Abstract
When cultured with sufficient nutrient supply, engineered cartilage synthesizes proteoglycans rapidly, producing an osmotic swelling pressure that destabilizes immature collagen and prevents the development of a robust collagen framework, a hallmark of native cartilage. We hypothesized that mechanically constraining the proteoglycan-induced tissue swelling would enhance construct functional properties through the development of a more stable collagen framework. To test this hypothesis, we developed a novel “cage” growth system to mechanically prevent tissue constructs from swelling while ensuring adequate nutrient supply to the growing construct. The effectiveness of constrained culture was examined by testing constructs embedded within two different scaffolds: agarose and cartilage-derived matrix hydrogel (CDMH). Constructs were seeded with immature bovine chondrocytes and cultured under free swelling (FS) conditions for 14 days with transforming growth factor-β before being placed into a constraining cage for the remainder of culture. Controls were cultured under FS conditions throughout. Agarose constructs cultured in cages did not expand after the day 14 caging while FS constructs expanded to 8 × their day 0 weight after 112 days of culture. In addition to the physical differences in growth, by day 56, caged constructs had higher equilibrium (agarose: 639 ± 179 kPa and CDMH: 608 ± 257 kPa) and dynamic compressive moduli (agarose: 3.4 ± 1.0 MPa and CDMH 2.8 ± 1.0 MPa) than FS constructs (agarose: 193 ± 74 kPa and 1.1 ± 0.5 MPa and CDMH: 317 ± 93 kPa and 1.8 ± 1.0 MPa for equilibrium and dynamic properties, respectively). Interestingly, when normalized to final day wet weight, cage and FS constructs did not exhibit differences in proteoglycan or collagen content. However, caged culture enhanced collagen maturation through the increased formation of pyridinoline crosslinks and improved collagen matrix stability as measured by α-chymotrypsin solubility. These findings demonstrate that physically constrained culture of engineered cartilage constructs improves functional properties through improved collagen network maturity and stability. We anticipate that constrained culture may benefit other reported engineered cartilage systems that exhibit a mismatch in proteoglycan and collagen synthesis.
Keywords: : cartilage tissue engineering, constrained culture, collagen, proteoglycans, pyridinoline
Introduction
To date, most engineered cartilage culture techniques produce constructs lacking the comprehensive functional properties of native cartilage, such as equilibrium and dynamic moduli and failure properties in compression and tension, as well as frictional and wear properties. These characteristics of native cartilage are essential to its biomechanical function; therefore, functional cartilage tissue engineering aims to grow replacement tissues that are biomechanically similar to the native articular cartilage.1 This limitation of growing functional replacement tissues for resurfacing osteoarthritic defects has hindered the clinical translation of cartilage tissue engineering.2,3
In particular, native cartilage has a dense type II collagen fibrillar network that provides the tissue with a high tensile modulus, and a negatively charged glycosaminoglycan (GAG) ground matrix that imparts a high fixed charge density and robust compressive properties to the tissue.4–6 The interaction between collagen and GAG gives native cartilage unique compressive and tensile moduli as well as friction and wear properties ideally suited for its physiological function within the joint.7,8 Current engineered cartilage culture protocols successfully develop native GAG content and compressive moduli9; however, achieving native collagen content and tensile properties remains elusive.10
Although the subphysiological functional properties of tissue-engineered cartilage constructs are often attributed to the low biosynthetic activity of chondrocytes, we have recently shown that chondrocytes seeded at the cell densities present in the developing joint can deposit native levels of collagen, at the expense of producing supraphysiological levels of GAG.11,12 This rapid and excessive GAG synthesis and deposition results in a high degree of tissue growth and swelling as negatively charged GAG attracts ions and fluid from the surrounding media bath, increasing the osmotic swelling pressure within the construct. As the nascent collagen framework of engineered tissues provides only modest tensile stiffness,10 constructs volumetrically expand under this swelling pressure. This expansion continues throughout culture as adequately nourished chondrocytes will continuously synthesize and deposit GAG.
In our earlier work, this process resulted in constructs volumetrically growing nearly 800% in wet weight (ww) over a 105 day culture.12 This study was also our first promising evidence of constructs synthesizing native collagen contents, which reached 15% day 0 ww (%D0-ww). This deposition, however, was effectively diluted because of the profound tissue swelling (∼1.75%ww). Moreover, collagen solubility, measured as the susceptibility of collagen to α-chymotrypsin digestion, increased during culture. Increases in collagen solubility are associated with collagen destabilization and remodeling phenomena, including collagen denaturation, immaturity, and damage, all of which may weaken the bulk mechanical properties.13–15 Therefore, although both GAG and collagen are necessary for obtaining native cartilage functionality, high GAG deposition outcompetes and destabilizes the collagen network in developing cartilage constructs, hindering their functional properties.
