Abstract
To investigate whether the endogenous neuropeptide nociceptin/orphanin FQ (N/OFQ) contributes to the death of dopamine neurons in Parkinson's disease, we undertook a genetic and a pharmacological approach using NOP receptor knockout (NOP−/−) mice, and the selective and potent small molecule NOP receptor antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). Stereological unbiased methods were used to estimate the total number of dopamine neurons in the substantia nigra of i) NOP−/− mice acutely treated with the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP), ii) naïve mice subacutely treated with MPTP, alone or in combination with SB-612111, iii) rats injected with a recombinant adeno-associated viral (AAV) vector overexpressing human mutant p.A53T α-synuclein, treated with vehicle or SB-612111. NOP−/− mice showed a 50% greater amount of nigral dopamine neurons spared in response to acute MPTP compared to controls, which was associated with a milder motor impairment. SB-612111, given 4 days after MPTP treatment to mimic the clinical condition, prevented the loss of nigral dopamine neurons and striatal dopaminergic terminals caused by subacute MPTP. SB-612111, administered a week after the AAV injections in a clinically-driven protocol, also increased by 50% both the number of spared nigral dopamine neurons and striatal dopamine terminals, and prevented accompanying motor deficits induced by α-synuclein. We conclude that endogenous N/OFQ contributes to dopamine neuron loss in pathogenic and etiologic models of Parkinson's disease through NOP receptor-mediated mechanisms. NOP receptor antagonists might prove effective as disease-modifying agents in Parkinson's disease, through the rescue of degenerating nigral dopamine neurons and/or the protection of the healthy ones.
Keywords: Adeno associated viral vectors, α-Synuclein, Nociceptin/orphanin FQ, MPP+, MPTP, Neuroprotection, NOP receptor, Parkinson's disease, SB-612111
1. Introduction
Nociceptin/orphanin FQ is an opioid-like neuropeptide that selectively activates the N/OFQ peptide (NOP) receptor (Meunier et al., 1995; Reinscheid et al., 1995). N/OFQ and its receptor are expressed in cortical and subcortical areas of the central nervous system (Neal et al., 2001; Neal et al., 1999a; Neal et al., 1999b), and contribute to the modulation of a number of central functions such as pain, reward, food intake, mood and locomotion (Calo et al., 2000; Lambert, 2008; Mogil and Pasternak, 2001). Mesencephalic dopamine (DA) neurons are involved in motor effects of N/OFQ (Florin et al., 1996; Kuzmin et al., 2004; Viaro et al., 2013). In fact, DA neurons projecting from substantia nigra compacta (SNc) and ventral-tegmental area to the strifatum and prefrontal cortex express the NOP receptor (Maidment et al., 2002; Norton et al., 2002). Activation of somato-dendritic NOP receptors causes inhibition of the firing activity along the nigro-striatal (Marti et al., 2004b) and meso-accumbal (Murphy and Maidment, 1999) pathways, while activation of presynaptic NOP receptors causes inhibition of DA neurosecretion (Flau et al., 2002). These effects have been associated with hypolocomotion (Murphy, 2010; Narayanan et al., 2004; Sakoori and Murphy, 2004; Viaro et al., 2013). In addition, blockade of NOP receptors in substantia nigra reticulata (SNr) evokes DA release in striatum, suggesting that endogenous N/OFQ exerts a tonically negative control over nigro-striatal DA neurons activity. In parkinsonian animals, destruction of DA cells located in SNc causes an increase of expression of the N/OFQ precursor (ppN/OFQ) (Di Benedetto et al., 2009; Gouty et al., 2010; Marti et al., 2005; Norton et al., 2002) and an elevation of extracellular N/OFQ levels in SNr (Marti et al., 2005). Such up-regulation contributes to parkinsonian-like hypokinesia since blockade of NOP receptors in SNr reverses motor deficits in rodent and non-human primate models of Parkinson's disease (PD) (Mabrouk et al., 2010; Marti et al., 2013; Marti et al., 2005; Marti et al., 2008; Marti et al., 2007; Viaro et al., 2008; Visanji et al., 2008; Volta et al., 2010). In addition, an increase in N/OFQ levels may also contribute to SNc DA cells loss, the PD hallmark (Brown et al., 2006; Marti et al., 2005), since mice lacking ppN/OFQ (ppN/OFQ−/− mice) are partially resistant to the 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP)-induced loss of nigral DA cells and striatal DA terminals (Brown et al., 2006; Marti et al., 2005). However, in addition to N/OFQ, at least two other peptides are generated by the cleavage of ppN/OFQ, namely N/OFQ II and nocistatin (Mogil and Pasternak, 2001). These peptides do not bind the NOP receptor but exert biological activity (Fantin et al., 2007; Florin et al., 1997; Okuda-Ashitaka et al., 1998; Okuda-Ashitaka et al., 2012), questioning the view that endogenous N/OFQ is the only culprit for DA neurons degeneration observed in ppN/OFQ−/− mice.
We therefore undertook a thorough study in pathogenic and etiologic models of PD to unequivocally demonstrate that endogenous N/OFQ causes DA neuron degeneration through the NOP receptor. In the first experiment, NOP receptor knockout (NOP−/−) mice and wild-type controls (NOP+/+) were acutely treated with MPTP, and nigral DA cell loss measured with stereological methods at 7 days after intoxication (i.e. when neurodegeneration has fully established) (Jackson-Lewis and Przedborski, 2007; Sundstrom et al., 1988). In the second experiment, the proof of concept that pharmacological blockade of NOP receptor might help protect/rescue DA cells was investigated in mice subacutely treated with MPTP in combination with the NOP receptor selective antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H–benzocyclohepten-5-ol (SB-612111) (Rizzi et al., 2007; Spagnolo et al., 2007; Zaratin et al., 2004). To finally demonstrate the neurotoxic role of endogenous N/OFQ and the neuroprotective potential of NOP receptor antagonists, SB-612111 was chronically administered in rats where human mutant p.A53T α-synuclein (hα-syn) was delivered into SNc through a recombinant adeno-associated viral (AAV) vector pseudotype 2/9 (AAV2/9 hα-syn) (Bourdenx et al., 2015). Indeed, AAV-mediated overexpression of hα-syn causes a slow and progressive neurodegeneration (Bezard et al., 2013; Bourdenx et al., 2015; Kirik et al., 2002; Ulusoy et al., 2010; Van der Perren et al., 2015), consistent with the pattern of slow nigrostriatal neurodegeneration observed in PD patients (Recasens et al., 2014) and with the human genetics of PD (Chartier-Harlin et al., 2004; Devine et al., 2011).
2. Materials and methods
2.1. Animals
Experiments were performed in accordance with the EU directive of September 22, 2010 (2010/63/EU)on the protection of animals used for scientific purposes. Male NOP−/− and NOP+/+ mice (Nishi et al., 1997), bred on a CD-1 background for 9 generations (Gavioli et al., 2007), and male C57BL/6J mice (10 weeks old, Harlan Italy, S. Pietro al Natisone, Italy) were housed with free access to food and water and kept under regular lighting conditions (12 h dark/light cycle), after approval of the experimental protocols by the Italian Ministry of Health (license 171/2010-B e 170/2013-B). Male Sprague–Dawley rats (180 g; Charles River Laboratories, France) were housed in the same conditions at the IMN (Bordeaux). The Institutional Animal Care and Use Committee of Bordeaux (CE50) approved these experiments under the license number 5012099-A.
2.2. Experimental design for in vivo experiments
2.2.1. Experiment #1
NOP+/+ and NOP−/− mice were trained for a week to perform specific motor tasks (bar, drag and rotarod tests) (Marti et al., 2005; Viaro et al., 2008) and then treated with MPTP (20 mg/kg × 4 i.p. administrations, 90 min apart) or with saline (Viaro et al., 2008). Motor activity was monitored before (baseline) and daily for 7 days after toxin administration, using the bar, drag and rotarod tests.
