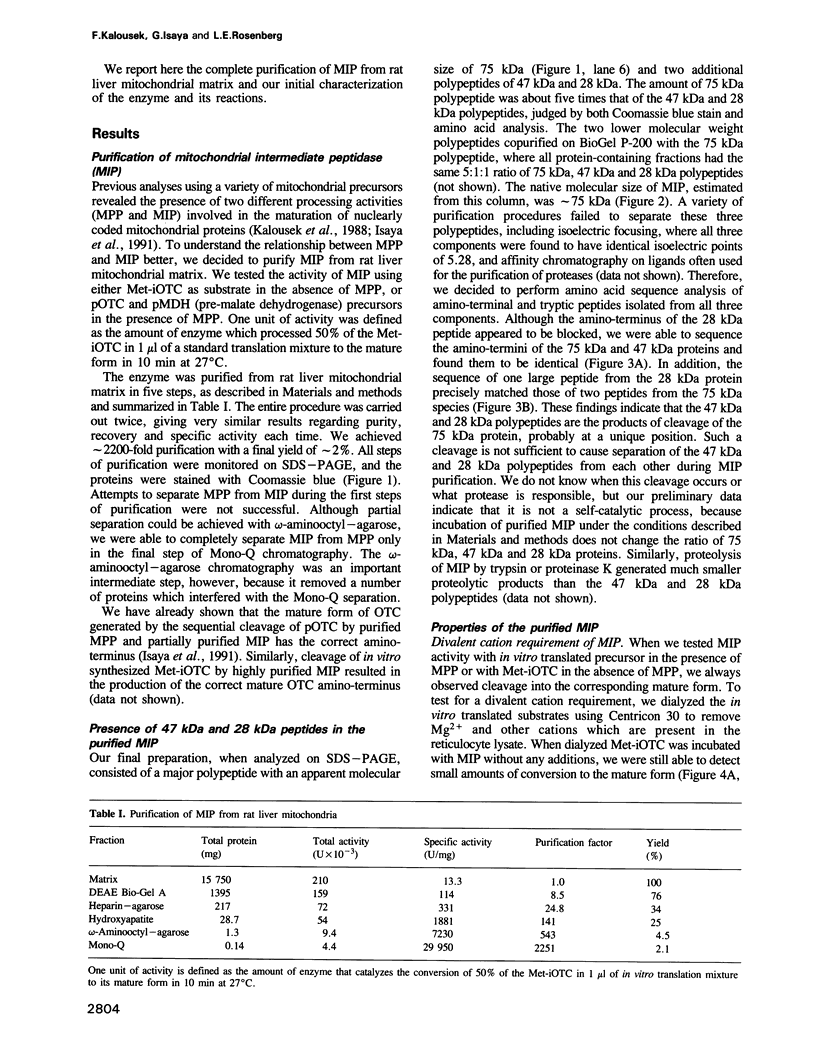
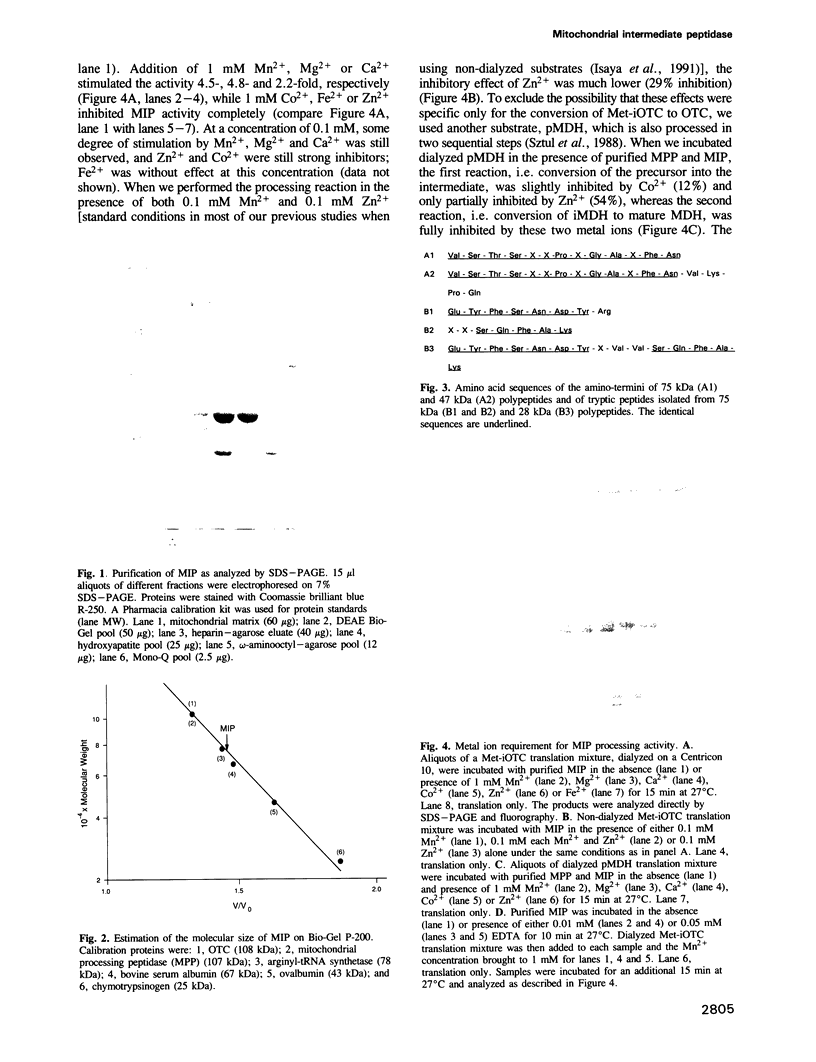
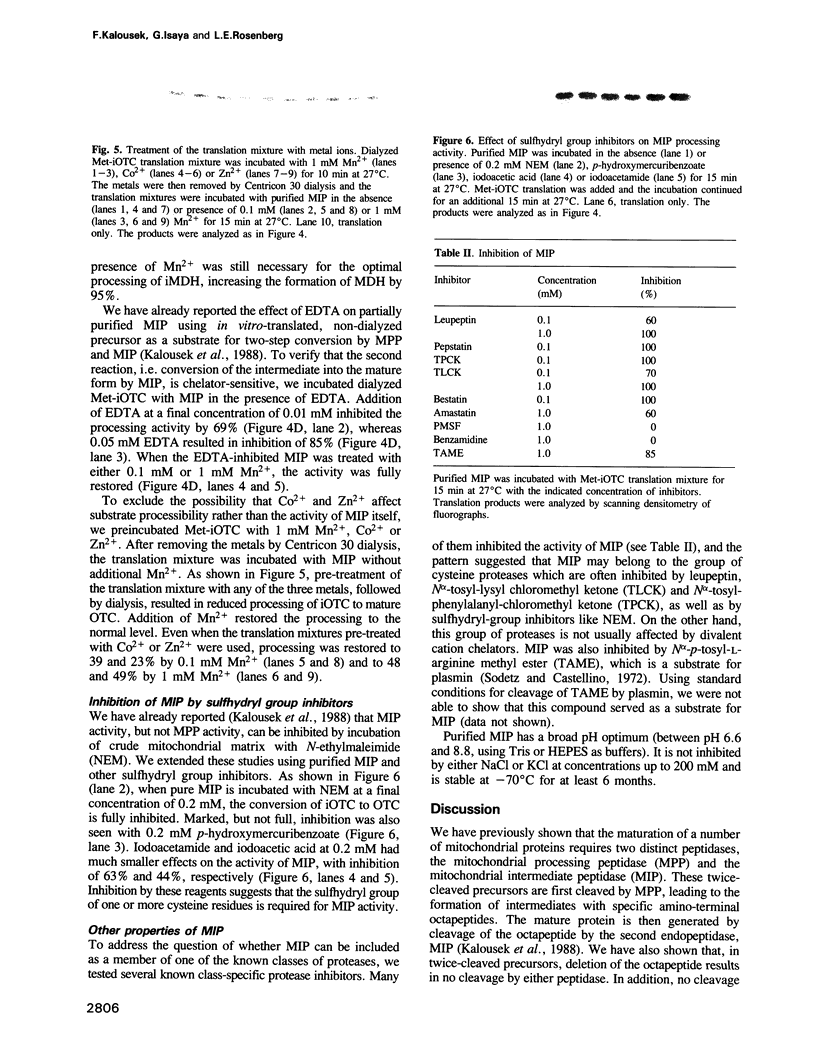
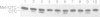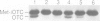Abstract
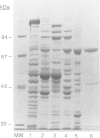
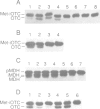
A number of nuclearly encoded mitochondrial protein precursors that are transported into the matrix and inner membrane are cleaved in two sequential steps by two distinct matrix peptidases, mitochondrial processing peptidase (MPP) and mitochondrial intermediate peptidase (MIP). We have isolated and purified MIP from rat liver mitochondrial matrix. The enzyme, purified 2250-fold, is a monomer of 75 kDa and cleaves all tested mitochondrial intermediate proteins to their mature forms. About 20% of the final MIP preparation consists of equimolar amounts of two peptides of 47 kDa and 28 kDa, which are apparently the products of a single cleavage of the 75 kDa protein. These peptides are not separable from the 75 kDa protein, nor from each other, under any conditions used in the purification. The peptidase has a broad pH optimum between pH 6.6 and 8.9 and is inactivated by N-ethylmaleimide (NEM) and other sulfhydryl group reagents. The processing activity is divalent cation-dependent; it is stimulated by manganese, magnesium or calcium ions and reversibly inhibited by EDTA. Zinc, cobalt and iron strongly inhibit MIP activity. This pattern of cation dependence and inhibition is not clearly consistent with that of any known family of proteases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conboy J. G., Fenton W. A., Rosenberg L. E. Processing of pre-ornithine transcarbamylase requires a zinc-dependent protease localized to the mitochondrial matrix. Biochem Biophys Res Commun. 1982 Mar 15;105(1):1–7. doi: 10.1016/s0006-291x(82)80002-8. [DOI] [PubMed] [Google Scholar]
- Fu W., Japa S., Beattie D. S. Import of the iron-sulfur protein of the cytochrome b.c1 complex into yeast mitochondria. J Biol Chem. 1990 Sep 25;265(27):16541–16547. [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Cleavage-site motifs in mitochondrial targeting peptides. Protein Eng. 1990 Oct;4(1):33–37. doi: 10.1093/protein/4.1.33. