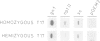Abstract
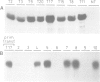
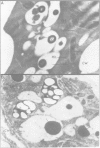
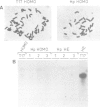
A chimeric construct containing the Nicotiana plumbaginifolia beta-1,3-glucanase gn1 gene was introduced into Nicotiana tabacum SR1 to produce high levels of the enzyme constitutively. We determined that the GN1 protein represents a basic beta-1,3-glucanase isoform which accumulates into the vacuoles of the transgenic plants. Analysis of the progeny of the transgenic plant with the highest levels of gn1 expression revealed an unexpected phenomenon of gene suppression. Plants hemizygous for the T-DNA locus contained high levels of gn1 mRNA and exhibited a 14-fold higher beta-1,3-glucanase activity than untransformed plants. However, the expression of gn1 was completely suppressed in the homozygous plants: no corresponding mRNA or protein could be detected. This suppression mechanism occurs at a post-transcriptional level and is under developmental control. In addition, by generating haploid plants we found that this silencing phenomenon is not dependent on allelic interaction between T-DNA copies present at the same locus of homologous chromosomes, but rather is correlated with the transgene dose in the plant genome. We postulate that high doses of GN1 protein relative to the level(s) of other still unknown plant products could trigger the cellular processes directed to suppress gn1 expression.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulcke M. V., Bauw G., Castresana C., Van Montagu M., Vandekerckhove J. Characterization of vacuolar and extracellular beta(1,3)-glucanases of tobacco: Evidence for a strictly compartmentalized plant defense system. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2673–2677. doi: 10.1073/pnas.86.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Castresana C., de Carvalho F., Gheysen G., Habets M., Inzé D., Van Montagu M. Tissue-specific and pathogen-induced regulation of a Nicotiana plumbaginifolia beta-1,3-glucanase gene. Plant Cell. 1990 Dec;2(12):1131–1143. doi: 10.1105/tpc.2.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté F., Cutt J. R., Asselin A., Klessig D. F. Pathogenesis-related acidic beta-1,3-glucanase genes of tobacco are regulated by both stress and developmental signals. Mol Plant Microbe Interact. 1991 Mar-Apr;4(2):173–181. doi: 10.1094/mpmi-4-173. [DOI] [PubMed] [Google Scholar]
- De Block M., Herrera-Estrella L., Van Montagu M., Schell J., Zambryski P. Expression of foreign genes in regenerated plants and in their progeny. EMBO J. 1984 Aug;3(8):1681–1689. doi: 10.1002/j.1460-2075.1984.tb02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq A., Vandewiele M., De Rycke R., Van Damme J., Van Montagu M., Krebbers E., Vandekerckhove J. Expression and Processing of an Arabidopsis 2S Albumin in Transgenic Tobacco. Plant Physiol. 1990 Apr;92(4):899–907. doi: 10.1104/pp.92.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loose M., Alliotte T., Gheysen G., Genetello C., Gielen J., Soetaert P., Van Montagu M., Inzé D. Primary structure of a hormonally regulated beta-glucanase of Nicotiana plumbaginifolia. Gene. 1988 Oct 15;70(1):13–23. doi: 10.1016/0378-1119(88)90100-x. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind Y., Edwards R., Mavandad M., Hedrick S. A., Ribak O., Dixon R. A., Lamb C. J. Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9057–9061. doi: 10.1073/pnas.87.22.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi S., Martin C., Vallée J. C. Hypersensibilité aux virus, température et protéines soubles chez le Nicotiana Xanthi n.c. Apparition de nouvelles macromolécules lors de la répression de la synthèse virale. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 11;270(19):2383–2386. [PubMed] [Google Scholar]
- Goring D. R., Thomson L., Rothstein S. J. Transformation of a partial nopaline synthase gene into tobacco suppresses the expression of a resident wild-type gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1770–1774. doi: 10.1073/pnas.88.5.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. Altered gene expression in plants due to trans interactions between homologous genes. Trends Biotechnol. 1990 Dec;8(12):340–344. doi: 10.1016/0167-7799(90)90220-r. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotan T., Ori N., Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell. 1989 Sep;1(9):881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M. A., Matzke A. J. Differential inactivation and methylation of a transgene in plants by two suppressor loci containing homologous sequences. Plant Mol Biol. 1991 May;16(5):821–830. doi: 10.1007/BF00015074. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Primig M., Trnovsky J., Matzke A. J. Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J. 1989 Mar;8(3):643–649. doi: 10.1002/j.1460-2075.1989.tb03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memelink J., Linthorst H. J., Schilperoort R. A., Hoge J. H. Tobacco genes encoding acidic and basic isoforms of pathogenesis-related proteins display different expression patterns. Plant Mol Biol. 1990 Feb;14(2):119–126. doi: 10.1007/BF00018553. [DOI] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M., Ahl-Goy P., Hinz U., Flores S., Meins F., Jr High-level expression of a tobacco chitinase gene in Nicotiana sylvestris. Susceptibility of transgenic plants to Cercospora nicotianae infection. Plant Mol Biol. 1991 Jan;16(1):141–151. doi: 10.1007/BF00017924. [DOI] [PubMed] [Google Scholar]
- Nitsch J. P., Nitsch C. Haploid plants from pollen grains. Science. 1969 Jan 3;163(3862):85–87. doi: 10.1126/science.163.3862.85. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pan S. Q., Ye X. S., Kuć J. Direct detection of beta-1,3-glucanase isozymes on polyacrylamide electrophoresis and isoelectrofocusing gels. Anal Biochem. 1989 Oct;182(1):136–140. doi: 10.1016/0003-2697(89)90730-6. [DOI] [PubMed] [Google Scholar]
- Potrykus I., Paszkowski J., Saul M. W., Petruska J., Shillito R. D. Molecular and general genetics of a hybrid foreign gene introduced into tobacco by direct gene transfer. Mol Gen Genet. 1985;199(2):169–177. doi: 10.1007/BF00330255. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Watson C. F., Bird C. R., Ray J., Schuch W., Grierson D. Expression of a truncated tomato polygalacturonase gene inhibits expression of the endogenous gene in transgenic plants. Mol Gen Genet. 1990 Dec;224(3):477–481. doi: 10.1007/BF00262443. [DOI] [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C., van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. "Samsun" and "Samsun NN". II. Changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970 Feb;40(2):190–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mur L. A., Beld M., Mol J. N., Stuitje A. R. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990 Apr;2(4):291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]