2.2.2. Experiment #2
Naïve C57BL/6 J mice were trained as above, then allotted in two groups treated with either MPTP (25 mg/kg, i.p.) or saline, once daily for 7 days (175 mg/kg cumulative), according to a slightly modified version of the subacute protocol described by Tatton&Kish (30 mg/kg for 5 days; 150 mg/kg cumulative) (Tatton and Kish, 1997). Four days after treatment onset (i.e. at the 4th MPTP dose), each treatment group was split in two subgroups, and mice administered twice daily for 10 days with either the NOP receptor antagonist SB-612111 (10 mg/kg, i.p.) or vehicle, in order to adopt a clinically-relevant design, i.e. to treat mice once degeneration had started (Bezard, 2003). SB-612111 was administered at 9.00 and 18.00 h. When co-administered with SB-612111 (days 4–7), MPTP was given 5 h after the first daily injection of SB-612111. Motor activity was monitored before (baseline) and at days 1, 5 and 10 after toxin administration, using the bar, drag and rotarod tests.
2.2.3. Experiment #3
Rats received a bilateral stereotaxic injection of AAV2/9-hα-syn in SNc as previously described (Bourdenx et al., 2015). Seven days later, animals were allotted in two treatment groups, which received either SB-612111 (1 mg/kg, twice daily) or its vehicle, twice daily for 8 weeks, for again complying with the concept of clinical relevance of experimental design (Bezard, 2003). SB-612111 was administered at 9.00 and 18.00 h. Motor performance was monitored before (baseline) and once a week for 8 weeks after surgery, using the stepping test. This test was performed just before the second SB-612111 administration. A group of 6 rats injected with AAV-GFP (green fluorescent protein) was used as a control to quantify the number of TH + neurons in SNc.
2.3. Behavioral motor studies
2.3.1. Bar test
This test, also known as the catalepsy test (Marti et al., 2005; Sanberg et al., 1988; Viaro et al., 2008), measures the ability of the mouse to respond to an externally imposed static posture. Each mouse was placed gently on a table and the right and left forepaws were placed alternatively on blocks of increasing heights (1.5, 3 and 6 cm). The immobility time (in sec) of each forepaw on the block was recorded (cut-off time of 20 s). Akinesia was calculated as total time spent on the blocks. Since values did not significantly differ between the left and right forepaw, data were pooled together.
2.3.2. Drag test
This test (modification of the “wheelbarrow” test) (Schallert et al., 1979), measures the ability of the mouse to balance its body posture using forelimbs in response to an externally imposed dynamic stimulus (backward dragging) (Marti et al., 2005; Marti et al., 2004b; Viaro et al., 2008). Each mouse was gently lifted from the tail (leaving the forepaws on the table) and dragged backwards at a constant speed (about 20 cm/s) for a fixed distance (100 cm). The number of touches made by each forepaw was counted by two separate observers. Since values did not significantly differ between the left and right forepaw, data were pooled together.
2.3.3. Rotarod test
This test analyzes the ability of the mouse to run on a rotating cylinder (diameter 8 cm) and provides information on different motor parameters such as coordination, gait, balance, muscle tone and motivation to run (Rozas and Labandeira Garcia, 1997). The fixed-speed rotarod test was employed according to a previously described protocol (Marti et al., 2004b; Viaro et al., 2008). Mice were tested in a stepwise mode at increasing speeds (usually from 5 to 45 rpm; 180 s each), and time spent on the rod calculated (in sec).
2.3.4. Stepping Test
This test assesses forelimb akinesia (Olsson et al., 1995). Rats were gently held and moved sideways at a constant speed for a fixed distance, to allow forehand and backhand steps count. Left and right limb performances were evaluated once a week, three times over 2 consecutive days. Since values did not significantly differ between the left and right forepaw, data were pooled together, and the average number of left/right backhand steps was averaged over the 3 sessions.
2.4. AAV2/9-hα-syn vector production and injection
Recombinant AAV2/9-hα-syn vectors were produced and purified as already described (Bourdenx et al., 2015; Engeln et al., 2013; Zolotukhin et al., 1999). Briefly, vectors were transfected into HEK-293T/17 cells (ATCC, Teddington, UK) for three times using a polyethylenimine solution. Seventy-two hours after transfection, cells were re-suspended in lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH 8.5), and then lysed using a freeze–thaw cycle (−80 °C/+37 °C). The obtained supernatant was purified by iodixanol gradient step centrifugation, and finally aliquoted and kept in stock at −80 °C.
Sixteen male Sprague–Dawley rats received a bilateral SNc stereotaxic injection of AAV2/9-hα-syn (6.9 × 1013 genome containing particles/ml; 2 µl), under isoflurane anesthesia. Virus was injected with a glass pipette at a flow rate of 0.25 µl/min and was left in place for additional 4 min to prevent backflush. Coordinates from bregma were (in mm): anteroposterior (AP) −4.9 and −5.4; medio-lateral (ML) ±2.2 and ±2.0; dorsoventral (DV) −7.8.
2.5. Post mortem brain processing
At the end of the experiments, animals were deeply anesthetized (ketamine 85 mg/kg, xylazine 15 mg/kg i.p., or chloral hydrate 15%, i.p.) and transcardially perfused with 0.9% NaCl solution followed by 4% paraformaldehyde in PBS (0.1 M, pH 7.4). Brains were removed, transferred to a 20% sucrose solution in PBS for cryoprotection and finally stored at −80 °C.
2.6. Immunohistochemistry
2.6.1. Experiment #1
Coronal sections (40 µM) encompassing the whole SNc (AP from −3.16 to −3.52 from bregma) (Paxinos and Franklin, 2001) were cut using a cryostat (Leica Microsystems, Wetzlar, Germany), and then collected free floating for immunohistochemistry. Serum containing TH antibody (polyclonal rabbit primary TH antibody, J. Boy, Reims, France) was diluted 1:2000 in PBS containing 0.3% Triton ×100 and 1% bovine serum albumin (BSA). Sections were incubated overnight at 4 °C with primary antibody, then for 1 h with biotinylated horse anti-rabbit antibody (universal secondary antibody, AbCys SA, Paris, France) diluted 1:200 in PBS containing 1% BSA and 0.3% Triton × 100, and finally revealed with 3,3′-diaminobenzidine tetrahydrochloride (DAB kit, Vector Laboratories). Sections were then mounted on gelatine-coated slides, counterstained with cresyl violet, dried with ethanol and xylene and coverslipped with mounting medium.
2.6.2. Experiment #2
Coronal sections (35 µm)of striatum (AP from + 1.42 to + 0.14 from bregma) and SNc (AP from −3.16 to −3.52 from bregma) (Paxinos and Franklin, 2001) were cut with a cryostat and then collected free floating in five different series for immunohistochemistry. After being rinsed in TBS (Tris Buffered Saline), serial SNc sections were incubated for 1 h with blocking solution (Bovin Serum Albumin; 3% in TBS), then incubated overnight with TH primary antibody (Purified rabbit polyclonal antibody; 1:500 in 1% BSA in TBS; Merck Millipore, Darmstadt, Germany) and with a fluorescent marker of Nissl Bodies (Neurotrace; 1:150 in 1% BSA in TBS; Life Technologies, Grand Island, NY, USA). Sections were then incubated with a secondary antibody (AlexaFluor 488, Goat anti-rabbit IgG; 1:500 in TBS; Life Technologies) for 40 min, mounted on slides and coverslipped with mounting medium. Three representative striatal sections were incubated for 1 h with blocking solution (Goat Serum 5% in TBS), then incubated overnight with TH primary antibody (Purified rabbit polyclonal antibody; 1:500 in 1% Goat Serum in TBS; Merck Millipore, Darmstadt, Germany). Finally, sections were incubated with a secondary antibody (AlexaFluor 488, Goat anti-rabbit IgG; 1:500 in TBS; Life Technologies) for 40 min, mounted on slides and coverslipped with mounting medium.
