Abstract
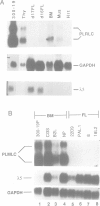
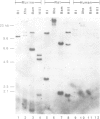
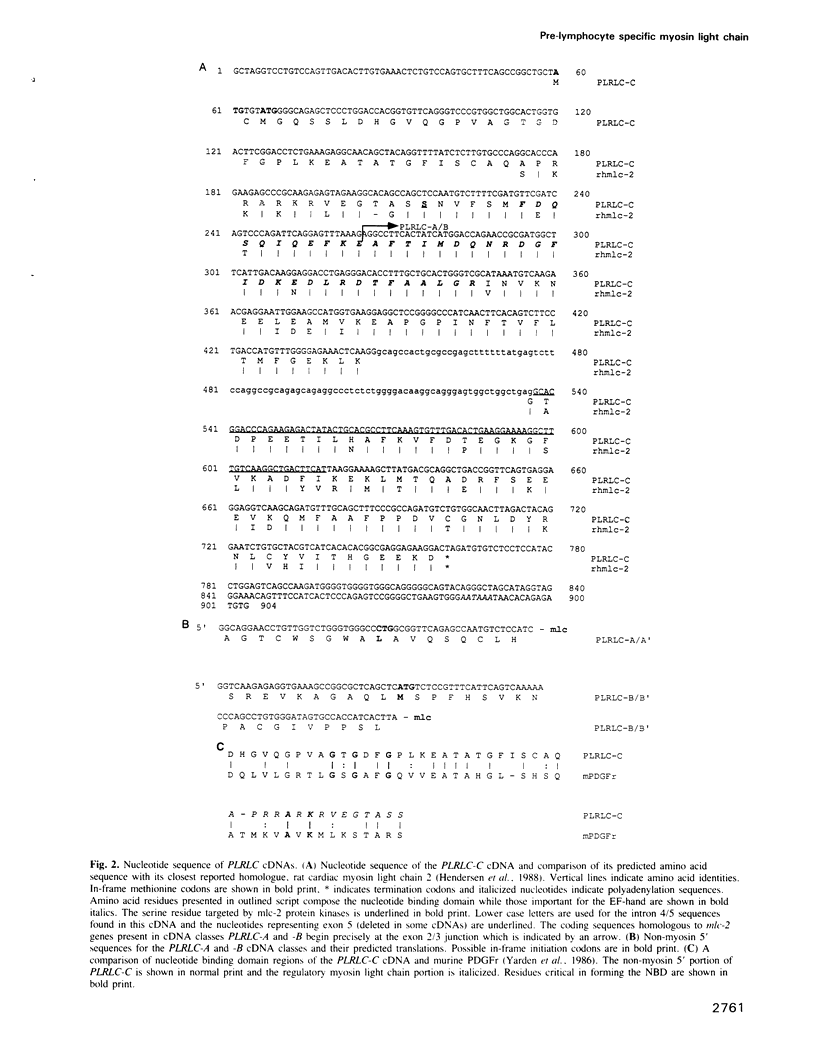
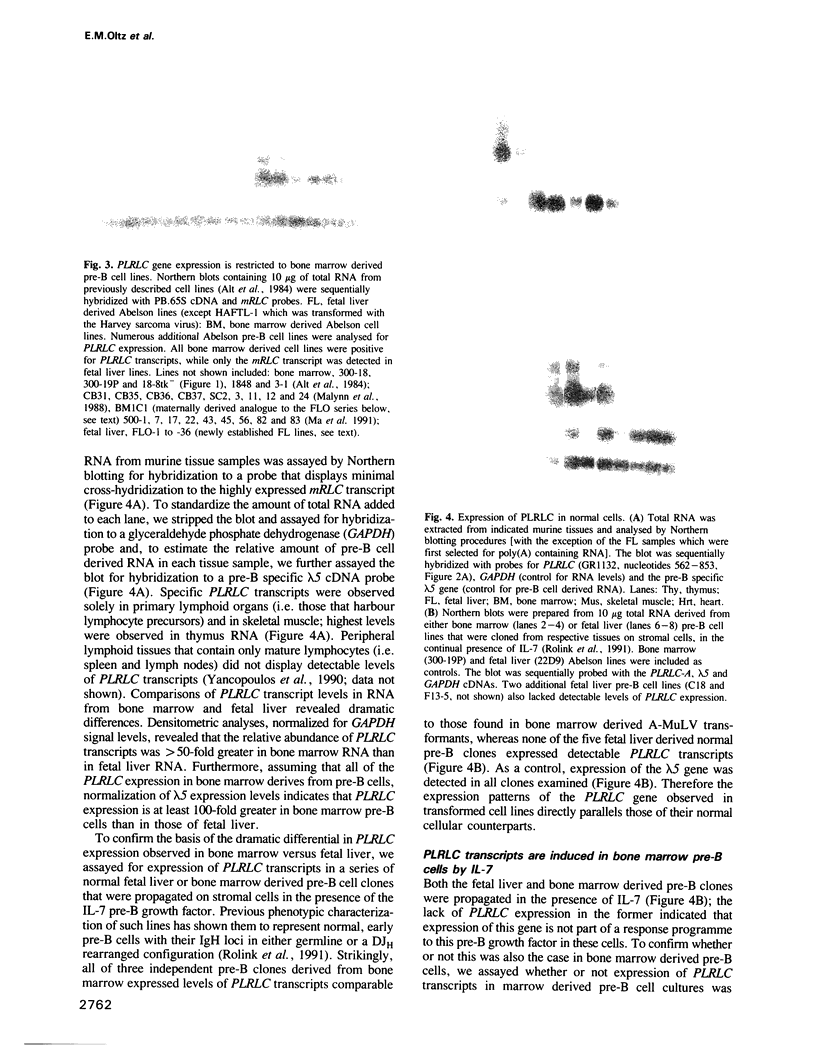
We describe a novel regulatory myosin light chain gene (termed precursor lymphocyte-specific regulatory light chain or PLRLC) that is expressed specifically in precursor B and T lymphocytes. PLRLC is the first example of a regulatory myosin light chain gene which displays specific expression in non-muscle cells. PLRLC is expressed in adult bone marrow derived normal and transformed pre-B cells; in the former, PLRLC expression levels are induced by the pre-B cell specific growth factor interleukin-7 (IL-7). PLRLC is not expressed in either transformed pre-B cells derived from fetal liver or in normal fetal liver pre-B clones grown in the presence of IL-7. Therefore this gene provides the first marker that clearly distinguishes these two pre-B subsets. Finally, several of the different PLRLC transcripts potentially encode regulatory myosin light chains with unique structural features. The unique distribution, regulation and structural features of the PLRLC gene products suggest an important role for PLRLC during lymphocyte development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernards A., de la Monte S. M. The ltk receptor tyrosine kinase is expressed in pre-B lymphocytes and cerebral neurons and uses a non-AUG translational initiator. EMBO J. 1990 Jul;9(7):2279–2287. doi: 10.1002/j.1460-2075.1990.tb07399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Bourguignon G. J. Capping and the cytoskeleton. Int Rev Cytol. 1984;87:195–224. doi: 10.1016/s0074-7696(08)62443-2. [DOI] [PubMed] [Google Scholar]
- Chazen G. D., Pereira G. M., LeGros G., Gillis S., Shevach E. M. Interleukin 7 is a T-cell growth factor. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5923–5927. doi: 10.1073/pnas.86.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechheimer M., Cebra J. J. Phosphorylation of lymphocyte myosin catalyzed in vitro and in intact cells. J Cell Biol. 1982 May;93(2):261–268. doi: 10.1083/jcb.93.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier P., Krippl B., Blackwell T. K., Furley A. J., Suh H., Winoto A., Cook W. D., Hood L., Costantini F., Alt F. W. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990 Jan;9(1):117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F., Rodgers M. E. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Henderson S. A., Xu Y. C., Chien K. R. Nucleotide sequence of full length cDNAs encoding rat cardiac myosin light chain-2. Nucleic Acids Res. 1988 May 25;16(10):4722–4722. doi: 10.1093/nar/16.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. L., Pierce J. H., Davidson W. F., Morse H. C., 3rd Murine hematopoietic cells with pre-B or pre-B/myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986 Aug 1;164(2):443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M., Koretz J., Hartshorne D. J. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988 May 5;263(13):6432–6437. [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Gee C. E., Alt F. W. Activated expression of the N-myc gene in human neuroblastomas and related tumors. Science. 1984 Dec 14;226(4680):1335–1337. doi: 10.1126/science.6505694. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Kanda N., Schreck R. R., Bruns G., Latt S. A., Gilbert F., Alt F. W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983 Dec;35(2 Pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Legouy E., DePinho R. A., Nisen P. D., Smith R. K., Gee C. E., Alt F. W. Human N-myc is closely related in organization and nucleotide sequence to c-myc. Nature. 1986 Jan 2;319(6048):73–77. doi: 10.1038/319073a0. [DOI] [PubMed] [Google Scholar]
- Lee G., Namen A. E., Gillis S., Ellingsworth L. R., Kincade P. W. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989 Jun 1;142(11):3875–3883. [PubMed] [Google Scholar]
- Lenz S., Lohse P., Seidel U., Arnold H. H. The alkali light chains of human smooth and nonmuscle myosins are encoded by a single gene. Tissue-specific expression by alternative splicing pathways. J Biol Chem. 1989 May 25;264(15):9009–9015. [PubMed] [Google Scholar]
- Ma A., Smith R. K., Tesfaye A., Achacoso P., Dildrop R., Rosenberg N., Alt F. W. Mechanism of endogenous myc gene down-regulation in E mu-N-myc tumors. Mol Cell Biol. 1991 Jan;11(1):440–444. doi: 10.1128/mcb.11.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Morrow M. A., Lee G., Gillis S., Yancopoulos G. D., Alt F. W. Interleukin-7 induces N-myc and c-myc expression in normal precursor B lymphocytes. Genes Dev. 1992 Jan;6(1):61–70. doi: 10.1101/gad.6.1.61. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Calvo J. M., Shani M., Levy Z. The nucleotide sequence of a rat myosin light chain 2 gene. Nucleic Acids Res. 1984 Sep 25;12(18):7175–7186. doi: 10.1093/nar/12.18.