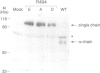
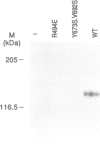
Abstract
Hepatocyte growth factor (HGF) is a potent mitogen for parenchymal liver, epithelial and endothelial cells. Structurally, it has similarities to kringle-containing serine proteases, although it does not possess proteolytic activity. A structure-activity relationship study of human HGF was performed by functional analysis of HGF substitution and deletion variants. Analysis of HGF variants was accomplished by defining their ability to induce DNA synthesis on hepatocytes in primary culture and to compete with wild-type HGF for binding to a soluble form of the HGF receptor. Three groups of variants were made: (i) substitutions at the cleavage site, (ii) substitutions within the protease-like domain and (iii) deletions of the beta-chain and/or kringle domains. Our results show that: (i) single-chain HGF is a zymogen-like promitogen in that cleavage into a two-chain form is required for biological activity, however, the single chain form of HGF still retains substantial receptor binding capacity; (ii) certain mutations in the protease-like domain result in variants that are completely defective for mitogenic activity, yet exhibit apparent receptor binding affinities similar to wild-type HGF (Kd approximately 50-70 pM); and (iii) a variant containing the N-terminal 272 residues of mature HGF showed only a 4-fold increase in Kd when compared with wild-type HGF indicating that a primary receptor binding determinant is located within this sequence.
Full text
PDF


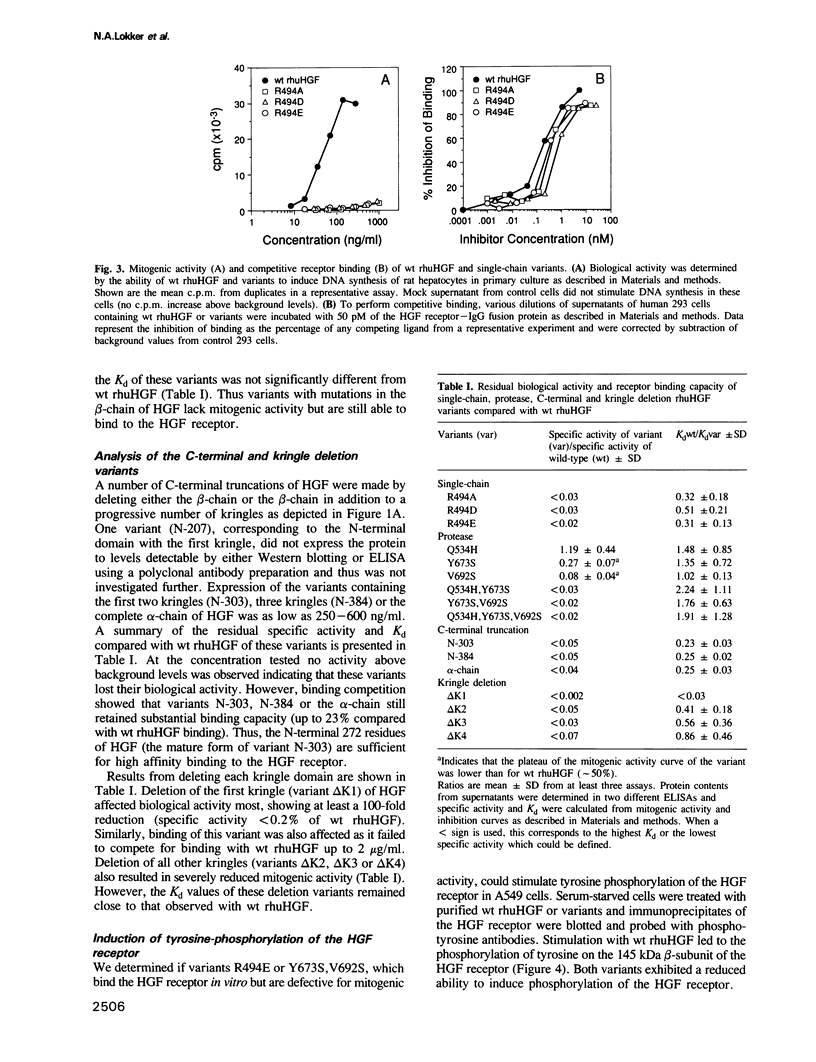




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami O., Ihara I., Shimidzu N., Shimizu S., Tomita Y., Ichihara A., Nakamura T. Purification and characterization of hepatocyte growth factor from injured liver of carbon tetrachloride-treated rats. J Biochem. 1991 Jan;109(1):8–13. [PubMed] [Google Scholar]
- Blevins R. A., Tulinsky A. The refinement and the structure of the dimer of alpha-chymotrypsin at 1.67-A resolution. J Biol Chem. 1985 Apr 10;260(7):4264–4275. doi: 10.2210/pdb5cha/pdb. [DOI] [PubMed] [Google Scholar]
- Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991 Feb 15;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Chan A. M., Rubin J. S., Bottaro D. P., Hirschfield D. W., Chedid M., Aaronson S. A. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991 Nov 29;254(5036):1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- DeBlasi A., O'Reilly K., Motulsky H. J. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989 Jun;10(6):227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Haynes R. C., Jr The hormonal control of gluconeogenesis by regulation of mitochondrial pyruvate carboxylation in isolated rat liver cells. J Biol Chem. 1975 Apr 25;250(8):2769–2777. [PubMed] [Google Scholar]
- Gohda E., Tsubouchi H., Nakayama H., Hirono S., Sakiyama O., Takahashi K., Miyazaki H., Hashimoto S., Daikuhara Y. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J Clin Invest. 1988 Feb;81(2):414–419. doi: 10.1172/JCI113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Stuart L. A., Degen S. J. Characterization of the DNF15S2 locus on human chromosome 3: identification of a gene coding for four kringle domains with homology to hepatocyte growth factor. Biochemistry. 1991 Oct 8;30(40):9768–9780. doi: 10.1021/bi00104a029. [DOI] [PubMed] [Google Scholar]
- Higuchi O., Nakamura T. Identification and change in the receptor for hepatocyte growth factor in rat liver after partial hepatectomy or induced hepatitis. Biochem Biophys Res Commun. 1991 Apr 30;176(2):599–607. doi: 10.1016/s0006-291x(05)80226-8. [DOI] [PubMed] [Google Scholar]