2.6.3. Experiment #3
Coronal sections (50 µm) of rat striatum (AP from +2.28 to −1.08 mm from bregma) and SNc (AP from −4.36 to −6.72 mm from bregma) (Paxinos and Franklin, 2001) were obtained using a cryostat. Serial SNc sections were incubated for 1 h with blocking solution, then overnight at 4 °C with mouse monoclonal antibody raised against TH. TH staining was then revealed with a specific mouse EnVision™ System (mouse HRP EnVision™ kit DAB+ DAKO). Sections were then mounted on gelatine coated slides, counterstained with 0.1% cresyl violet, dried with xylene and coverslipped with mounting medium. In these brains, expression of hα-syn and p-hα-syn was also quantified in SNc. Selected sections were incubated overnight at room temperature with mouse monoclonal antibody against hα-syn or against p-α-syn (clone syn211 Thermo Scientific, 1:1000; mouse monoclonal [p-syn 81/A], Abcam, 1:1000), and then revealed with an anti-mouse peroxidase EnVision™ system (DAKO) followed by DAB substrate.
2.7. Stereological cell count in SNc
2.7.1. Experiments #1 and #3
To count TH positive (TH +) neurons (phenotypic marker) and cresyl violet stained cells (structural marker) in SNc, an unbiased stereological sampling method (Larsen et al., 1998; West and Gundersen, 1990), based on optical fractionator stereological probe was used, as previously described (Bezard et al., 2003; Fernagut et al., 2014; Gross et al., 2003). TH+ neurons were counted using a Leica DM6000B motorized microscope coupled with the Mercator Pro Software (Mercator Digital Imaging System, Explora Nova, La Rochelle, France). Five sections were used for each brain. For each animal, SNc boundaries were delimited at low magnification (2.5×) by examining the size and shape of the different groups of TH+ neurons and their axonal projections, as well as nearby fiber bundles (Paxinos and Franklin, 2001), and probes for stereology were applied. Any TH+ cell within the probe, or intersecting an acceptance line delimiting the probe (green line) that came into focus at 40× magnification was counted. The optical fractionator method was then used to estimate the total number of TH+ cells in the SNc of each animal. Mean estimated number of neurons and SEM were then calculated for each group.
2.8. Experiment #2
Images were taken with a fluorescence microscope (Leica Microsystems, Wetzlar, Germany) equipped with a motorized Z and X-Y stage. Five serial SNc slices were used in order to analyze the entire SNc volume, and the area was identified with 10× magnification according to a mouse brain atlas (Paxinos and Franklin, 2001). The area was then divided into 3 rectangles (350 × 260 µm) and each rectangle was then amplified (40×). To count all TH+ positive neurons, 8 images of the same rectangle were taken on different levels of the Z axis. The images were then mounted and analyzed off-line using ImageJ (NIH, USA). Every neuron that appeared on focus was counted and the number obtained for each brain multiplied by five to approximate the total number of SNc TH+ neurons.
2.9. TH quantification in striatum
2.9.1. Experiments #2 and #3
Images were taken at 10× magnification with a fluorescence microscope (Experiment #2; see above) or at 20× magnification with NanoZoomer 2.0 HT (BIC facility, Bordeaux) (Experiment #3), and optical densitometry analyzed off-line as gray level with ImageJ using the cerebral cortex as background.
2.10. Quantification of hα-syn and p-α-syn expression
Images were obtained using the NanoZoomer 2.0 HT, and later analyzed with the Mercator Pro Software using a color threshold method. SNc limits were delineated to obtain a representative surface of hα-syn and p-α-syn expression. Finally, the immunopositive surface as percentage of total SNc area was calculated, in order to compare the different treatments.
2.11. Data presentation and statistical analysis
Motor performance in the bar, drag and rotarod test was presented as percentage of baseline (calculated as time on bar or on rod in sec, number of steps) and analyzed by one-way ANOVA followed by the Newman–Keuls test (Fig. 1) whereas motor performance in the stepping test was presented as absolute data and analyzed by two-way ANOVA followed by the Bonferroni test (Fig. 4). Data obtained from stereological counting were expressed as absolute values (number of cells) and analyzed by one-way ANOVA followed by the Newman–Keuls test (Figs. 2 and 3),or by the Student t-test when only two groups were compared (Fig. 5). Striatal TH density was expressed as absolute data (mean absolute value of gray scale between the two striata), and analyzed by one-way ANOVA followed by the Newman–Keuls test (Fig. 3), or by the Student t-test (Fig. 5). Density of α-syn aggregates was analyzed using the Student t-test (Fig. 6).
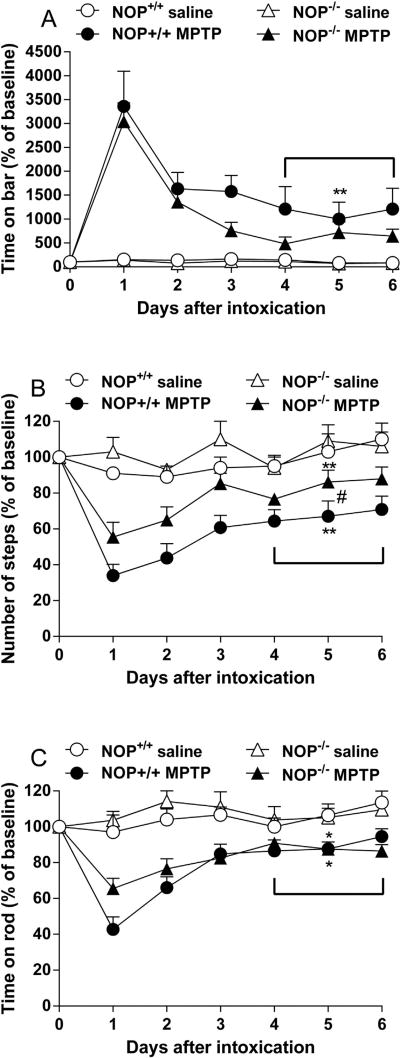
Fig 1.
NOP−/− mice are less susceptible than NOP+/+ mice to develop hypokinesia in response to MPTP. Motor activity was measured using the bar (A), drag (B), and rotarod (C) tests, before (day 0) and for up to 6 days after systemic administration of MPTP (20 × 4 mg/kg, i.p.). Data are mean ± SEM of 6–9 mice per group. Statistical analysis was performed on the average performance of the last three days of observation (D4-D6) *p < 0.05, **p < 0.01 different from saline-treated mice of the same genotype; #p < 0.05, different from MPTP-treated NOP−/− mice (one-way ANOVA followed by Newman–Keuls test for multiple comparisons).
Fig 4.
SB-612111 attenuates motor deficits induced by AAV2/9 A53T human α-syn (hα-syn) injection in rats. SB-612111 (1 mg/kg, s.c., twice daily) or its vehicle, were administered for 8 weeks starting one week after AAV2/9-hα-syn injection, and motor activity assessed using the stepping test once a week. Data are expressed as number of backhand steps and are mean ± SEM of n = 7 (Vehicle) and n = 8 (SB-612111) rats per group. ##p < 0.01 (two-way ANOVA followed by Bonferroni test for multiple comparisons).