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D. J., Schmierer A. E., Dower S. K., Namen A. E. Murine interleukin 7 (IL-7) receptor. Characterization on an IL-7-dependent cell line. J Exp Med. 1990 Apr 1;171(4):1073–1089. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C., Spudich J. A., Elson E. L. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989 Oct 12;341(6242):549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Nagai K., Kendrick-Jones J. Site-directed mutagenesis of the regulatory light-chain Ca2+/Mg2+ binding site and its role in hybrid myosins. Nature. 1986 Jul 3;322(6074):80–83. doi: 10.1038/322080a0. [DOI] [PubMed] [Google Scholar]
- Rolink A., Kudo A., Karasuyama H., Kikuchi Y., Melchers F. Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J. 1991 Feb;10(2):327–336. doi: 10.1002/j.1460-2075.1991.tb07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Sherwood P. J., Weissman I. L. The growth factor IL-7 induces expression of a transformation-associated antigen in normal pre-B cells. Int Immunol. 1990;2(5):399–406. doi: 10.1093/intimm/2.5.399. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Stobo J. D., Green I. Immunoglobulin and theta-bearing murine leukemias and lymphomas. J Immunol. 1972 May;108(5):1146–1151. [PubMed] [Google Scholar]
- Taubman M. B., Grant J. W., Nadal-Ginard B. Cloning and characterization of mammalian myosin regulatory light chain (RLC) cDNA: the RLC gene is expressed in smooth, sarcomeric, and nonmuscle tissues. J Cell Biol. 1987 Jun;104(6):1505–1513. doi: 10.1083/jcb.104.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer M. B., Morrissey P. J., Namen A. E., Voice R. F., Watson J. D. Interleukin 7 stimulates growth of fetal thymic precursors of cytolytic cells: induction of effector function by interleukin 2 and inhibition by interleukin 4. Int Immunol. 1990;2(11):1055–1061. doi: 10.1093/intimm/2.11.1055. [DOI] [PubMed] [Google Scholar]
- Wu Q., Li L., Cooper M. D., Pierres M., Gorvel J. P. Aminopeptidase A activity of the murine B-lymphocyte differentiation antigen BP-1/6C3. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):676–680. doi: 10.1073/pnas.88.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Oltz E. M., Rathbun G., Berman J. E., Smith R. K., Lansford R. D., Rothman P., Okada A., Lee G., Morrow M. Isolation of coordinately regulated genes that are expressed in discrete stages of B-cell development. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5759–5763. doi: 10.1073/pnas.87.15.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]