Abstract
The genome of Fusarium oxysporum (Fo) consists of a set of eleven ‘core’ chromosomes, shared by most strains and responsible for housekeeping, and one or several accessory chromosomes. We sequenced a strain of Fo f.sp. radicis-cucumerinum (Forc) using PacBio SMRT sequencing. All but one of the core chromosomes were assembled into single contigs, and a chromosome that shows all the hallmarks of a pathogenicity chromosome comprised two contigs. A central part of this chromosome contains all identified candidate effector genes, including homologs of SIX6, SIX9, SIX11 and SIX 13. We show that SIX6 contributes to virulence of Forc. Through horizontal chromosome transfer (HCT) to a non-pathogenic strain, we also show that the accessory chromosome containing the SIX gene homologs is indeed a pathogenicity chromosome for cucurbit infection. Conversely, complete loss of virulence was observed in Forc016 strains that lost this chromosome. We conclude that also a non-wilt-inducing Fo pathogen relies on effector proteins for successful infection and that the Forc pathogenicity chromosome contains all the information necessary for causing root rot of cucurbits. Three out of nine HCT strains investigated have undergone large-scale chromosome alterations, reflecting the remarkable plasticity of Fo genomes.
Introduction
Fusarium oxysporum Schlechtend.: Fr. f.sp. radicis-cucumerinum Vakalounakis (Forc) is the causal agent of root and stem rot in cucurbits resulting in severe damage, particularly in greenhouse cucumber (Cucumis sativus) and muskmelon (C. melo). The disease was first described in Greece in 1989 by Vakalounakis, who identified the pathogen as a new forma specialis of F. oxysporum (Fo)1. Forc has since been recorded in several other countries including Canada, France, Spain, China, Turkey and Israel2–6. Unlike Fusarium wilt caused by Fo f.sp. cucumerinum (Foc) or Fo f.sp. melonis (Fom), the main symptoms caused by Forc are external rotting of the root and stem and profuse sporulation in the rotted tissue1, 3. Still, the infection mechanism appears to be the same: the fungus invades the roots and colonizes the xylem vessels of the plant (Video S1).
Formae speciales of Fo typically have a very narrow host range, often restricted to a single plant species7, 8. Forc is exceptional because its host range includes not only cucumber and melon, but also additional Cucurbitaceae species such as watermelon (Citrullus lanatus), squash (Cucurbita pepo) and gourd (Luffa aegyptiaca)1, 3, 6. The genetic mechanism underlying the difference in disease symptoms (root rot versus wilt) as well as the extended host range of this pathogen are unknown, but may be associated with the suite of effector genes present in the genome of this forma specialis 9. We found that Forc strains possess four Secreted In Xylem (SIX) gene homologs: SIX6, SIX9, SIX11 and SIX13, which encode small secreted proteins originally identified in tomato-infecting strains10–12. Additionally, we found several other genes encoding candidate effectors based on small size, predicted secretion signal, and vicinity to a “miniature impala” (mimp) transposable element, including a secreted astacin-like metalloprotease9.
The genome of Fo is typically divided into a set of eleven ‘core’ chromosomes, with sequences generally conserved in all Fusarium species, and responsible for housekeeping, and one or several transposon-rich and gene-poor ‘accessory’ chromosomes13. In Fo f.sp. lycopersici (Fol), one of these accessory chromosomes was shown to be required for pathogenicity towards tomato14. Moreover, it can be horizontally transferred to the non-pathogenic strain Fo47, thereby transforming this strain into a tomato pathogen15, 16. In de novo Illumina assemblies, accessory chromosomes are typically dispersed over many contigs or scaffolds due to their high repeat-content, making it impossible to determine how many accessory chromosomes are present in a strain. Three Forc strains have been sequenced so far, each resulting in assemblies of several hundred scaffolds9. A solution to the high level of fragmentation of Fo assemblies could be long-read sequencing technology, such as PacBio Single Molecule Real-Time (SMRT) sequencing. This would allow the multiple kb-sized repetitive elements to be spanned by individual reads, leading to much larger contigs.
The aims of this study were to (i) determine the genome structure of Forc, (ii) investigate whether Forc, like wilt-causing strains of Fo, relies on effector proteins for successful colonization and (iii) identify which part(s) of the Forc genome are necessary for the root- and shoot-rot phenotype as well as the extended host range of Forc. To reach these aims, we applied SMRT sequencing of a representative strain of Forc (strain Forc016) as well as Fom (Fom001; NRRL26406) as a step towards answering the question what differentiates Forc from strains causing wilt.
Results
A corrected SMRT assembly of Forc contains 33 sequences including 12 (near) full-length chromosomes
In order to obtain a better understanding of the genome composition of Forc, an HGAP.3 de novo assembly was generated for Forc016, a strain previously sequenced by Illumina9. The initial SMRT assembly consisted of 41 contigs, including seven contigs that contained ribosomal DNA (rDNA) repeats. Two of these show rDNA sequences at one end and telomeric repeats (CCCTAA) on the other end, indicating that they together form chromosome 213. The rDNA copy number was estimated through Illumina read coverage (~98 copies), and the two contigs were joined to reconstruct chromosome 2 (N.B. numbering of core chromosomes follows the Fol4287 reference genome). Three rDNA repeats of each contig were kept. The 91 copies in between were filled with the first rDNA repeat of the first contig.
Chromosome 13 was also assembled into two contigs, but an overlap of 13,396 nucleotides and synteny to the SMRT assemblies of Fom001 (Fig. S1), as well as a related Fusarium species, F. subglutinans, were found (B. Brankovics, personal communication). This allowed us to merge these sequences into chromosome 13.
Contour-clamped homogeneous electric field (CHEF) electrophoretic karyotyping followed by Southern blotting and hybridization with a radioactive probe generated from a Fol-SIX6 Polymerase Chain Reaction (PCR) product revealed that the SIX6 sequence is present on a ~2.5 megabase (Mb)-sized chromosome in Forc016 (Fig. 1, Fig. S2). This chromosome is present in the SMRT assembly as two separate contigs (13 and 17) of which the ends have an overlap of 586 nucleotides. Comparison to the Fom001 SMRT assembly revealed that this chromosome is largely syntenic to contig 127 of Fom001, but with a large (1.448 Mb) inversion between inverted, highly similar regions of about 200 kb (Fig. S3). Either end of this region matched the end of Forc016 contig 17. However, when the 1.448 Mb region was manually inverted, not a single nucleotide polymorphism (SNP) was found in the pairwise sequence alignment, whereas in the original assembly three single nucleotide InDel mismatches were identified (data not shown). We therefore conclude that it is more likely that the 1.448 Mb region is in the reverse orientation and we adjusted this manually. One contig containing the mitochondrial DNA (mtDNA) sequence was identified by BLAST, removed from the SMRT assembly and the 47,541 nucleotide-long (annotated) mitogenome generated through Illumina reads by the GRAbB program17 was added (Brankovics et al., submitted).
Figure 1.
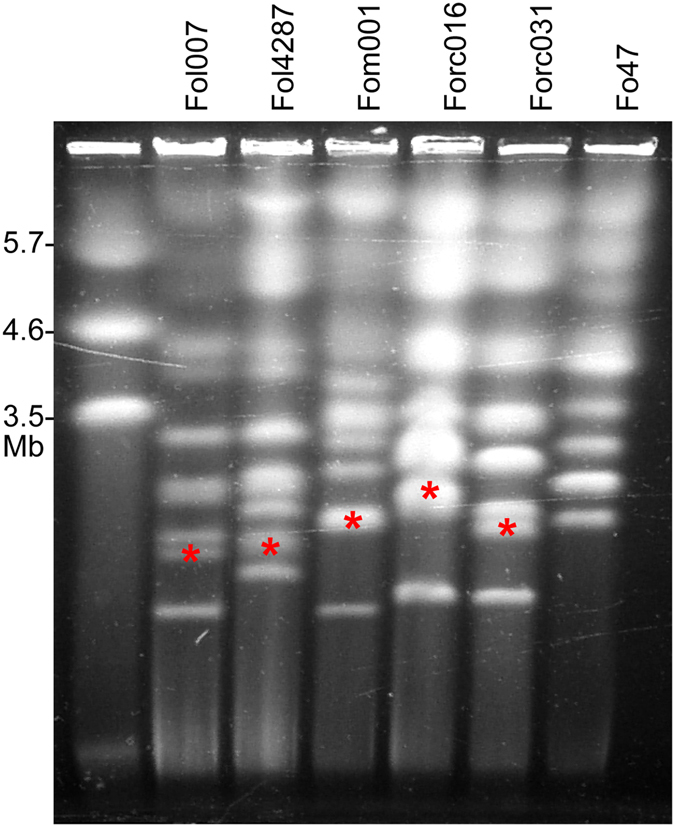
Electrophoretic karyotypes of strains belonging to F. oxysporum f.sp. lycopersici (Fol007, Fol4287), melonis (Fom001) or radicis-cucumerinum (Forc016, Forc031) and non-pathogenic strain Fo47. A red asterisk indicates the location of the radioactive Fol-SIX6 probe hybridization signal, identifying the chromosomes potentially involved in pathogenicity. Fo47, a non-pathogenic strain, does not have a SIX6 homolog. The left lane shows the karyotype of Schizosaccharomyces pombe, applied as a marker. This image is cropped, the original gel photograph can be found in Supplementary Fig. S11.