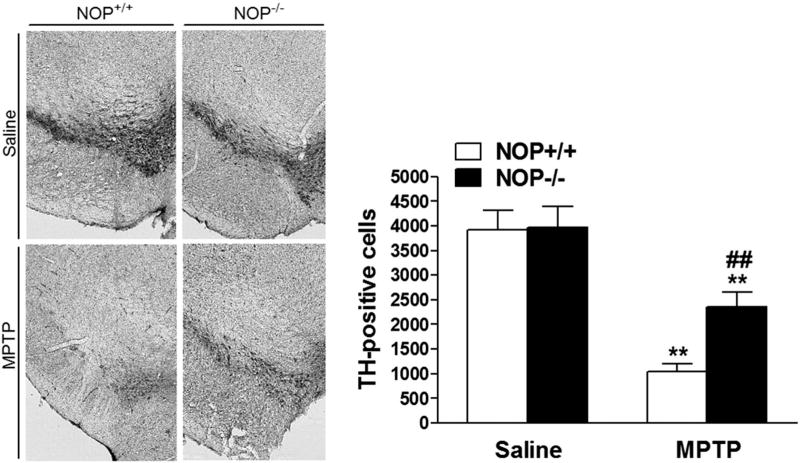
Fig 2.
NOP−/− mice are more resistant than NOP+/+ mice to MPTP-induced dopamine cell loss. Representative microphotographs (left) of tyrosine hydroxylase (TH) positive neurons in substantia nigra compacta (SNc) of NOP+/+ and NOP−/− mice, at 7 days after saline or MPTP (20 × 4 mg/kg, i.p.) administration. Stereological quantification of dopamine neurons in SNc (right). Data are mean ± SEM of 6–9 mice per group. **p < 0.01, different from saline-treated mice of the same genotype; ##p < 0.01, different from MPTP-treated NOP+/+ mice (one-way ANOVA followed by the Newman–Keuls test for multiple comparisons).
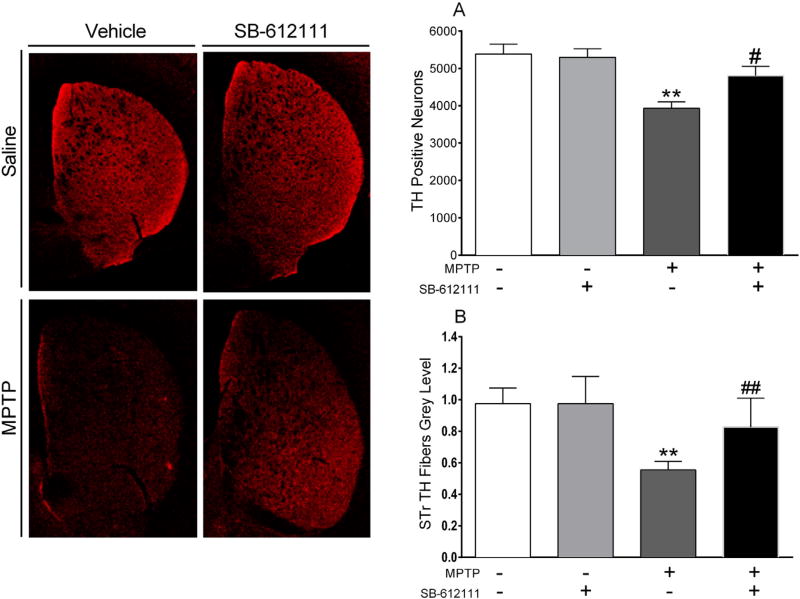
Fig 3.
SB-612111 attenuates subacute MPTP-induced neurotoxicity. Representative microphotographs (left) of tyrosine hydroxylase (TH) positive fibers in striatum. Stereological count of TH positive neurons in substantia nigra compacta (A) and quantification of TH positive terminals in striatum (B) of mice treated with MPTP (25 mg/kg, once daily for 7 days, i.p.) and/or SB-612111 (10 mg/kg, twice daily for 10 days, i.p.). Immunohistochemistry was performed 8 days after the last MPTP injection. Data are expressed as number of positive neurons (A) or mean gray level of TH striatal immunoreactivity (B), and are mean ± SEM of n = 7 mice per group. p** < 0.01 different from Saline/vehicle; #p < 0.05, ##p < 0.01 different from MPTP/vehicle (one-way ANOVA followed by the Newman–Keuls test for multiple comparisons).
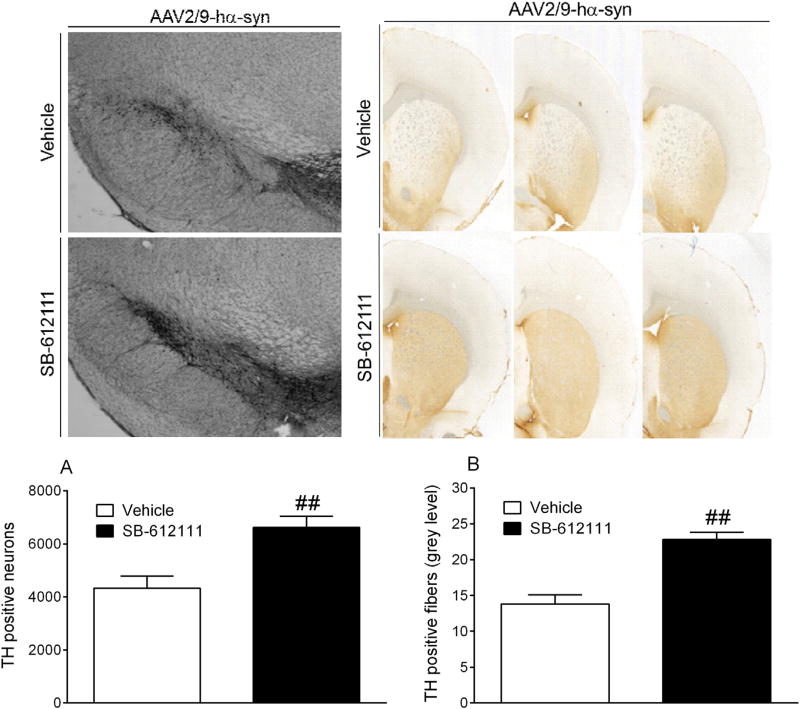
Fig 5.
SB-612111 attenuates neurodegeneration induced by AAV2/9 A53T human α-syn (hα-syn) injection in rats. Stereological count of TH positive DA neurons in the substantia nigra compacta (A) and quantification of TH positive terminals in the striatum (B) of rats injected with AAV2/9-hα-syn and treated with SB-612111 (1 mg/kg, twice daily for 8 weeks, s.c.) or vehicle. Data are expressed as number of TH positive neurons (A) or mean gray level of TH immunoreactivity (B), and are mean±SEM of n= 7(Vehicle) and n = 8(SB-612111) rats per group. ##p < 0.01 (Student t-test).
Fig 6.