- Kan M., Zhang G. H., Zarnegar R., Michalopoulos G., Myoken Y., McKeehan W. L., Stevens J. I. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991 Jan 15;174(1):331–337. doi: 10.1016/0006-291x(91)90524-b. [DOI] [PubMed] [Google Scholar]
- Kossiakoff A. A., Spencer S. A. Direct determination of the protonation states of aspartic acid-102 and histidine-57 in the tetrahedral intermediate of the serine proteases: neutron structure of trypsin. Biochemistry. 1981 Oct 27;20(22):6462–6474. doi: 10.1021/bi00525a027. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lindroos P. M., Zarnegar R., Michalopoulos G. K. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991 Apr;13(4):743–750. [PubMed] [Google Scholar]
- Matsumoto K., Tajima H., Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991 Apr 15;176(1):45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Houck K. A., Dolan M. L., Leutteke N. C. Control of hepatocyte replication by two serum factors. Cancer Res. 1984 Oct;44(10):4414–4419. [PubMed] [Google Scholar]
- Miyazawa K., Kitamura A., Naka D., Kitamura N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur J Biochem. 1991 Apr 10;197(1):15–22. doi: 10.1111/j.1432-1033.1991.tb15876.x. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Tsubouchi H., Naka D., Takahashi K., Okigaki M., Arakaki N., Nakayama H., Hirono S., Sakiyama O., Takahashi K. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989 Sep 15;163(2):967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991 Nov 29;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nawa K., Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L., Vigna E., Narsimhan R. P., Gaudino G., Zarnegar R., Michalopoulos G. K., Comoglio P. M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991 Apr;6(4):501–504. [PubMed] [Google Scholar]
- Park M., Dean M., Kaul K., Braun M. J., Gonda M. A., Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. A., Naujokas M. A., Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol. 1991 Jun;11(6):2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Chan A. M., Bottaro D. P., Burgess W. H., Taylor W. G., Cech A. C., Hirschfield D. W., Wong J., Miki T., Finch P. W. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. E., McGowan J. A., Bucher N. L. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984 May;119(2):183–192. doi: 10.1002/jcp.1041190207. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Seki T., Ihara I., Sugimura A., Shimonishi M., Nishizawa T., Asami O., Hagiya M., Nakamura T., Shimizu S. Isolation and expression of cDNA for different forms of hepatocyte growth factor from human leukocyte. Biochem Biophys Res Commun. 1990 Oct 15;172(1):321–327. doi: 10.1016/s0006-291x(05)80212-8. [DOI] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Hagiya M., Nishizawa T., Seki T., Shimonishi M., Shimizu S., Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate K. M., Higgins D. L., Holmes W. E., Winkler M. E., Heyneker H. L., Vehar G. A. Functional role of proteolytic cleavage at arginine-275 of human tissue plasminogen activator as assessed by site-directed mutagenesis. Biochemistry. 1987 Jan 27;26(2):338–343. doi: 10.1021/bi00376a002. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Weidner K. M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990 Nov;111(5 Pt 1):2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T. C. Plasminogen activation: biochemistry, physiology, and therapeutics. Crit Rev Biotechnol. 1988;8(2):131–148. doi: 10.3109/07388558809150542. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Arakaki N., Naka D., Takahashi K., Hirono S., Kondo J., Nakayama H., Gohda E., Kitamura N., Tsubouchi H. Identification of the N-terminal residue of the heavy chain of both native and recombinant human hepatocyte growth factor. Biochem Biophys Res Commun. 1991 Mar 15;175(2):660–667. doi: 10.1016/0006-291x(91)91616-k. [DOI] [PubMed] [Google Scholar]