The final, manually corrected assembly of Forc016 is composed of eleven core chromosomes, one pathogenicity chromosome, twenty unpositioned sequences with a cumulative size of 2.572 Mb and the mitochondrial genome (Table 1). Nine of the unpositioned sequences end in telomeric repeats, indicating that they should probably be attached to the ends of chromosome-sized contigs that lack a telomere. The assembly is of a very high quality, with the L90 being reached with only eleven sequences.
Table 1.
Comparison of the Forc016 genome assembly generated with Illumina HiSeq 2500 reads with the manually corrected SMRT HGAP.3 assembly and the raw SMRT HGAP.3 assembly of Fom001.
| Forc016 Illumina HiSeq | Forc016 SMRT | Fom001 SMRT | |
|---|---|---|---|
| Assembly size | 50,061,337 | 52,860,752 | 60,704,002 |
| Ambiguous bases (Ns) | 85,353 | 0 | 0 |
| Gaps | 902 | 0 | 0 |
| GC (%) | 47.63 | 47.69 | 47.65 |
| Mean sequence lengtha | 59,244 | 1,651,898 | 632,333 |
| Shortest sequence lengtha | 505 | 4587 | 5,714 |
| Longest sequence lengtha | 2,409,929 | 6,470,671 | 6,402,286 |
| L50 (kbp) | 575.0 (n = 21) | 4,490.1 (n = 5) | 4,357.5 (n = 6) |
| L70 (kbp) | 237.5 (n = 49) | 3,661.0 (n = 8) | 2,962.0 (n = 10) |
| L90 (kbp) | 37.7 (n = 149) | 2,466.0 (n = 11) | 761.3 (n = 17) |
| Coverage | 91 X | 72 X | 59 X |
| # Sequencesa | 845 | 32 + mtDNA | 96 |
| # Sequences having telomeric repeats on both endsa | 0 | 5 | 0 |
| # Sequences having telomeric repeats on one enda | 0 | 15 | 19 |
| # Sequences having no telomeric repeatsa | 845 | 12 | 77 |
a‘Sequences’ refers to scaffolds (Illumina assembly) or contigs (SMRT assembly).
Two large sequence duplications are present on contig 53 of the SMRT assembly (Fig. S4E). Because of its size, this contig is likely a large part of one of the small (±1–1.5 Mb) accessory chromosome shown in Fig. 1. This is supported by the fact that it contains a GC-content drop typical of a centromeric region (Fig. S4B).
Comparison of the Forc016 SMRT assembly to that of Fol4287, the reference genome of F. oxysporum, revealed that (i) the eleven core chromosomes are highly syntenic between the strains (with 98.9% sequence identity), (ii) the Forc016 assembly has six contigs that contain sequences that align to known Fol accessory regions – likely due to the presence of similar transposable elements (TEs) in both and (iii) one of these six contigs is a putative pathogenicity chromosome on which the SIX6 sequence was identified earlier (Fig. 1) with a high number of repeats and effector candidates, that we named chrRC (Fig. 2). Gene ontology (GO) terms related to metabolism, protein ADP-ribosylation and DNA integrity were found to be overrepresented among the predicted genes on chrRC (Fig. S5, Table S1). We further focused on this chromosome.
Figure 2.
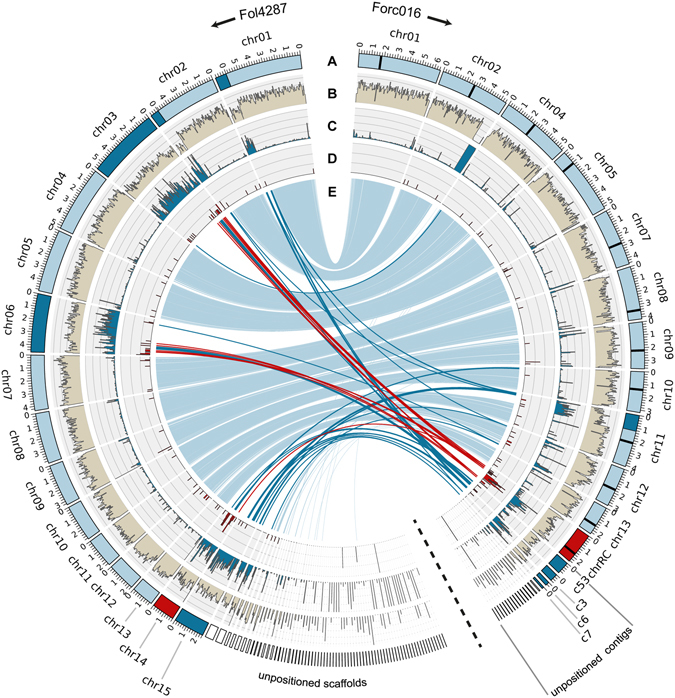
The SMRT genome assembly of Forc016 includes eleven core chromosomes, several repeat-rich, gene-poor accessory regions and one chromosome enriched in candidate effector genes. Comparison of the Forc016 assembly to that of Fol4287 reveals (A) eleven conserved core chromosomes (light blue), one putative pathogenicity chromosome (red) and several other accessory sequences (dark blue). Accessory regions typically have (B) low gene density and (C) high repeat density, both calculated here in 50 kb windows. The putative pathogenicity chromosome is marked by (D) the presence of many candidate effector genes. (E) Indicates nucmer alignments with the Fol4287 reference assembly: in red alignments to the putative pathogenicity chromosome of Forc016, in dark blue alignments from known accessory regions in Fol4287 (chr1B; chr2B; chr3; chr6; chr14; chr15) and in light blue the remaining alignments, mostly between core regions in both genomes.
Most candidate effector genes reside in a subregion of chrRC
The putative pathogenicity chromosome of Forc016, chrRC, is highly similar (99.8%) to sequences present in the two other previously sequenced Forc strains9. Surprisingly, high similarity (>99%) was also observed with sequences in the genomes of Fom001, Fom004, Fom005, Fom006, Fom012, Fom13, Fom016 but not Fom009, Fom010, Fom011 (data not shown). A de novo HGAP.3 assembly for Fom001 was generated and we found that synteny is mostly preserved between chrRC and Fom001 contig 127 (Fig. 3A). A notable exception is a central, ~700 kb region of the chromosome, which is exceptionally repeat-rich and of which ~300 kb is absent in Fom001 contig 127, flanked by several multi-kb inversions (Fig. 3B). Within the 700 kb region, 195 genes were predicted in the Forc016 SMRT assembly. 185 of these have a highly similar homolog in Fom001 (average nucleotide sequence identity is 99.1%). 135 of these genes (including SIX6 and SIX11) are almost identical between the two strains, returning a BLAST hit percentage of 99.8% or higher, suggesting that they have been reshuffled recently. Only ten genes could not be identified with BLASTN (e-value <1e-20; perc_identity >90%; query coverage >70%) in Fom001: SIX9 (g15834), three beta-lactamases (g15883, g15832, g15833), three hypothetical proteins (g15854, g15957, g15835), a cytochrome p450 (g15902), a putative lysine decarboxylase (g15903) and an NADH-flavin oxidoreductase (g15826). One or several of these genes may contribute to the ability of Forc to cause root rot in several cucurbit species.
Figure 3.
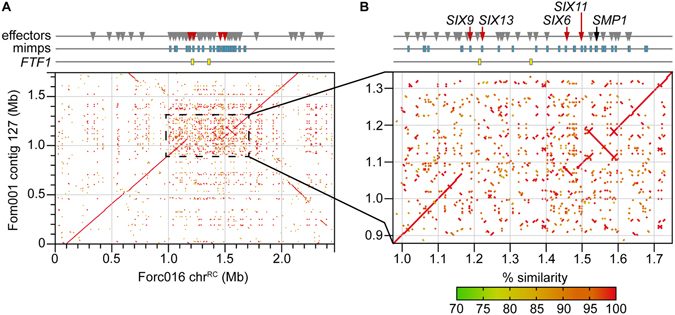
Comparison of chrRC with Fom001 contig 127 reveals a highly dynamic central region containing the majority of the miniature impala (mimp) TEs and candidate effector genes of the Forc genome. (A) The chromosomes of the Fom and Forc strains are mostly syntenic, with large stretches showing 100% identity in this nucmer alignment. (B) The dynamic central region of the chromosome, about 700 kb in size, has the highest repeat density, 30 of the 35 mimps of the Forc genome and the majority of the Forc candidate effector genes, including SIX9, 13, 6 and 11 and SMP1. Additionally, the two FTF1 homologs present in the Forc genome are found here.
Interestingly, this is exactly the region where 30 of the 35 full-size miniature impala (mimp) elements were found in the Forc genome. Two other mimps were found on chr12, one on chr11 and two more on contig 14, a contig of only 22.4 kb. Mimps are contextually associated with effector genes in F. oxysporum 10, 18. Indeed, the majority (51 out of 98) of candidate effector genes identified by BLAST from the list of 104 candidates that we identified earlier9 are localized in this region (Fig. 3B). Among these are the four SIX homologs that are present in Forc: SIX6 (g15909), SIX9 (g15834), SIX11 (g16807) and SIX13 (g15844). These were previously shown to be expressed during plant infection9. Additionally, two homologs of the FTF1 transcription factor, associated with effector gene expression19, 20, are found here (g15884 and g15843).