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Fenton W. A., Rosenberg L. E. Cleavage of precursors by the mitochondrial processing peptidase requires a compatible mature protein or an intermediate octapeptide. J Cell Biol. 1991 Apr;113(1):65–76. doi: 10.1083/jcb.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Rosenberg L. E. Amino-terminal octapeptides function as recognition signals for the mitochondrial intermediate peptidase. J Biol Chem. 1992 Apr 15;267(11):7904–7910. [PubMed] [Google Scholar]
- Jensen R. E., Yaffe M. P. Import of proteins into yeast mitochondria: the nuclear MAS2 gene encodes a component of the processing protease that is homologous to the MAS1-encoded subunit. EMBO J. 1988 Dec 1;7(12):3863–3871. doi: 10.1002/j.1460-2075.1988.tb03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Hendrick J. P., Rosenberg L. E. Two mitochondrial matrix proteases act sequentially in the processing of mammalian matrix enzymes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7536–7540. doi: 10.1073/pnas.85.20.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber J., Kalousek F., Swaroop M., Rosenberg L. E. The general mitochondrial matrix processing protease from rat liver: structural characterization of the catalytic subunit. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7978–7982. doi: 10.1073/pnas.87.20.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto T., Ito A., Omura T. Characterization of a mitochondrial matrix protease catalyzing the processing of adrenodoxin precursor. J Biochem. 1986 Jul;100(1):247–254. doi: 10.1093/oxfordjournals.jbchem.a121700. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Ristevski S., Höj P., Hoogenraad N. High-level expression of a mitochondrial enzyme, ornithine transcarbamylase from rat liver, in a baculovirus expression system. DNA Cell Biol. 1991 Jul-Aug;10(6):443–449. doi: 10.1089/dna.1991.10.443. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Ou W. J., Ito A., Okazaki H., Omura T. Purification and characterization of a processing protease from rat liver mitochondria. EMBO J. 1989 Sep;8(9):2605–2612. doi: 10.1002/j.1460-2075.1989.tb08400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N. M., Maloy W. L., Guy H. R., Zasloff M. A novel endopeptidase from Xenopus that recognizes alpha-helical secondary structure. Cell. 1991 Aug 9;66(3):541–554. doi: 10.1016/0092-8674(81)90017-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Fenton W. A., Horwich A. L., Kalousek F., Kraus J. P. Targeting of nuclear-encoded proteins to the mitochondrial matrix: implications for human genetic defects. Ann N Y Acad Sci. 1986;488:99–108. doi: 10.1111/j.1749-6632.1986.tb46550.