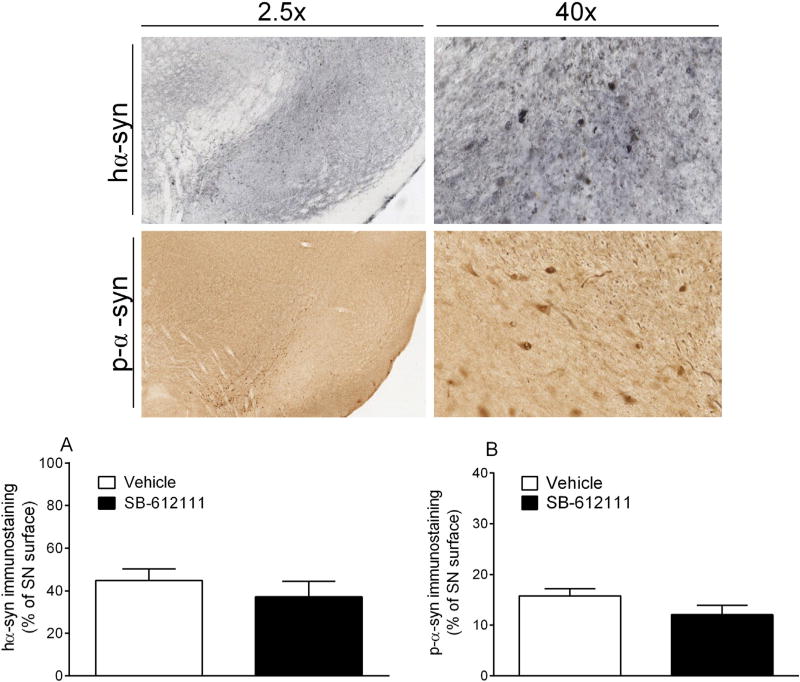
SB-612111 does not change the load of hα-syn (A) and phosphorylated-α-syn (p-α-syn) (B) in the SNc of AAV A53T human α-syn injected rats. Data are expressed as immunopositive surface percentage of total nigral surface, and are mean ± SEM of n = 7 (Vehicle) and n = 8 (SB-612111) rats per group.
2.12. Substances
MPTP was purchased from Sigma Aldrich (St. Louis, MO, USA) and dissolved in 0.9% NaCl saline solution. SB-612111 used in Experiment #2 was synthetized by Dr. Zaveri (Astraea Therapeutics, Mountain View, CA, USA), whereas SB-612111 used in Experiment #3 was purchased from Sequoia Research Product Ltd. (Pangbourne, UK). SB-612111 was dissolved in 3% DMSO in saline solution.
3. Results
3.1. NOP−/− mice are partially resistant to MPTP-induced hypolocomotion and neurodegeneration (Experiment #1)
As an initial screening to prove the role of endogenous N/OFQ and the NOP receptor in contributing to parkinsonian-like neurodegeneration, NOP−/− mice were acutely treated with MPTP, in parallel with NOP+/+ controls. Basal immobility time in the bar test was similar between NOP+/+ and NOP−/− mice (0.3 ± 0.1 vs 0.1 ± 0.1 s, respectively). Conversely, NOP−/− mice outperformed NOP+/+ mice in the drag test (13.2 ± 0.7 vs 10.8 ± 0.2 steps, respectively; p = 0.0012) and rotarod test (1926.6 ± 50.6 vs 1517.6 ± 54.6 s, respectively; p < 0.0001). MPTP treatment caused motor impairment in both genotypes, although NOP−/− mice were less affected (Fig. 1). ANOVA on the average performance of the last three testing days (i.e. when mice motor activity was stable), revealed a significant treatment effect on bar test (Fig. 1A; F3,25 = 6.74, p = 0.0017), drag test (Fig. 1B; F3,25 = 8.24, p = 0.0006), and rotarod test (Fig. 1C; F3,25 = 5.50, p = 0.0048) values. Post hoc analysis revealed that MPTP significantly enhanced immobility time in NOP+/+ but not NOP−/− mice (Fig. 1A), and reduced stepping activity in both genotypes, although NOP−/− mice were less affected than NOP+/+ mice (p < 0.05; Fig. 1B). Conversely, MPTP equally reduced rotarod performance in both genotypes (Fig. 1C).
Stereology revealed that under control conditions (saline treatment) both NOP−/− and NOP+/+ mice showed similar estimated numbers of tyrosine hydroxylase TH + neurons in SNc (Fig. 2). ANOVA showed that total number of cells significantly differed between genotypes and treatments (F3,26 = 16.84, p < 0.0001). Post hoc analysis revealed that MPTP caused a marked loss of TH+ cells in both genotypes. However, the number of TH + cells surviving MPTP treatment was 50% higher in NOP−/− than NOP+/+ mice (p < 0.01; Fig. 2).
3.2. Pharmacological blockade of the NOP receptor protects from MPTP-induced neurodegeneration (Experiment #2)
Data obtained in NOP−/− mice indicate that endogenous N/OFQ contributes to parkinsonian-like neurodegeneration via the NOP receptor, further suggesting that pharmacological blockade of the NOP receptor could provide some degree of neuroprotection/neurorescue to DA cells. To prove this concept, we subacutely treated mice with MPTP in combination with 10 mg/kg SB-612111, a dose found effective in producing NOP-specific central effects in the mouse (Khroyan et al., 2009; Rizzi et al., 2007). SB-612111 was administered in a clinically-driven experimental design (Bezard et al., 2013), i.e. starting from the 4th day of MPTP administration.
No differences in motor performance were detected among groups before, during and after treatments (Supplementary Fig. 1). Stereology revealed that mice treated with SB-612111/vehicle and saline/vehicle had a similar estimated number of TH+ neurons in SNc (Fig. 3). ANOVA showed that subacute MPTP treatment caused significant DA neuron loss when given alone (treatment: F3,24 = 8.050; p = 0.0007). However, delayed administration of SB-612111 prevented MPTP-induced dopaminergic neurodegeneration (Fig. 3A; p < 0.05). Accordingly, MPTP induced a significant loss of TH staining in striatum (treatment: F3,24 = 14.42; p < 0.0001; Fig. 3B) and this effect was prevented by combined administration of SB-612111 (p < 0.01).
3.3. Pharmacological blockade of the NOP receptor protects from hα-syn induced neurodegeneration (Experiment #3)
The AAV2/9-hα-syn rat model was used to test the ability of the NOP antagonist SB-612111 to also counteract α-synuclein-induced degeneration, shedding further light on the protective potential of this class of compounds in PD. The stepping test revealed that rats chronically treated with SB-612111 (1 mg/kg, s.c., twice daily) displayed a better motor performance, i.e. a higher number of adjusting steps, over the course of the experiment compared to vehicle-treated animals (Fig. 4) (Bourdenx et al., 2015). ANOVA showed a significant effect of treatment (F1,117 = 52.00, p < 0.0001), time (F8,117 = 20.68, p < 0.0001) and time × treatment interaction (F8,117 = 3.055, p = 0.0037). Post hoc analysis revealed that SB-612111 significantly attenuated motor impairment from week 6 onward, the maximal difference between groups being observed at week 8(6.9±0.3 vs 10.0 ± 0.4 steps; p < 0.01).In line with behavioral data, the number of TH+ cells was ~50% higher in the SNc of SB-612111-treated than in the vehicle-treated rats (6623 ± 422 vs 4330 ± 464; t = 3.66, df = 13, p = 0.0029; Fig. 5A), demonstrating strong neuroprotection in this etiologic model of PD. Nonetheless, the neuroprotection provided by SB-612111 was partial since in the group of control animals injected with AAV-GFP the number of nigral TH+ neurons was 12,614 ± 960 (n = 6).
The greater number of TH+ cells in the SNc of SB-612111-treated rats was paralleled by a significantly higher mean gray level value in the striatum (t = 5.61, df = 13, p < 0.001; Fig. 5B), suggesting a significant sparing of striatal DA terminals as well.
To check for the efficiency of AAV2/9 injections and to investigate whether SB-612111 interferes with α-syn aggregation, the expression patterns of hα-syn and Ser129 phosphorylated α-syn (p-α-syn) were finally evaluated in the two groups. SB-612111 treatment did not affect the load of hα-syn (Fig. 6A) or p-α-syn (Fig. 6B) in SNc.