In Fom001, homologs of SIX1 (contig 22), SIX6 (contig 127), SIX11 (two copies; contig 10 and 127) and SIX13 (contig 10) are present. Fom contigs 10 and 22 are 2.962 and 1.268 Mb in size, respectively, suggesting that Fom001 may have more than one chromosome associated with pathogenicity. The sequences of SIX6 and SIX11 that are located on Fom contig 127 are identical between Forc016 and Fom001. From the list of candidate Fom effectors in Schmidt et al.18, only candidate 1 A is present in the Forc016 assembly on chrRC (100% identical between Fom and Forc). None of the other candidates, including AVRFOM2, is present in Forc.
Six6 contributes to virulence of Forc
In order to investigate the role candidate effectors play in Forc pathogenicity, knockout strains were generated through homologous recombination with a hygromycin resistance marker. Although this process is very inefficient in regions with many repeats, such as the region depicted in Fig. 4B, successful knockout was achieved for SIX6, SIX9 and an astacin-like Secreted MetalloProtease gene (which we named SMP1 (g15931); Fig. 3). All three genes are single copy in the Forc016 genome. Bioassays were conducted with cucumber, melon and watermelon plants to evaluate whether the fungus had become less pathogenic to one or several host plants upon loss of these genes.
Figure 4.
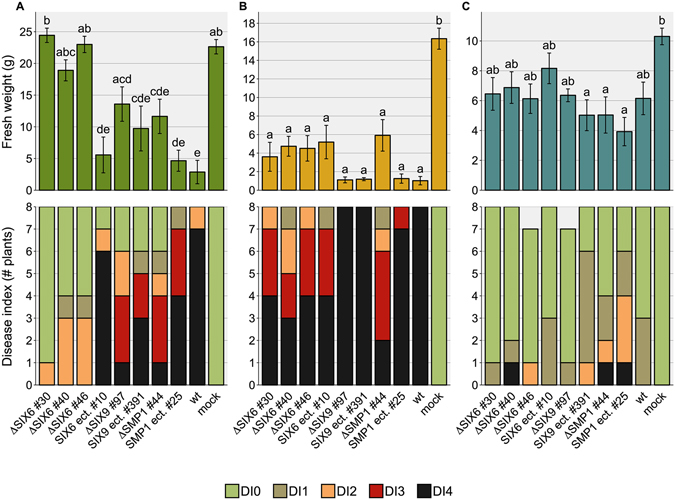
Three independent SIX6 deletion strains cause less symptom development in cucumber. Fresh weight (±S.E.) and disease index (DI) of (A) cucumber, (B) melon and (C) watermelon plants were scored 14 days post inoculation. An ANOVA followed by a Tukey HSD test (p < 0.05) was performed to determine significance of the differences in the fresh weight measurements (significance categories shown with letters above the bars). Under the tested conditions (106 spores/ml, 25 °C), three independent SIX6 deletion strains caused reduced symptoms in cucumber compared to wildtype (wt) and an ectopic transformant (SIX6 ect. #10). Knockout of the two other candidate virulence genes SIX9 and SMP1 did not have a significant effect on virulence. Under these conditions (25 °C), Forc causes only mild symptoms on watermelon.
When inoculated with 106 spores/ml at an ambient temperature of 25 °C, the three independent SIX6 knockout strains caused reduced disease symptoms in cucumber compared to a transformant with an ectopic integration of the T-DNA, as well as to the wild type Forc016 strain and the two other knockout strains (Fig. 4; pictures shown in Fig. S6). When tested at a lower ambient temperature of 18–20 °C, all strains caused quick and uniform death to all plants of the three tested species, indicating that these conditions are more favourable for Forc disease development and do not require Six6 (data not shown). Disruption of SIX9 or SMP1 did not significantly affect virulence under the tested conditions (Fig. 4). The absence of SIX9 in Fom001 is therefore not responsible for the phenotypic difference between Fom and Forc.
Cell wall degrading enzymes in rot symptom development
In a comparison between Forc and Foc strains, it was found that isolates of Forc have more pectolytic enzyme activity6. This may, in part, account for the crown rot and tissue maceration seen in root and shoot rot disease caused by Forc. Production of cell wall degrading enzymes (CWDEs) by Fo is well documented (reviewed in refs 21 and 22) and was shown to be positively correlated with virulence in Fo f.sp. dianthi 23. Individual knockout of CWDE- or protease-encoding genes, however, usually does not result in a detectable reduction in virulence in Fo21, 22. Site-directed mutagenesis of three amino acid residues located at the putative active site of an endopolygalacturonase from F. verticillioides (formerly F. moniliforme) did result in reduced macerating activity on potato medullary tissues24. This led Reignault et al. to hypothesize that pectinases are important for necrosis and maceration (e.g. by Forc), but are less important for vascular wilt disease25.
In total, 179 gene products are predicted to have proteolytic activity (ontology term GO:0006508) in the Forc genome, of which four are encoded on chrRC. Two of these possess a predicted signal peptide: SMP1 and a subtilase gene. Polygalacturonase activity (GO:0004650) was predicted for 11 genes, none of which resides on chrRC. Likewise, none of six pectinesterase-encoding genes in the Forc genome (GO:0030599) resides on chrRC. Since knockout of SMP1 did not result in reduced virulence, there may be functional redundancy with other proteases. Despite these observations, protease or CWDE activity may still be important during plant colonization and rot symptom development.
ChrRC is a mobile chromosome
ChrRC is similar to the mobile Fol pathogenicity chromosome15 in that it is repeat-rich, gene-poor and contains most candidate effector genes, of which at least one (SIX6) contributes to virulence towards cucumber. In order to assess whether this chromosome could be horizontally transferred to other strains, a co-cultivation experiment was performed. Forc016∆SIX6#46 was chosen as the potential chromosome donor strain, since it carries the HPH hygromycin-resistance marker on chrRC. Spores from this strain were mixed with spores from three different ‘recipient’ strains: Fo47, Fol4287 and Fom001, all tagged by random insertion of the BLE zeocin-resistance gene. Double-resistant colonies were recovered only in the combination with Fo47. Nine such strains were saved and used for further analysis. All were shown by PCR to contain both HPH and BLE genes.
To assess whether indeed chrRC from Forc016 had been transferred to Fo47, a CHEF gel was run (Fig. 5). This revealed that all nine double-resistant strains displayed the karyotype of Fo47, with an additional chromosome presumably resulting from horizontal chromosome transfer (HCT). In the cases of HCT-derived strains #2, #4, #5, #6, #7 and #9 this chromosome is similar in size to a chromosome in the Forc016 donor strain (~2.5 Mb). However, since it is roughly the same size as the smallest chromosome of Fo47, the two co-migrated through the gel, resulting in a band with double intensity. In the three other cases (HCT #1, #3 and #8), this double band was absent and instead other new chromosomes were observed (Fig. 5, white arrowheads).
Figure 5.
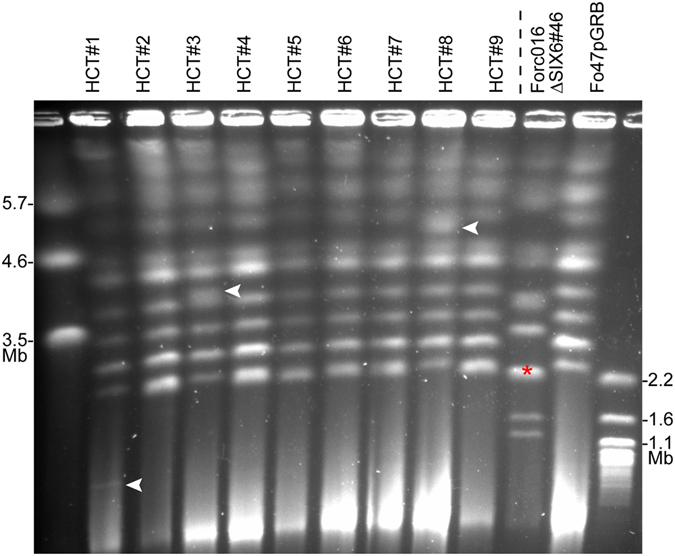
Nine strains derived from a HCT experiment between Forc016∆SIX6#46 and Fo47pGRB contain new chromosomes in the Fo47 background. Lanes 1–9 show the karyotype of HCT-derived strains, resembling that of Fo47pGRB (lane 11). Most of these strains have a double band at the size of chrRC (~2.5 Mb), marked in Forc016∆SIX6#46 with a red asterisk (lane 10). Strains #1, #3 and #8 do not have this double band, but instead have at least one novel chromosome that is not found in either parental strain (white arrowheads). The left and right lanes show the karyotypes of S. pombe and S. cerevisiae, respectively, applied as markers. This image is cropped, the original gel photograph can be found in Supplementary Fig. S12.