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Behrens M., Scherer P., Pratje E., Michaelis G., Schatz G. Inner membrane protease I, an enzyme mediating intramitochondrial protein sorting in yeast. EMBO J. 1991 Feb;10(2):247–254. doi: 10.1002/j.1460-2075.1991.tb07944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Arretz M., Wachter E., Neupert W. Matrix processing peptidase of mitochondria. Structure-function relationships. J Biol Chem. 1990 Jun 15;265(17):9881–9887. [PubMed] [Google Scholar]
- Sodetz J. M., Castellino F. J. A comparison of steady- and presteady-state kinetics of bovine and human plasmins. Biochemistry. 1972 Aug 15;11(17):3167–3171. doi: 10.1021/bi00767a005. [DOI] [PubMed] [Google Scholar]
- Stone K. L., Elliott J. I., Peterson G., McMurray W., Williams K. R. Reversed-phase high-performance liquid chromatography for fractionation of enzymatic digests and chemical cleavage products of proteins. Methods Enzymol. 1990;193:389–412. doi: 10.1016/0076-6879(90)93429-o. [DOI] [PubMed] [Google Scholar]
- Sztul E. S., Chu T. W., Strauss A. W., Rosenberg L. E. Import of the malate dehydrogenase precursor by mitochondria. Cleavage within leader peptide by matrix protease leads to formation of intermediate-sized form. J Biol Chem. 1988 Aug 25;263(24):12085–12091. [PubMed] [Google Scholar]
- Sztul E. S., Hendrick J. P., Kraus J. P., Wall D., Kalousek F., Rosenberg L. E. Import of rat ornithine transcarbamylase precursor into mitochondria: two-step processing of the leader peptide. J Cell Biol. 1987 Dec;105(6 Pt 1):2631–2639. doi: 10.1083/jcb.105.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Yang M. J., Geli V., Oppliger W., Suda K., James P., Schatz G. The MAS-encoded processing protease of yeast mitochondria. Interaction of the purified enzyme with signal peptides and a purified precursor protein. J Biol Chem. 1991 Apr 5;266(10):6416–6423. [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]