of TcR, CD4 and CD8 gene expression during activation of normal human T lymphocytes. EMBO J. 1990 Jun;9(6):1867–1872. doi: 10.1002/j.1460-2075.1990.tb08312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Daniels J. D., Auerbach H. S., De Schryver-Kecskemeti K., Winter H. S., Alpers D. H. The alpha 1-antitrypsin gene is expressed in a human intestinal epithelial cell line. J Biol Chem. 1989 Jun 5;264(16):9485–9490. [PubMed] [Google Scholar]
- Pringault E., Arpin M., Garcia A., Finidori J., Louvard D. A human villin cDNA clone to investigate the differentiation of intestinal and kidney cells in vivo and in culture. EMBO J. 1986 Dec 1;5(12):3119–3124. doi: 10.1002/j.1460-2075.1986.tb04618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Gregory R. J., Anderson M. P., Manavalan P., Smith A. E., Welsh M. J. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991 Jul 12;253(5016):205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robine S., Huet C., Moll R., Sahuquillo-Merino C., Coudrier E., Zweibaum A., Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci U S A. 1985 Dec;82(24):8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens J. M., Dho S., Bear C. E., Kartner N., Kennedy D., Riordan J. R., Tsui L. C., Foskett J. K. cAMP-inducible chloride conductance in mouse fibroblast lines stably expressing the human cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7500–7504. doi: 10.1073/pnas.88.17.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986 Sep;68(9):1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Rydell E., Magnusson K. E., Sjö A., Axelsson K. Protein kinase C and casein kinase II activities in two human colon carcinoma cell lines, HT-29 and CaCo-2: possible correlation with differentiation. Biosci Rep. 1990 Jun;10(3):293–299. doi: 10.1007/BF01117245. [DOI] [PubMed] [Google Scholar]
- Sebastio G., Hunziker W., O'Neill B., Malo C., Ménard D., Auricchio S., Semenza G. The biosynthesis of intestinal sucrase-isomaltase in human embryo is most likely controlled at the level of transcription. Biochem Biophys Res Commun. 1987 Dec 16;149(2):830–839. doi: 10.1016/0006-291x(87)90442-6. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Fargon F., McNaughton P. A. K+ and Cl- currents in enterocytes isolated from guinea-pig small intestinal villi. J Physiol. 1991 Mar;434:351–367. doi: 10.1113/jphysiol.1991.sp018474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W. Expression of digestive and absorptive function in differentiating enterocytes. Annu Rev Physiol. 1985;47:247–260. doi: 10.1146/annurev.ph.47.030185.001335. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991 Aug 15;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Zeitlin P. L., Chu C. S., Yoshimura K., Nakamura H., Guggino W. B., Bargon J., Banks T. C., Dalemans W., Pavirani A. Down-regulation of cystic fibrosis gene mRNA transcript levels and induction of the cystic fibrosis chloride secretory phenotype in epithelial cells by phorbol ester. J Biol Chem. 1991 Jun 5;266(16):10319–10323. [PubMed] [Google Scholar]
- Trezise A. E., Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991 Oct 3;353(6343):434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Trugnan G., Rousset M., Chantret I., Barbat A., Zweibaum A. The posttranslational processing of sucrase-isomaltase in HT-29 cells is a function of their state of enterocytic differentiation. J Cell Biol. 1987 May;104(5):1199–1205. doi: 10.1083/jcb.104.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Chu C. S., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991 Oct 11;19(19):5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. The cystic fibrosis gene has a "housekeeping"-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991 May 15;266(14):9140–9144. [PubMed] [Google Scholar]
- Zweibaum A., Triadou N., Kedinger M., Augeron C., Robine-Léon S., Pinto M., Rousset M., Haffen K. Sucrase-isomaltase: a marker of foetal and malignant epithelial cells of the human colon. Int J Cancer. 1983 Oct 15;32(4):407–412. doi: 10.1002/ijc.2910320403. [DOI] [PubMed] [Google Scholar]