Stringent Illumina short-read mapping of HCT strains #2, #4, #8 and #9 on the Forc016 assembly showed that these strains indeed contain the full chrRC sequence but no other Forc016-derived sequences (the core genomes of Forc016 and Fo47 have an average SNP density of 0.4% (more towards the telomeres), reducing mapping of reads to ~85%) (Fig. 6). This confirms that chrRC now resided in a Fo47 core genome background. Remarkably, HCT#8 showed a relative depth of coverage of chrRC about two times higher than the other HCT strains, indicating that the chrRC sequences are present twice. Since a double band at the expected size of chrRC (~2.5 Mb) is missing in this strain but a double-sized band of ~5 Mb is visible (Fig. 5), this duplication appears to have resulted in a single chromosome twice the size of chrRC. From the normalized read coverage of HCT strains #1 and #3 it is clear that large-scale rearrangements also took place along chrRC in these strains (Fig. S7). Assembly of the mitochondrial genome with GRABb17 and comparison to the mitogenomes of Fo47 and Forc016 showed that the mitochondrial DNA of the Fo47 acceptor strain had been retained in all cases (data not shown).
Figure 6.
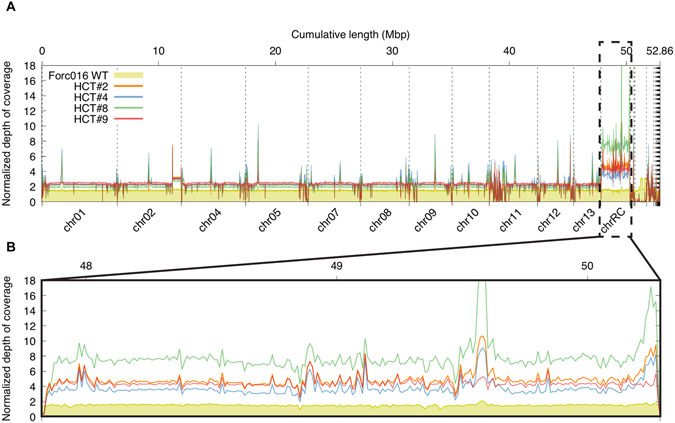
Normalized Illumina read mapping to the SMRT assembly of Forc016 confirms horizontal transfer of chrRC in a Fo47 background. (A) Reads mapped more abundantly to the transferred chrRC sequence than the rest of the assembly. (B) HCT strains #2, #4, #9 showed a relative (compared to total # mapped reads) depth of coverage of ~4 on chrRC, whereas the relative coverage of HCT#8 sequences was ~8 along the entire chromosome. This indicates a chromosomal duplication, in accordance with the ~5Mb-sized band in the CHEF gel in Fig. 5.
ChrRC is capable of turning Fo47 into a cucurbit root rot pathogen
To assess whether the HCT-strains, carrying chrRC in a Fo47 background, are pathogenic on cucurbits, a bioassay was performed on cucumber, melon and watermelon plants. This time, the assay was done under conditions ideal for Forc infection, with a relatively low ambient temperature of 18–20 °C. All four tested strains (#2, #4, #8, #9) caused abundant symptom development in each of the three plant species, comparable to the control strain, Forc016∆SIX6#46 (Fig. 7; Fig. S8). This shows that the biocontrol strain Fo47 can be transformed into a radicis-cucumerinum strain, capable of infecting multiple host plants and causing root and shoot rot, by a single chromosome of Forc (Fig. S8).
Figure 7.
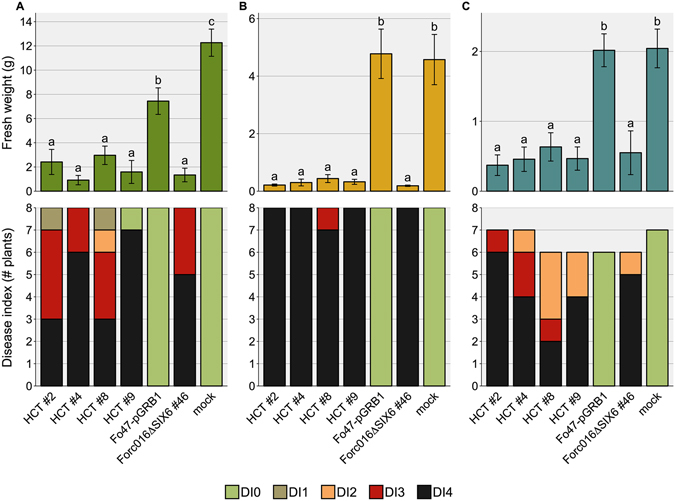
Horizontal transfer of chrRC transforms the non-pathogenic strain Fo47 into a root and shoot rot pathogen of cucurbits. Fresh weight (±S.E.) and disease index (DI) of (A) cucumber, (B) melon and (C) watermelon plants were scored 14 days post inoculation. An ANOVA followed by a Tukey HSD test (p < 0.05) was performed to determine the significance of differences in the fresh weight measurements (significance categories shown with letters above the bars). Under the tested conditions (107 sp/ml, 20 °C), HCT strains #2, #4, #8 and #9 caused a similar level of disease severity in all three cucurbit species as the chrRC donor strain (Forc016∆SIX6#46).
ChrRC and the two smaller accessory chromosomes are conditionally dispensable
Incubation of the Forc016∆SIX9 strain (harboring the HPH hygromycin resistance gene on chrRC) in medium containing 12.5 µg/ml benomyl yielded five strains that had become hygromycin sensitive, indicating a loss of the genomic region containing the HPH gene. Electrophoretic karyotyping showed that in all five cases, chrRC had been completely lost (Fig. 8, Fig. S9). Chromosome loss strain #2 had additionally lost the two smaller accessory chromosomes of ~1.1 and 1.6 Mb. When tested in a bioassay, none of these strains caused symptoms in any of the three tested host plants (Fig. 9, Fig. S10). No growth rate alteration compared to their parent strain was found when the strains were grown on CDA or PDA medium. This demonstrates that chrRC is required for pathogenicity of Forc016 towards multiple cucurbit species.
Figure 8.
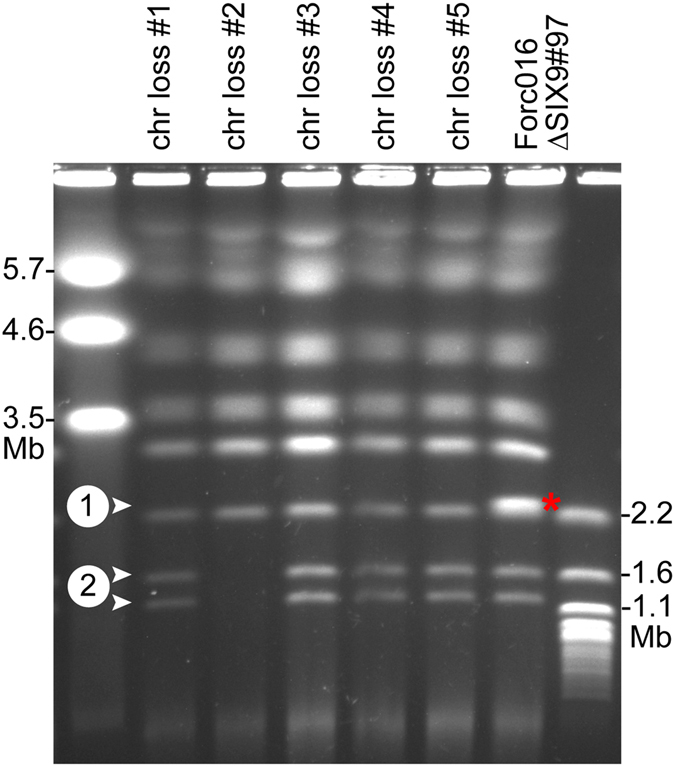
ChrRC and the two smaller accessory chromosomes of Forc016 are conditionally dispensable. Electrophoretic karyotyping shows complete absence of chrRC (marked with a red asterisk in lane 6) in all five hygromycin sensitive strains (arrowhead 1, lanes 1–5). Additionally, chromosome loss strain #2 displays absence of the two smallest chromosomes (arrowheads 2). The left and right lanes show the karyotypes of S. pombe and S. cerevisiae, respectively, applied as markers. This image is cropped, the original gel photograph can be found in Supplementary Fig. S13.
Figure 9.
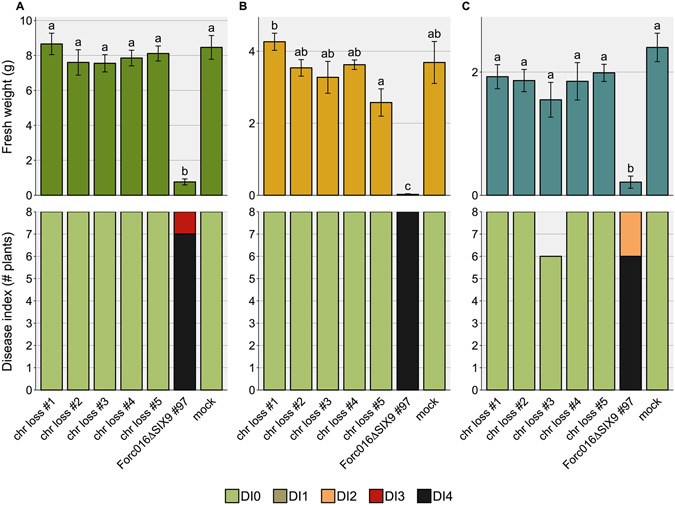
Forc016 strains without chrRC are completely avirulent. Fresh weight (±S.E.) and disease index (DI) of (A) cucumber, (B) melon and (C) watermelon plants were scored 14 days post inoculation. An ANOVA followed by a Tukey HSD test (p < 0.05) was performed to determine the significance of differences in the fresh weight measurements (significance categories shown with letters above the bars). Under the tested conditions (107 spores/ml, 20 °C), none of the chromosome loss strains were able to cause disease symptoms in cucurbit plants, while their parent strain (Forc016∆SIX9#97) was.