4. Discussion
Both genetic deletion and pharmacological blockade of the NOP receptor confer protection against MPTP-induced toxicity in the mouse. Blockade of the NOP receptor also protects DA neurons and striatal DA terminals in a progressive rat model of PD, where DA cell degeneration is achieved via overexpression of hα-syn in SNc. These data extend previous evidence in MPTP-treated ppN/OFQ−/− mice (Brown et al., 2006; Marti et al., 2005), and unequivocally prove that, in addition to contributing to parkinsonian-like hypokinesia (Marti et al., 2005; Marti et al., 2010; Marti et al., 2007; Viaro et al., 2008; Volta et al., 2010), endogenous N/OFQ is involved in the mechanisms that cause degeneration of nigral DA neurons in experimental parkinsonism.
The MPTP mouse model of PD is still considered a reference rodent model for studying mechanisms of parkinsonian neurodegeneration as well as for screening for neuroprotective agents, since it replicates some of the key neuropathological features of PD (Jackson-Lewis and Przedborski, 2007; Meredith and Rademacher, 2011; Przedborski et al., 2001). Nonetheless, the contribution of the different neurotoxicity mechanisms/pathways varies depending on the protocol of MPTP administration. Indeed, in the acute MPTP model, DA neurons die rapidly, within 4 days from toxin administration (Jackson-Lewis and Przedborski, 2007; Sundstrom et al., 1988), through non-apoptotic (Jackson-Lewis and Przedborski, 2007), perhaps microglia-mediated inflammatory (Furuya et al., 2004; Liberatore et al., 1999) mechanisms. Conversely, in the subacute MPTP model, neuronal death is delayed, and apoptosis along with astrocyte activation is clearly observed (Serra et al., 2002; Tatton and Kish, 1997).
In line with previous studies (Jackson-Lewis and Przedborski, 2007; Thomas et al., 2007), we report a larger nigral DA cell loss following acute MPTP (~70%) compared to subacute MPTP (~30%) administration. Genetic removal of the NOP receptor provided partial neuroprotection in the acute MPTP model whereas pharmacological blockade of the NOP receptor provided full protection in the subacute model. This suggests that the role of endogenous N/OFQ is more prominent and pharmacological blockade of the NOP receptor more effective when the MPTP insult is milder and cell death more progressive.
Although we did not directly measure MPTP metabolism and uptake in NOP−/− mice nor did we assess whether SB-612111 could alter these parameters (Jackson-Lewis and Przedborski, 2007), the possibility that the neuroprotection in mice was due to reduced MPTP conversion to MPP+ or impaired MPP+ uptake in DA neurons seems remote. In fact, MPTP metabolism and uptake are unaltered in ppN/OFQ−/− mice (Marti et al., 2005). Moreover, neuroprotection was also observed in an etiologic model of PD, where MPTP was not employed. Therefore, it seems likely that endogenous N/OFQ acting via the NOP receptor mediates events that directly or indirectly lead to neurotoxicity.
We previously showed that N/OFQ administered exogenously in the SNr of awake rats can elevate glutamate release (Marti et al., 2002). Moreover, NOP receptor antagonists reduce glutamate levels in the SNr of 6-OHDA-hemilesioned rats (Marti et al., 2005; Marti et al., 2004a; Marti et al., 2008; Marti et al., 2007; Volta et al., 2011) and MPTP-treated mice (Mabrouk et al., 2010). Therefore, it would be tempting to speculate that endogenous N/OFQ causes DA neuron cell death through excitotoxic mechanisms (Brown et al., 2006; Marti et al., 2005). In fact, changes in mitochondrial potential due to inhibition of complex I by MPP+ (the active metabolite of MPTP) lead to oxidative stress and glutamate-mediated excitotoxicity (Meredith and Rademacher, 2011; Serra et al., 2002). Interestingly, exogenous N/OFQ has been shown to exacerbate, whereas NOP receptor antagonists protect, from ibotenate-induced excitotoxic lesions of the periventricular murine white matter in vivo, without affecting the simultaneous loss of cortical gray matter (Laudenbach et al., 2001). This suggests that rather than potentiating a direct NMDA receptor-mediated toxic insult on cortical neurons, exogenous and endogenous N/OFQ modulates microglial activation, which underlies astrocyte cell death and white matter lesion (Laudenbach et al., 2001). Since that study, multiple lines of evidence show that N/OFQ modulates the inflammatory and microglial response, although both pro- and anti-inflammatory effects of N/OFQ have also been described, at the peripheral and central level (Mallimo and Kusnecov, 2013). Therefore, an unequivocal role of N/OFQ in neuroinflammation cannot be established. Finally, we cannot rule out the possibility that endogenous N/OFQ exerts direct detrimental effects on DA neurons, since it inhibits the survival and growth of DA neurons in cultures through NOP receptor-mediated activation of the p38-MAPK pathway (Collins et al., 2015).
It could be argued that, at face-validity, the MPTP model does not fully recapitulate PD neurodegeneration occurring in humans. In fact, mice treated with this toxin do not present Lewy bodies, which are a hallmark of PD, and the neurodegenerative process triggered by MPTP directly targets cell bodies and is complete within few days from administration (Jackson-Lewis and Przedborski, 2007; Meredith and Rademacher, 2011). This is at variance with what is believed to be the pattern of neurodegeneration in PD patients, i.e. a slow and progressive process detected first at the level of striatal axon terminals (Burke and O'Malley, 2013; Kordower et al., 2013). However, in line with that observed in MPTP-treated mice, SB-612111 was able to spare a significant amount of TH+ cells in SNc and fibers in striatum even in rats overexpressing hα-syn. In this model, although providing only partial neuroprotection, SB-612111 increased by 50% the number of surviving DA cells, which is a relevant effect if we consider that SB-612111 administration started a week after AAV2/9-hα-syn injection, i.e. when DA neurodegeneration had already reached 50% of DA neurons (Bourdenx et al., 2015). Moreover, a lower SB-612111 dose was used in rats (1 mg/kg) compared to mice (10 mg/kg). This dose was chosen based on a previous study in rats where SB-612111 potently reversed 6-OHDA-induced motor deficits, and caused maximal effects at 1 mg/kg (Marti et al., 2013). Due the lack of available information on SB-612111 pharmacokinetics, we do not know the level of NOP receptor occupancy achieved by this dose. Therefore, we cannot rule out that a higher dose would provide a higher degree of neuroprotection. We should emphasize that while MPTP has identified a lot of false positive, the AAV-hα-syn model is very stringent, and very few strategies have proven effective in this model (Bezard et al., 2013). In addition, not many studies have provided evidence of efficacy for the same strategy, administered with a clinically-relevant schedule in both pathogenic and etiologic models of PD, which further increases our confidence that NOP receptor blockade indeed has potential as a novel disease-modifying strategy in PD.
The neuroprotective effect of SB-612111 is physiologically relevant, since it translated into a better stepping activity in rats. On the basis of a previous study in 6-OHDA-hemilesioned rats (Marti et al., 2013), it could be argued that the better stepping activity of SB-612111-treated AAV2/9-hα-syn injected rats might be due to a symptomatic effect rather than preservation of dopaminergic function. Indeed, in 6-OHDA-hemilesioned rats (Marti et al., 2013) the acute symptomatic effects of 1 mg/kg SB-612111 lasted (at least) up to 90 min after administration. Although this possibility cannot be ruled out, we should note that stepping activity was performed 9 h after the first SB-612111 administration, and in 6-OHDA-hemilesioned rats subacutely treated with 1 mg/kg SB-612111 (once daily for 15 days) no carryover effect was observed when motor activity was monitored 24 h after the last SB-612111 injection (Marti et al., 2013).
Regarding the mechanism of action of SB-612111 in AAV2/9-hα-syn injected rats, SB-612111 does not appear to target α-syn in a direct manner, i.e. α-syn clearance or aggregation, since no differences in hα-syn and p-α-syn load in SNc were found between SB-612111-treated and untreated animals. Therefore, the mechanism underlying neuroprotection could lie downstream, and impact other aspects of α-syn toxicity, such as the activation of immune response or the mitochondrial damage that are features of this (Ulusoy et al., 2010; Yamada et al., 2004) as well as the MPTP model (see above).