Discussion
We show that Forc016 has 11 core chromosomes, one horizontally transferrable 2.446 Mb pathogenicity chromosome (chrRC) and two smaller accessory chromosomes. We conclude that chrRC is necessary and sufficient for the root- and shoot-rot phenotype in several cucurbit species when infected by Forc. More specifically, the middle region of chrRC, which differs from Fom001’s homologous chromosome, might play a decisive role in both the extended host range and rotting symptoms caused by Forc.
Are effectors important for Forc pathogenicity?
Since the identification of SIX1, the first effector gene from F. oxysporum f.sp. lycopersici 26, thirteen more SIX genes have been described10, 27. For several of these, including SIX6, a role in virulence has been shown12, 26, 28–31. SIX6 homologs have been found in Fo f.sp. lycopersici, cucumerinum, radicis-cucumerinum, melonis, niveum, pisi, passiflorae, cubense and vasinfectum 9, 32–34, as well as in Fo f.sp. momordicae, luffae, Fusarium hostae 35 and in Colletotrichum spp.36. Strains belonging to the same forma specialis typically have the same sequence types for effector genes, even when core genes are not identical in sequence9. This is likely the result of horizontal inheritance of dispensable genomic regions34 and is corroborated by the incongruent phylogeny of SIX genes compared to the housekeeping gene EF1α reported by Rocha et al.37. Interestingly, Forc shares its SIX6 sequence type with strains belonging to Fo f.sp. melonis (Fom) as well as some – but not all – Fo f. sp. niveum (Fon) strains9. This may be indicative of a (partially) shared ancestry of cucurbit-infection between these formae speciales.
Deletion of SIX6 in Fol marginally compromises virulence in the Fol-tomato pathosystem. Additionally, Six6 suppresses I-2-mediated cell death upon transient expression in N. benthamiana, but does not compromise the activity of other cell-death-inducing genes12. Three individual Fol strains with a partial deletion of chromosome 14, thereby losing SIX6, SIX9 and SIX11, as well as ORX1 encoding an in xylem-secreted oxidoreductase, did not show a significant reduction in disease severity, indicating that these genes are largely dispensable for Fol pathogenicity14. In the Fon-watermelon pathosystem, however, Six6 has been reported to be involved in virulence38.
We find a clear reduction in virulence of three independent Forc strains in which the SIX6 locus was disrupted (Fig. 4). However, this phenotype could only be observed at relatively high ambient temperatures (25 °C) in cucumber, while at lower temperatures all plants died. In contrast to most wilt-causing Fo pathogens like Fol, Foc, Fom and Fon, Forc symptoms develop most efficiently at temperatures below 20 °C6, 39, 40, particularly during seedling infection, when plants may be under physiological stress6. We conclude that Six6 contributes to virulence only under non-optimal conditions and only in cucumber.
The other tested effector candidate knockout strains (ΔSIX9 and ΔSMP1) did not display a reduction in virulence towards cucumber, melon or watermelon compared to Forc-wt and ectopic transformant strains. Six9 and Smp1 are therefore, by themselves, not important for disease development caused by Forc.
HCT of chrRC contributes to genome evolution in Fo
HCT has so far been described for Fol chromosomes 7, 8, 14 and the smallest chromosome of Fol00715, 16, but was until now not shown for other formae speciales of Fo. Non-pathogenic recipient strain Fo47 became pathogenic towards tomato upon receiving Fol chromosome 14, albeit less than the Fol donor strain. A higher aggressiveness of HCT-strains was observed when another accessory chromosome co-migrated, potentially due to the influence of transcription factors located on that chromosome19. Interestingly, two copies of FTF1, a transcription factor associated with effector gene expression19, 20, 41, are located in the effector-rich central part of chrRC (Fig. 3), potentially indicating a partial transcriptional autonomy of chrRC.
Horizontal transfer of chrRC was accomplished with Forc016∆SIX6 as a donor and Fo47 as a recipient strain. Nine double-drug resistant colonies were recovered after co-cultivation and electrophoretic karyotyping of these HCT strains (Fig. 5) showed that six strains gained chrRC while three strains (#1, #3, #8) had undergone chromosome rearrangements. HCT strain #8 had a double relative coverage of chrRC compared to that of the other strains and a band at twice the size of chrRC (~5 Mb, Fig. 5) in its electrophoretic karyotype. The chromosome apparently duplicated but remained present as a single entity, pointing to a high level of genome plasticity.
A recent study by Vlaardingerbroek et al.16 also showed chromosomal plasticity in horizontal transfer experiments of the Fol pathogenicity chromosome. Transformation for marker insertion on this chromosome resulted in a larger (estimated 250 kb) pathogenicity chromosome in a donor strain that was used for HCT towards Fo47. Selection for loss of this chromosome in another study14 resulted in several strains that only partially lost the chromosome. Interestingly, deletions within a chromosome and chromosomal breaks appeared to happen non-randomly at so-called ‘deletion hotspots’. Whether something similar happened to HCT strains #1 and #3 in this study remains to be seen. It is clear that genomes of F. oxysporum, particularly the accessory parts defining host virulence, are highly plastic. This could result in accelerated genetic diversification, possibly facilitating adaptation to new environments including new host plants.
In Forc, two vegetative compatibility groups (VCGs) have been described: VCG0260 (to which Forc016 belongs) and VCG026142. RAPD fingerprinting analyses and concatenated sequence alignment of 1195 conserved core genes showed that the two VCGs are very similar and appear to be clonally related9, 39. The other two sequenced Forc strains, Forc031 (VCG0261) and Forc024 (VCG0260), both possess the chrRC sequence and their effector gene content is nearly identical9. Interestingly, large parts of chrRC were also identified in two out of three sequenced Fom VCGs, including Fom001 (VCG0136, Fig. 3), whose core genome is highly similar to that of the Fol4287 reference strain and other Fol strains in VCG00309. This is a strong indication that the Forc and Fom pathogenicity chromosomes evolved from a shared ancestor. Integration of the highly diverse central region in an ancestral chromosome from an unknown source potentially gave rise to chrRC and the new forma specialis radicis-cucumerinum. The suite of candidate effector genes found in Forc, concentrated in the central region of chrRC (Fig. 3), is most similar to that of strains belonging to Fo f.sp. cucumerinum 9. Systematic comparative and functional analysis of the accessory genomic regions of multiple cucurbit-infecting formae speciales will be necessary to reconstruct the evolutionary paths that led to host-specificity of Fo towards this plant family.
The wider host range of Forc compared to Fom could be caused by the absence of avirulence genes. SIX1 has been reported as an avirulence gene in the Fol-tomato interaction (AVR3) and could potentially be recognized by cucumber and watermelon, triggering a defence response by these plants upon colonization by Fom. This is the only SIX gene homolog that is consistently present in the Fom genome but is not found in Forc.
Assembly of highly repetitive genomes benefits from long-read sequencing technology
Repetitive regions, including centromeres of Fusarium, are difficult to assemble using short-read sequencing technologies such as Illumina. F. oxysporum’s compartmentalized genome is a good example of a genome that can benefit greatly from longer read sequencing techniques, such as the PacBio SMRT sequencing technology employed here (median read length 15 kb) as well as the development of novel technologies such as Oxford Nanopore sequencing43. Manual curation of the assembly improved it to the point where five chromosomes are complete (telomere-to-telomere), fifteen contigs have telomeric repeats on only one end and 20 contigs are left with no telomeric repeats on either end. The estimated chromosome count of Forc016 is 14, which is most clearly visible in Fig. 6 where the separation in the 1–1.5 Mb region shows that Forc016 possesses two small accessory chromosomes. Considering their size and accessory-like appearance (high TE and low gene content), these possibly correspond to contig 53 and the non-conserved region that is probably erroneously attached to chromosome 11 in the assembly. This is supported by the read mapping of chromosome loss strain #2, where no coverage was found for the mentioned two regions, as well as contig 3 and several of the smaller unplaced contigs (Fig. S9B). This strain lost these two chromosomes in addition to chrRC, showing that they are conditionally dispensable. Moreover, the pathogenicity of the chrRC chromosome transfer strains demonstrates that the two smallest Forc016 chromosomes are not required for pathogenicity. Comparison of the read coverage from chromosome loss strain #2 to wildtype will allow us to see which contigs belong to these chromosomes.
Even though PacBio SMRT sequencing is a great improvement to short-read technologies, it does not as yet allow for completely closed assemblies for F. oxysporum if not combined with other techniques like optical mapping44, 45. Nevertheless, the assembly of the core chromosomes as well as the pathogenicity chromosome of Forc016 were of sufficient quality to answer the biological questions addressed here.
Conclusions
We report here the near-complete genome assembly of Fo f.sp. radicis-cucumerinum strain Forc016 and horizontal transfer of its pathogenicity chromosome, chrRC, to the non-pathogenic strain Fo47. This is the first time HCT has been accomplished using a donor strain from a forma specialis other than lycopersici. The virulence of the progeny strains deriving from this experiment is identical to that of the Forc chromosome donor, indicating that chrRC is sufficient for root and shoot rot disease development. Complete loss of virulence of the five strains that lost chrRC shows that chrRC is also required for pathogenicity of Forc016. The experimental evidence presented here provides compelling confirmation that horizontal transfer of genetic material plays a crucial role in the adaptation to new host ranges of pathogenic isolates within the F. oxysporum species complex.
Methods
Fungal strains
F. oxysporum strains Forc016 (‘33’; CBS141123)9, 46 and Fom001 (NRRL26406)47 were sequenced with SMRT sequencing technology. Fo47pGRB116 was used as a chromosome recipient in HCT experiments.
Cloning
pPDh was constructed by introducing a KpnI-KpnI fragment containing a multiple cloning site (MCS) and the eGFP coding sequence followed by the SIX1 terminator sequence, amplified from pPZP200-pSIX1GFP48, into the binary vector pRW2h49. Additionally, a HindIII-HindIII fragment containing a MCS and the Herpes Simplex Virus thymidine kinase (HSVtk) gene under the control of the C. heterostrophus glyceraldehyde-3-phosphate dehydrogenase (ChGPD) gene promoter and the N. crassa β-tubulin gene terminator was inserted into the vector as a conditional negative selection marker against ectopic transformants50.
For knockout constructs, two ~1 kb fragments flanking the gene of interest were amplified using the primers listed in Table S2. The fragments were digested with PacI-SpeI and AscI-Sbf1 (SIX6; SMP1) or PacI-SpeI and AscI-BstEII (SIX9) and subsequently inserted on either side of the GFP / HPH cassette of pPDh.
Forc gene knockout
F. oxysporum strain Forc016 was transformed by Agrobacterium mediated transformation as described previously51. Following monosporing of hygromycin-resistant colonies, the transformants were grown in 96-well plates containing in each well 150 µl PDB supplemented with hygromycin and 5 µM 5-Fluoro-2-deoxyuridine (Alfa-Aesar) for pre-selection of in locus transformation50. Successful knockout of the genes was confirmed by PCR, using primers inside the T-DNA and outside the 1 kb flanking region.
Disease assays
Pathogenicity tests were performed using the root dip method52. In short, conidia were isolated from five-day-old cultures NO3-medium (0.17% yeast nitrogen base, 3% sucrose, 100 mM KNO3) by filtering through miracloth (Merck; pore size of 22–25 μm). Spores were centrifuged, resuspended in sterile MilliQ water, counted and brought to a final concentration of 106 (effector KO assay) or 107 spores/mL (chromosome transfer and loss assays). When the first true leaves were emerging (after ±10 days), 6–8 seedlings per treatment were uprooted, inoculated, individually potted and kept at 25 °C (effector KO assay) or 20 °C (HCT assay) in the greenhouse. The following plant cultivars were used: Cucumis sativus cv. Paraiso, Cucumis melo cv. Cha-T, Citrullus lanatus cv. Black Diamond. Two weeks after inoculation, disease was scored using a disease index from 0–4 (0, no symptoms; 1, slight root rot symptoms, only at tip of main root; 2, root rot symptoms and stem lesions visible aboveground; 3, very clear root rot symptoms of the entire root system, often with a large lesion extending above the cotyledons; 4, plant either dead or very small and wilted).
Chromosome transfer and loss
Chromosome transfer from Forc016ΔSIX6#46 to Fo47pGRB16 was performed through co-cultivation of the strains53. 1 × 105 microconidia from each of the two strains were mixed and co-incubated on PDA plates for six days. Newly formed spores were washed from the co-incubation plate using 5 ml sterile MilliQ, filtered through sterile miracloth and pipetted on a double selective PDA plate containing 0.1 M Tris pH 8 supplemented with 100 µg/ml hygromycin (Duchefa) and 100 µg/ml zeocin (InvivoGen). Double drug resistant colonies were selected after six days and monospored by spreading on a fresh plate supplemented with both antibiotics. After two days of growth, single-spore colonies were selected and transferred to fresh plates.
Chromosome loss was induced as previously described54 with some modifications. Forc016∆SIX9#97 was grown on PDA supplemented with hygromycin for 4 to 10 days. A Forc016 ∆SIX9#97 mycelial agar block was incubated in M100 broth54 containing 12.5 µg/ml benomyl (methyl 1-(butylcarbamoyl)-2-benzimidazolecarbamate, Aldrich) for 4 days, 175 rpm at 25 °C. The culture was filtered through sterile miracloth. Conidia were collected by centrifugation and resuspended in 5 ml sterile water. 100 µl of a 100-fold dilution of conidia suspension was spread on M100 plates containing 0.04% Triton X-100 (Sigma). The plates were overlaid with a sterile filter paper and plates and conidia were incubated at 25 °C for 2 days. The paper was transferred from M100 plates to PDA with hygromycin. After 1–2 days, the paper was removed and the colonies surviving only on M100 were selected and transferred to fresh PDA plates for further analysis.
Electrophoretic karyotyping and Southern analysis
Preparation of protoplasts and running of pulsed-field gel elecrophoresis was performed as described previously16, 55. F. oxysporum was cultured in 100 ml NO3 medium for five days. Next, microconidia were collected by filtration through a double layer of sterile miracloth. 5 × 108 spores were transferred to 40 ml PDB (BD Biosciences) and grown for 13 h at 25 °C, followed by incubation at 30 °C for 13–16 h in MgSO4 solution (1.2 M MgSO4, 50 mM sodium citrate, pH 5.8) supplemented with 50 mg/ml Glucanex (Sigma). Protoplasts were filtered through a double layer of miracloth, collected by centrifugation and cast in InCert agarose (Lonza) plugs at a concentration of 1 × 108 protoplasts per ml. Plugs were treated with Pronase E and chromosomes were separated by running for 260 hours in 1% Seakem Gold agarose (Lonza) at 1.5 V/cm in a CHEF-DRII system (Biorad) in 0.5 × TBE at 4 °C with switch times between 1200 and 4800 s. The gels were stained with ethidium bromide and de-stained using 0.5 × TBE.
DNA was blotted to a Hybond N + membrane (Amersham Pharmacia) by alkaline transfer with 0.4 N NaOH. A 793 bp PCR product containing the FolSIX6 open reading frame was generated with primers FP1490 and FP1491 (Table S2). This fragment was radioactively labelled with [α32P]-dATP using the DecaLabel DNA labeling kit (Thermo Scientific). Hybridization was performed overnight at 65 °C in Church and Gilbert buffer containing 0.5 M phosphate, 7% SDS and 1 mM EDTA at pH 7.2. Blots were washed at 65 °C with 0.5 × SSC, 0.1% SDS. The position of chromosomal sequences to which the SIX6 probe hybridized was visualized by phosphoimaging (Storm 840, Molecular Dynamics).
DNA isolation, genome sequencing and assembly
DNA isolation was performed on freeze-dried mycelium ground in liquid nitrogen as starting material, using multiple rounds of phenol-chloroform extraction and precipitation, as well as treatment with RNase A and proteinase K.
SMRT sequencing was performed at Keygene N.V. (Wageningen, the Netherlands). PacBio libraries were prepared and size-selected at ~20 Kb using Blue Pippin prep. Sequencing of 5 SMRT cells was performed using the P6-C4 polymerase-chemistry combination, ≥4 hr movie time, stage start. This resulted in a sum of 4772 Mb (Forc016) and 4846 Mb (Fom001) filtered data. De novo assembly was performed with the Hierarchical Genome Assembly Process v3 (HGAP.3, Pacific Biosciences) within the SMRT Portal environment (v1.87.139483). Default values were kept and the expected genome size was set to 60 Mb.
The raw assembly was manually improved by removing contigs originating from mtDNA and rDNA repeats. Two contigs that ended in telomeric repeats on one end and rDNA repeats on the other were joined together with in total 97 rDNA repeats in between (based on Illumina read mapping and coverage estimation on 10 rDNA repeats). Chromosome 13 could be reconstructed by joining two contigs that showed conserved synteny in Fom001 and the SMRT assembly of F. subglutinans. The two contigs were merged at the position of an overlapping region of 13,396 nt.
The mitochrondrial DNA was assembled from Illumina reads using GRAbB17 by specifying the mitochondrial genome of F. oxysporum F11 as reference and employing SPAdes as assembler. Annotation of the mitogenome was performed as described in Brankovics et al. (submitted) using a combination of MFannot (http://megasun.bch.umontreal.ca/cgi-bin/mfannot/mfannotInterface.pl), tRNAscan-SE56, NCBI ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder), InterPro57 and CD-Search58.