5. Concluding remarks
Our results show that genetic deletion or pharmacological blockade of the NOP receptor provides significant neuroprotection in the acute and subacute MPTP mouse models and/or in the AAV2/9 hα-syn rat model of PD. These data extend previous findings in MPTP-treated ppN/PFQ−/− mice (Brown et al., 2006; Marti et al., 2005), endorsing the view that endogenous N/OFQ plays a pathogenic role in PD-like neurodegeneration. Since the contribution of N/OFQ appears to be independent of the species (mouse vs. rats) and the model (pathogenic or etiologic) used, N/OFQ should be considered a key player in the degeneration of DA neurons associated with PD. Perhaps more relevant from a clinical perspective, systemic administration of the potent and NOP-selective antagonist SB-612111, administered in a clinically-driven schedule, not only protected but also rescued DA neurons from toxic insult. This is the first evidence for a neuroprotective/neurorescue effect of a NOP receptor antagonist in a PD model, and further extends our previous studies showing the symptomatic effect of NOP receptor antagonists in rodent and nonhuman primate models of PD, alone and in combination with L-DOPA (Mabrouk et al., 2010; Marti et al., 2013; Marti et al., 2005; Marti et al., 2008; Marti et al., 2007; Viaro et al., 2008; Visanji et al., 2008; Volta et al., 2010). While these new findings require confirmation with other NOP antagonists, our studies suggest a new approach for PD treatment, in which chronic therapy with a NOP receptor antagonist in association with subthreshold doses of L-DOPA may not only provide symptomatic relief and delay the appearance of dyskinesia, but also delay the neurodegenerative process associated with the disease (Marti et al., 2013; Marti et al., 2005; Marti et al., 2007).
Supplementary Material
Acknowledgments
This work has been supported by the Italian Ministry of University (FIRB Internazionalizzazione grant n. RBIN047W33 to M.M.), the Italian Society of Pharmacology (SIF; “Bando borse di ricerca per soggiorni di studio allestero 2015” to L.A.), the Emilia-Romagna and Region Aquitaine cooperation project n. 2255/2010 and 2011/2589 (to M.M. and E.B.), the University of Bordeaux, the Centre National de la Recherche Scientifique, the Agence Nationale de la Recherche (Grants ANR-12-BSV4-0001-01 and LABEX BRAIN ANR-10-LABX-43 to E.B.).
Abbreviations
- AAV2/9
adeno-associated viral vector pseudotype2/9
- DA
dopamine
- hα-syn
p.A53T human α-synuclein
- MPTP
1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine
- N/OFQ
nociceptin/orphanin FQ
- NOP
N/OFQ peptide receptor
- 6-OHDA
6-hydroxydopamine
- p-α-syn
phosphorylated α-synuclein
- PD
Parkinson's disease
- ppN/OFQ
N/OFQ precursor
- SNc
substantia nigra compacta
- SNr
substantia nigra reticulata
- TH
tyrosine hydroxylase
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2016.01.016.
References
- Bezard E. Neuroprotection for Parkinson's disease: a call for clinically driven experimental design. Lancet Neurol. 2003;2:393. doi: 10.1016/s1474-4422(03)00432-0. [DOI] [PubMed] [Google Scholar]
- Bezard E, et al. Enriched environment confers resistance to 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J. Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, et al. Animal models of Parkinson's disease: limits and relevance to neuroprotection studies. Mov. Disord. 2013;28:61–70. doi: 10.1002/mds.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M, et al. Lack of additive role of ageing in nigrostriatal neurodegenera- tion triggered by alpha-synuclein overexpression. Acta Neuropathol. Commun. 2015;3:46. doi: 10.1186/s40478-015-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, et al. Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J. Neurochem. 2006;98:495–505. doi: 10.1111/j.1471-4159.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- Burke RE, O'Malley K. Axon degeneration in Parkinson's disease. Exp. Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, et al. Pharmacology of nociceptin and its receptor: a novel therapeutic target Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Collins LM, et al. Nociceptin/orphanin FQ Inhibits the survival and axon growth of midbrain dopaminergic neurons through a p38-MAPK dependent mechanism. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9611-6. http://dx.doi.org/10.1007/s12035-015-9611-6. [DOI] [PubMed]
- Devine MJ, et al. Parkinson's disease and alpha-synuclein expression. Mov. Disord. 2011;26:2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto M, et al. Alterations of N/OFQ and NOP receptor gene expression in the substantia nigra and caudate putamen of MPP+ and 6-OHDA lesioned rats. Neuropharmacology. 2009;56:761–767. doi: 10.1016/j.neuropharm.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeln M, et al. Levodopa gains psychostimulant-like properties after nigral dopaminergic loss. Ann. Neurol. 2013;74:140–144. doi: 10.1002/ana.23881. [DOI] [PubMed] [Google Scholar]
- Fantin M, et al. Nocistatin inhibits 5-hydroxytryptamine release in the mouse neocortex via presynaptic Gi/o protein linked pathways. Br. J. Pharmacol. 2007;152:549–555. doi: 10.1038/sj.bjp.0707377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, et al. Age-related motor dysfunction and neuropathology in a transgenic mouse model of multiple system atrophy. Synapse. 2014;68:98–106. doi: 10.1002/syn.21719. [DOI] [PubMed] [Google Scholar]
- Flau K, et al. Inhibition of striatal and retinal dopamine release via nociceptin/orphanin FQ receptors. Br. J. Pharmacol. 2002;137:1355–1361. doi: 10.1038/sj.bjp.0704998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin S, et al. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- Florin S, et al. Orphan neuropeptide NocII, a putative pronociceptin maturation product, stimulates locomotion in mice. Neuroreport. 1997;8:705–707. doi: 10.1097/00001756-199702100-00025. [DOI] [PubMed] [Google Scholar]
- Furuya T, et al. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J. Neurosci. 2004;24:1865–1872. doi: 10.1523/JNEUROSCI.3309-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli EC, et al. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28:1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Gouty S, et al. MPTP treatment increases expression of pre-pro-nociceptin/orphanin FQ mRNA in a subset of substantia nigra reticulata neurons. Neuroscience. 2010;169:269–278. doi: 10.1016/j.neuroscience.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CE, et al. Pattern of levodopa-induced striatal changes is different in normal and MPTP-lesioned mice. J. Neurochem. 2003;84:1246–1255. doi: 10.1046/j.1471-4159.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, et al. Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J. Pharmacol. Exp. Ther. 2009;331:946–953. doi: 10.1124/jpet.109.156711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J. Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, et al. Evidence in locomotion test for the functional heterogeneity of ORL-1 receptors. Br. J. Pharmacol. 2004;141:132–140. doi: 10.1038/sj.bjp.0705583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Larsen JO, et al. Global spatial sampling with isotropic virtual planes: estimators of length density and total length in thick, arbitrarily orientated sections. J. Microsc. 1998;191:238–248. doi: 10.1046/j.1365-2818.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- Laudenbach V, et al. Nociceptin/orphanin FQ exacerbates excitotoxic white-matter lesions in the murine neonatal brain. J. Clin. Invest. 2001;107:457–466. doi: 10.1172/JCI9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Mabrouk OS, et al. Endogenous nociceptin/orphanin FQ (N/OFQ) contributes to haloperidol-induced changes of nigral amino acid transmission and parkinsonism: a combined microdialysis and behavioral study in naive and nociceptin/orphanin FQ receptor knockout mice. Neuroscience. 2010;166:40–48. doi: 10.1016/j.neuroscience.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Maidment NT, et al. Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport. 2002;13:1137–1140. doi: 10.1097/00001756-200207020-00013. [DOI] [PubMed] [Google Scholar]