Illumina sequencing (150 bp paired-end, insert size ~450 bp) of HCT strains was performed on a HiSeq. 2500 machine by the Hartwig Medical Foundation (Amsterdam, the Netherlands) at ~100X coverage, resulting in 5.0–5.6 Mb of sequence data per sample.
Genome annotation
Repeats were identified with RepeatMasker v4.0.6 (with -engine ncbi -species “ascomycota”)59. Gene prediction was executed on the repeat-masked genome assembly by running BRAKER1 v1.960, using RNA-seq read mappings (both in vitro and 10 days post inoculation in planta conditions) as additional evidence and supplying the following flags: –fungus –useexisting=“fusarium_graminearum”. Repeats and genes were counted over 50 kb windows along the genome.
InterProScan v5.18–57.0 was used to assign functional annotation (including GO terms) to predicted genes. In order to find overrepresented GO terms on chrRC versus the whole genome, a hypergeometric test was performed on the GO term frequencies using the ‘phyper’ function in R. The p values were adjusted for multiple comparisons using ‘p.adjust’ and selecting the Bonferroni method in R. The results were visualized using REVIGO61.
Read mapping and genome analysis
For coverage plots, reads were trimmed to remove low-quality bases and adapter sequences using fastq-mcf v1.04.807 (-q 20) and mapped against the Forc genome assembly with Bowtie2 v2.2.5 (DNAseq) or Tophat2 v2.1.0 (RNAseq). Optical duplicates were removed using PicardTools MarkDuplicates v2.7.1 and coverage per 10 kb (HCT plots) or 50 kb (circos plots) windows was calculated with the samtools v1.3.1 mpileup command.
Whole genome or chromosome alignments were performed using nucmer (with –maxmatch) from the MUMmer v3.23 package62. Comparison to the Fol4287 reference genome was done against an approximate chromosome-level assembly in which we concatenated scaffolds as assigned to chromosomes in refs 10 and 15, separated by 1000 Ns. We kept accessory regions of chromosomes 1 and 2 as separate sequences (for visualisation in Fig. 2E).
Identification of candidate effectors was done with BLASTN using the list of 104 candidates from van Dam et al.9 as a query fasta. Mimps were identified by searching the genome for a consensus sequence of the mimp inverted repeat (IR), “TT[TA]TTGCNNCCCACTGNN”. If two were found within 400 nt from each other in the correct orientation, they were marked as the ends of an intact mimp element.
Data availability
The Whole-Genome Shotgun projects for the resequenced strains have been deposited at Genbank under the BioProjects PRJNA389503 and PRJNA389439. The genome assemblies can be found on GenBank under accession numbers MABQ01000000 (Forc016 Illumina assembly), MABQ02000000 (Forc016 SMRT assembly) and NJCY01000000 (Fom001 SMRT assembly). Raw SMRT sequence data, Illumina read data of the HCT and chromosome loss strains and RNAseq reads have been deposited into the Sequence Read Archive under the accession number SRP108975. Illumina paired-end read data for Forc016 is available under accession number SRP067515 (DNAseq).
Electronic supplementary material
Acknowledgements
We are grateful to M. Paauw for help in the horizontal chromosome transfer experiments. We wish to thank B. Lievens and D.J. Vakalounakis for providing the Forc016 (‘33’) strain. Furthermore, we are grateful to ENZA seeds for kindly providing cucumber and melon seeds and Rijk Zwaan for kindly providing watermelon seeds. Finally, we would like to thank L. Tikovsky and H. Lemereis for their help in the greenhouse and H.C. van der Does for help with the CHEF gel. This work was supported by the Horizon programme (project 93512007) of the Netherlands Genomics Initiative (NGI) through a grant to M. Rep.
Author Contributions
P.V.D. wrote the main manuscript text. P.V.D., L.F. and B.B. performed sequencing data analysis. P.V.D., Y.A., P.H., M.V.D.G. and A.T.H. performed the experiments. T.A. and M.R. secured funding and helped in text review. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07995-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vakalounakis DJ. Root and Stem Rot of Cucumber Caused by Fusarium oxysporum f.sp. radicis-cucumerinum f.sp. nov. Plant Dis. 1996;80:313–316. doi: 10.1094/PD-80-0313. [DOI] [PubMed] [Google Scholar]
- 2.Vakalounakis DJ, Wang Z, Fragkiadakis GA, Skaracis GN, Li D-B. Isolates Obtained from Cucumber in China by Pathogenicity, VCG, and RAPD. Plant Dis. 2004;88:645–649. doi: 10.1094/PDIS.2004.88.6.645. [DOI] [PubMed] [Google Scholar]
- 3.Cohen R, et al. Differences in the responses of melon accessions to fusarium root and stem rot and their colonization by Fusarium oxysporum f. sp. radicis-cucumerinum. Plant Pathol. 2015;64:655–663. doi: 10.1111/ppa.12286. [DOI] [Google Scholar]
- 4.Karaca G, Kahveci E. First report of Fusarium oxysporum f. sp. radicis-cucumerinum on cucumbers in Turkey. Plant Pathol. 2010;59:1173–1174. doi: 10.1111/j.1365-3059.2009.02242.x. [DOI] [Google Scholar]
- 5.Moreno A, et al. First report of Fusarium oxysporum f. sp. radicis-cucumerinum on cucumber in Spain. Plant Dis. 2001;85:1206–1206. doi: 10.1094/PDIS.2001.85.11.1206A. [DOI] [PubMed] [Google Scholar]
- 6.Punja ZK, Parker M. Development of fusarium root and stem rot, a new disease on greenhouse cucumber in British Columbia, caused by Fusarium oxysporum f. sp. radicis-cucumerinum. Can. J. Plant Pathol. 2000;22:349–363. doi: 10.1080/07060660009500453. [DOI] [Google Scholar]
- 7.Armstrong, G. M. & Armstrong, J. K. In Fusarium: diseases, biology, and taxonomy 391–399 (Pennsylvania State University Press, University Park, PA, 1981).
- 8.Kistler HC, et al. Systematic Numbering of Vegetative Compatibility Groups in the Plant Pathogenic Fungus Fusarium oxysporum. Phytopathology. 1998;88:30–32. doi: 10.1094/PHYTO.1998.88.1.30. [DOI] [PubMed] [Google Scholar]
- 9.van Dam P, et al. Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ. Microbiol. 2016;18:4087–4102. doi: 10.1111/1462-2920.13445. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt SM, et al. MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC Genomics. 2013;14:119. doi: 10.1186/1471-2164-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houterman PM, et al. The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol. Plant Pathol. 2007;8:215–221. doi: 10.1111/j.1364-3703.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 12.Gawehns F, et al. The Fusarium oxysporum Effector Six6 Contributes to Virulence and Suppresses I-2-Mediated Cell Death. Mol. Plant-Microbe Interact. 2014;27:336–348. doi: 10.1094/MPMI-11-13-0330-R. [DOI] [PubMed] [Google Scholar]
- 13.Ma L-J. Horizontal chromosome transfer and rational strategies to manage fusarium vascular wilt diseases. Mol. Plant Pathol. 2014;15:1–8. doi: 10.1111/mpp.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlaardingerbroek, I., Beerens, B., Schmidt, S. M., Cornelissen, B. J. C. & Rep, M. Dispensable chromosomes in Fusarium oxysporum f. sp lycopersici. Mol. Plant Pathol., doi:10.1111/mpp.12440 1–12 (2016). [DOI] [PMC free article] [PubMed]
- 15.Ma L-J, et al. Comparative analysis reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464:367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlaardingerbroek, I. et al. Exchange of core chromosomes and horizontal transfer of lineage-specific chromosomes in Fusarium oxysporum. Environ. Microbiol., doi:10.1111/1462-2920.13281 (2016). [DOI] [PubMed]
- 17.Brankovics B, et al. GRAbB: Selective Assembly of Genomic Regions, a New Niche for Genomic Research. PLoS Comput. Biol. 2016;12:1–9. doi: 10.1371/journal.pcbi.1004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt SM, et al. Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom-2 resistance gene in melon. New Phytol. 2016;209:307–318. doi: 10.1111/nph.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Does HC, et al. Transcription Factors Encoded on Core and Accessory Chromosomes of Fusarium oxysporum Induce Expression of Effector Genes. PLOS Genet. 2016;12:e1006401. doi: 10.1371/journal.pgen.1006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niño-Sánchez, J. et al. The FTF gene family regulates virulence and expression of SIX effectors in Fusarium oxysporum. Mol. Plant Pathol. 1–18, doi:10.1002/ana.23937 (2016). [DOI] [PMC free article] [PubMed]
- 21.Michielse CB, Rep M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009;10:311–324. doi: 10.1111/j.1364-3703.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Pietro A, Madrid MP, Caracuel Z, Delgado-Jarana J, Roncero MIG. Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant Pathol. 2003;4:315–325. doi: 10.1046/j.1364-3703.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 23.Baayen RP, Schoffelmeer EAM, Toet S, Elgersma DM. Fungal polygalacturonase activity reflects susceptibility of carnation cultivars to fusarium wilt. Eur. J. Plant Pathol. 1997;103:15–23. doi: 10.1023/A:1008616008804. [DOI] [Google Scholar]
- 24.Caprari C, et al. Mutagenesis of endopolygalacturonase from Fusarium moniliforme: histidine residue 234 is critical for enzymatic and macerating activities and not for binding to polygalacturonase-inhibiting protein (PGIP) Mol. Plant. Microbe. Interact. 1996;9:617–624. doi: 10.1094/MPMI-9-0617. [DOI] [PubMed] [Google Scholar]
- 25.Reignault P, Valette-Collet O, Boccara M. The importance of fungal pectinolytic enzymes in plant invasion, host adaptability and symptom type. Eur. J. Plant Pathol. 2008;120:1–11. doi: 10.1007/s10658-007-9184-y. [DOI] [Google Scholar]
- 26.Rep M, et al. A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 2004;53:1373–1383. doi: 10.1111/j.1365-2958.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 27.Takken F, Rep M. The arms race between tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010;11:309–314. doi: 10.1111/j.1364-3703.2009.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houterman PM, et al. The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. Plant J. 2009;58:970–978. doi: 10.1111/j.1365-313X.2009.03838.x. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Cornelissen BJC, Takken FLW. A nuclear localization for Avr2 from Fusarium oxysporum is required to activate the tomato resistance protein I-2. Front. Plant Sci. 2013;4:94. doi: 10.3389/fpls.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thatcher LF, Gardiner DM, Kazan K, Manners JM. A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol. Plant. Microbe. Interact. 2012;25:180–90. doi: 10.1094/MPMI-08-11-0212. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, et al. The AVR2-SIX5 gene pair is required to activate I-2-mediated immunity in tomato. New Phytol. 2015;208:507–518. doi: 10.1111/nph.13455. [DOI] [PubMed] [Google Scholar]
- 32.Chakrabarti A, et al. Variation in potential effector genes distinguishing Australian and non-Australian isolates of the cotton wilt pathogen Fusarium oxysporum f.sp. vasinfectum. Plant Pathol. 2011;60:232–243. doi: 10.1111/j.1365-3059.2010.02363.x. [DOI] [Google Scholar]
- 33.Guo L, et al. Genome and Transcriptome Analysis of the Fungal Pathogen Fusarium oxysporum f. sp. cubense Causing Banana Vascular Wilt Disease. PLoS One. 2014;9:e95543. doi: 10.1371/journal.pone.0095543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams AH, et al. Comparative genomics and prediction of conditionally dispensable sequences in legume–infecting Fusarium oxysporum formae speciales facilitates identification of candidate effectors. BMC Genomics. 2016;17:191. doi: 10.1186/s12864-016-2486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dam, P. & Rep, M. The Distribution of Miniature Impala Elements and SIX Genes in the Fusarium Genus is Suggestive of Horizontal Gene Transfer. J. Mol. Evol. doi:10.1007/s00239-017-9801-0 (2017). [DOI] [PMC free article] [PubMed]
- 36.Kleemann J, et al. Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012;8:e1002643. doi: 10.1371/journal.ppat.1002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha, L. O. et al. Putative effector genes detected in Fusarium oxysporum from natural ecosystems of Australia. Plant Pathol. 65, 914–929, doi:10.1111/ppa.12472 (2015).
- 38.Niu X, et al. The FonSIX6 gene acts as an avirulence effector in the Fusarium oxysporum f. sp. niveum - watermelon pathosystem. Sci. Rep. 2016;6:28146. doi: 10.1038/srep28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vakalounakis DJ, Fragkiadakis GA. Genetic Diversity of Fusarium oxysporum Isolates from Cucumber: Differentiation by Pathogenicity, Vegetative Compatibility, and RAPD Fingerprinting. Phytopathology. 1999;89:161–168. doi: 10.1094/PHYTO.1999.89.2.161. [DOI] [PubMed] [Google Scholar]
- 40.Tok FM, Kurt Ş. Pathogenicity, vegetative compatibility and amplified fragment length polymorphism (AFLP) analysis of Fusarium oxysporum f. sp. radicis-cucumerinum isolates from Turkish greenhouses. Phytoparasitica. 2010;38:253–260. doi: 10.1007/s12600-010-0099-3. [DOI] [Google Scholar]
- 41.Ramos B, et al. The gene coding for a new transcription factor (ftf1) of Fusarium oxysporum is only expressed during infection of common bean. Fungal Genet. Biol. 2007;44:864–876. doi: 10.1016/j.fgb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Katan T. Current status of vegetative compatibility groups in Fusarium oxysporum. Phytoparasitica. 1999;27:51–64. doi: 10.1007/BF02980727. [DOI] [Google Scholar]
- 43.Datema, E. et al. The megabase-sized fungal genome of Rhizoctonia solani assembled from nanopore reads only. bioRxiv, doi:10.1101/084772 (2016).
- 44.Van Kan, J. A. L. et al. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 1–15, doi:10.1111/mpp.12384 (2016). [DOI] [PMC free article] [PubMed]
- 45.Faino L, et al. Single-Molecule Real-Time Sequencing Combined with Optical Mapping Yields Completely Finished Fungal Genome. MBio. 2015;6:e00936–15. doi: 10.1128/mBio.00936-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lievens B, Claes L, Vakalounakis DJ, Vanachter ACRC, Thomma BPHJ. A robust identification and detection assay to discriminate the cucumber pathogens Fusarium oxysporum f. sp. cucumerinum and f. sp. radicis-cucumerinum. Environ. Microbiol. 2007;9:2145–2161. doi: 10.1111/j.1462-2920.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 47.Ma L-J, Shea T, Young S, Zeng Q, Kistler HC. Genome sequence of Fusarium oxysporum f. sp. melonis strain NRRL 26406, a fungus causing wilt disease on melon. Genome Announc. 2014;2:e00730–14. doi: 10.1128/genomeA.00730-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Does HC, et al. Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genet. Biol. 2008;45:1257–1264. doi: 10.1016/j.fgb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Houterman PM, Cornelissen BJC, Rep M. Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 2008;4:e1000061. doi: 10.1371/journal.ppat.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khang CH, Park S-Y, Lee Y-H, Kang S. A dual selection based, targeted gene replacement tool for Magnaporthe grisea and Fusarium oxysporum. Fungal Genet. Biol. 2005;42:483–492. doi: 10.1016/j.fgb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Takken FLW, et al. A one-step method to convert vectors into binary vectors suited for Agrobacterium-mediated transformation. Curr. Genet. 2004;45:242–248. doi: 10.1007/s00294-003-0481-5. [DOI] [PubMed] [Google Scholar]
- 52.Wellman FL. A technique for studying host resistance and pathogenicity in tomato Fusarium wilt. Phytopathology. 1939;29:945–956. [Google Scholar]
- 53.van der Does, H. C. & Rep, M. Horizontal transfer of supernumerary chromosomes in fungi. Plant Fungal Pathog. Methods Protoc. 427–437, doi:10.1007/978-1-61779-501-5_26 (2012). [DOI] [PubMed]
- 54.VanEtten H, Jorgensen S, Enkerli J, Covert SF. Inducing the loss of conditionally dispensable chromosomes in Nectria haematococca during vegetative growth. Curr. Genet. 1998;33:299–303. doi: 10.1007/s002940050340. [DOI] [PubMed] [Google Scholar]
- 55.Teunissen HAS, Verkooijen J, Cornelissen BJC, Haring MA. Genetic exchange of avirulence determinants and extensive karyotype rearrangements in parasexual recombinants of Fusarium oxysporum. Mol. Genet. Genomics. 2002;268:298–310. doi: 10.1007/s00438-002-0747-5. [DOI] [PubMed] [Google Scholar]
- 56.Lowe TM, Eddy SR. TRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1996;25:955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell A, et al. The InterPro protein families database: The classification resource after 15 years. Nucleic Acids Res. 2015;43:D213–D221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchler-Bauer A, Bryant SH. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004;32:327–331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smit, A. F. A., Hubley, R. & Green, P. RepeatMasker Open-4.0. 2013-2015. Inst. Syst. Biol. (2015).
- 60.Hoff, K. J., Lange, S., Lomsadze, A., Borodovsky, M. & Stanke, M. BRAKER1: Unsupervised RNA-Seq-Based Genome Annotation with GeneMark-ET and AUGUSTUS. Bioinformatics btv661 (2015). [DOI] [PMC free article] [PubMed]
- 61.Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 62.Delcher AL, Phillippy A, Carlton J, Salzberg SL. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 2002;30:2478–2483. doi: 10.1093/nar/30.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Whole-Genome Shotgun projects for the resequenced strains have been deposited at Genbank under the BioProjects PRJNA389503 and PRJNA389439. The genome assemblies can be found on GenBank under accession numbers MABQ01000000 (Forc016 Illumina assembly), MABQ02000000 (Forc016 SMRT assembly) and NJCY01000000 (Fom001 SMRT assembly). Raw SMRT sequence data, Illumina read data of the HCT and chromosome loss strains and RNAseq reads have been deposited into the Sequence Read Archive under the accession number SRP108975. Illumina paired-end read data for Forc016 is available under accession number SRP067515 (DNAseq).