- Mallimo EM, Kusnecov AW. The role of orphanin FQ/nociceptin in neuroplasticity: relationship to stress, anxiety and neuroinflammation. Front. Cell. Neurosci. 2013;7:173. doi: 10.3389/fncel.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, et al. Nociceptin/orphanin FQ receptors modulate glutamate extracellular levels in the substantia nigra pars reticulata. A microdialysis study in the awake freely moving rat. Neuroscience. 2002;112:153–160. doi: 10.1016/s0306-4522(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Marti M, et al. Blockade of nociceptin/orphanin FQ transmission in rat substantia nigra reverses haloperidol-induced akinesia and normalizes nigral glutamate release. J. Neurochem. 2004a;91:1501–1504. doi: 10.1111/j.1471-4159.2004.02843.x. [DOI] [PubMed] [Google Scholar]
- Marti M, et al. Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. J. Neurosci. 2004b;24:6659–6666. doi: 10.1523/JNEUROSCI.0987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, et al. Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson's disease. J. Neurosci. 2005;25:9591–9601. doi: 10.1523/JNEUROSCI.2546-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, et al. The nociceptin/orphanin FQ receptor antagonist J-113397 and L-DOPA additively attenuate experimental parkinsonism through overinhibition of the nigrothalamic pathway. J. Neurosci. 2007;27:1297–1307. doi: 10.1523/JNEUROSCI.4346-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, et al. The novel nociceptin/orphanin FQ receptor antagonist Trap-101 alleviates experimental parkinsonism through inhibition of the nigro-thalamic pathway: positive interaction with L-DOPA. J. Neurochem. 2008;107:1683–1696. doi: 10.1111/j.1471-4159.2008.05735.x. [DOI] [PubMed] [Google Scholar]
- Marti M, et al. Brain interstitial nociceptin/orphanin FQ levels are elevated in Parkinson's disease. Mov. Disord. 2010;25:1723–1732. doi: 10.1002/mds.23271. [DOI] [PubMed] [Google Scholar]
- Marti M, et al. Acute and chronic antiparkinsonian effects of the novel nociceptin/orphanin FQ receptor antagonist NiK-21273 in comparison with SB-612111. Br. J. Pharmacol. 2013;168:863–879. doi: 10.1111/j.1476-5381.2012.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Rademacher DJ. MPTP mouse models of Parkinson's disease: an update. J. Parkinsons Dis. 2011;1:19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Murphy NP. The nociceptin/orphanin FQ system as a target for treating alcoholism. CNS Neurol. Disord. Drug Targets. 2010;9:87–93. doi: 10.2174/187152710790966713. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Narayanan S, et al. Orphanin FQ/nociceptin suppresses motor activity through an action along the mesoaccumbens axis in rats. J. Psychiatry Neurosci. 2004;29:116–123. [PMC free article] [PubMed] [Google Scholar]
- Neal CR, Jr, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J. Comp. Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, et al. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Neal CR, Jr, et al. Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J. Chem. Neuroanat. 2001;22:219–249. doi: 10.1016/s0891-0618(01)00135-1. [DOI] [PubMed] [Google Scholar]
- Nishi M, et al. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton CS, et al. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J. Comp. Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, et al. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, et al. Identification of NIPSNAP1 as a nocistatin-interacting protein involving pain transmission. J. Biol. Chem. 2012;287:10403–10413. doi: 10.1074/jbc.M111.271866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, et al. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Przedborski S, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J. Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Recasens A, et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, et al. Orphanin-Fq - a neuropeptide that activates an opioid-like G-protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rizzi A, et al. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrah ydro-5 H-benzocyclohepten-5-ol]: in vivo studies. J. Pharmacol. Exp. Ther. 2007;321:968–974. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- Rozas G, Labandeira Garcia JL. Drug-free evaluation of rat models of parkinsonism and nigral grafts using a new automated rotarod test. Brain Res. 1997;749:188–199. doi: 10.1016/S0006-8993(96)01162-6. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology. 2004;172:129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, et al. The catalepsy test: its Ups and downs. Behav. Neurosci. 1988;102:748–759. doi: 10.1037//0735-7044.102.5.748. [DOI] [PubMed] [Google Scholar]
- Schallert T, et al. Excessive bracing reactions and their control by atropine and L-DOPA in an animal analog of parkinsonism. Exp. Neurol. 1979;64:33–43. doi: 10.1016/0014-4886(79)90003-7. [DOI] [PubMed] [Google Scholar]
- Serra PA, et al. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces apoptosis in mouse nigrostriatal glia. Relevance to nigral neuronal death and striatal neurochemical changes. J. Biol. Chem. 2002;277:34451–34461. doi: 10.1074/jbc.M202099200. [DOI] [PubMed] [Google Scholar]
- Spagnolo B, et al. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrah ydro-5 H-benzocyclohepten-5-ol]: in vitro studies. J. Pharmacol. Exp. Ther. 2007;321:961–967. doi: 10.1124/jpet.106.116764. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, et al. Time course of MPTP-induced degeneration of the nigrostriatal dopamine system in C57 BL/6 mice. Brain Res. Bull. 1988;21:257–263. doi: 10.1016/0361-9230(88)90240-7. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Thomas B, et al. MPTP and DSP-4 susceptibility of substantia nigra and locus coeruleus catecholaminergic neurons in mice is independent of parkin activity. Neurobiol. Dis. 2007;26:312–322. doi: 10.1016/j.nbd.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, et al. Viral vector-mediated overexpression of alpha-synuclein as a progressive model of Parkinson's disease. Prog. Brain Res. 2010;184:89–111. doi: 10.1016/S0079-6123(10)84005-1. [DOI] [PubMed] [Google Scholar]
- Van der Perren A, et al. Longitudinal follow-up and characterization of a robust rat model for Parkinson's disease based on overexpression of alpha-synuclein with adeno-associated viral vectors. Neurobiol. Aging. 2015;36:1543–1558. doi: 10.1016/j.neurobiolaging.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Viaro R, et al. Nociceptin/orphanin FQ receptor blockade attenuates MPTP-induced parkinsonism. Neurobiol. Dis. 2008;30:430–438. doi: 10.1016/j.nbd.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaro R, et al. Pharmacological and genetic evidence for pre- and postsynaptic D2 receptor involvement in motor responses to nociceptin/orphanin FQ receptor ligands. Neuropharmacology. 2013;72:126–138. doi: 10.1016/j.neuropharm.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Visanji NP, et al. The nociceptin/orphanin FQ (NOP) receptor antagonist J-113397 enhances the effects of levodopa in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov. Disord. 2008;23:1922–1925. doi: 10.1002/mds.22086. [DOI] [PubMed] [Google Scholar]
- Volta M, et al. Further evidence for an involvement of nociceptin/orphanin FQ in the pathophysiology of Parkinson's disease: a behavioral and neurochemical study in reserpinized mice. J. Neurochem. 2010;115:1543–1555. doi: 10.1111/j.1471-4159.2010.07061.x. [DOI] [PubMed] [Google Scholar]
- Volta M, et al. Dopamine-nociceptin/orphanin FQ interactions in the substantia nigra reticulata of hemiparkinsonian rats: involvement of D2/D3 receptors and impact on nigro-thalamic neurons and motor activity. Exp. Neurol. 2011;228:126–137. doi: 10.1016/j.expneurol.2010.12.024. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J. Comp. Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Yamada M, et al. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J. Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Zaratin PF, et al. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahy dro-5H–benzocyclohepten-5-ol (SB-612111) J. Pharmacol. Exp. Ther. 2004;308:454–461. doi: 10.1124/jpet.103.055848. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.