Although the mismatch in GAG and collagen synthesis and deposition was originally observed in agarose scaffolds,16–24 an open question is whether matrix deposition mismatches may be scaffold dependent. Differences in scaffold ultrastructure and pore size may lead to different rates of matrix deposition and retention. However, a review of engineered cartilage systems suggests that GAG deposition exceeds collagen deposition in the majority of systems and that matrix deposition ratios do not match mature cartilage in any system (Table 1)9,25–33; thus although the field has been able to successfully promote chondrocyte matrix synthesis, engineered tissues are biochemically distinct from native cartilage.
Table 1.
Glycosaminoglycan and Collagen Deposition Levels and Ratios from the Literature Using Chondrocytes and Mesenchymal Stem Cells
| Study | Cell type | Scaffold | Cell density (106 cell/mL) | GAG level (%ww) | COL level (%ww) | COL:GAG ratio |
|---|---|---|---|---|---|---|
| Engineered cartilage: chondrocytes | ||||||
| Vunjak-Novakovic et al.25 | 2–3 w.o. Bov | 3% PGA | 127 | 4.5 | 3.75 | 0.83:1 |
| Makris et al.30 | 4–8 w.o. Bov | Self-assembled | 117a | 1.45b | 1.75b | 1.2:1 |
| Mauck et al.26 | 3–6 m.o. Bov | 2% agarose | 60c | 1.7 | 1.8 | 1.1:1 |
| Ng et al.28 | 2–5 y.o. K9 | 2% agarose | 30c | 4.27d | 1.06d | 0.25:1 |
| Mesallati et al.32 | 4 m.o. Por | 2% agarose | 23 | 1.8 | 0.96 | 0.55:1 |
| Byers et al.9 | 4–6 m.o. Bov | 2% agarose | 6 | 6.6 | 3.3 | 0.5:1 |
| Engineered cartilage: mesenchymal stem cells | ||||||
| Bhumiratana et al.31 | Human | Self-assembled | 103 | 7.5f | 9.5e,f | 1.26:1 |
| Erickson et al.29 | Bov | 1% MeHA | 60c | 4.8 | 4.8 | 1:1 |
| Chung et al.27 | Human | 1:1 MeHA | 15 | 2.5f | 0.83f | 0.32:1 |
| Native cartilage: medial femoral condyle | ||||||
| Williamson et al.33 | 1–3 w.o. Bov | N/A | 66 | 2.1e | 6.9e | 3.2:1 |
| Williamson et al.33 | 1–2 y.o. Bov | N/A | 39 | 1.8e | 7.9e | 4.4:1 |
Data are arranged by cell type and density.
Native biochemical content in juvenile and adult bovine cartilage is listed in the two bottom rows.
Based on an earlier study.
Average between control groups of the two engineered cartilage studies. Cell densities were obtained either from the cell content reported, the DNA content reported and converted here based on a ratio of 7.7 pg DNA/cell, or, when experimental data were not reported, the nominal cell density; nominal cell densities are denoted with c.
Based on the day 28 mixed animal data.
Assumes an OHP:collagen mass ratio of 1:7.6.
Normalization reported in the study on a tissue ww (mg or g) basis and converted here to %ww assuming a density of 1 g/mL.
GAG, glycosaminoglycan; COL, collagen; Bov, bovine; K9, canine; Por, porcine; ww, wet weight; w.o., weeks old; y.o., years old; PGA, polyglycolic acid; MeHA, methacrylate hyaluronic acid; N/A, not applicable.
This trend seems to be present across scaffolds and cell types, importantly suggesting that clinical translational will remain limited if systems are not developed to address matrix synthesis mismatch. The field of cartilage tissue engineering has increasingly turned to extracellular matrix-based scaffolds to mimic the native cartilage environment34–39; of these, cartilage-derived and collagen-based scaffolds are widely used in both research and preclinical settings.36,40 Although results are promising because of their high initial matrix content, these scaffolds are prone to initial contraction and it is unclear whether they can produce a functional collagen network.
We hypothesize that suppressing GAG-induced construct swelling and growth, while maintaining nutrient access and matrix synthesis, can enhance collagen integrity and thereby lead to enhanced functional properties. To this end, we developed a constraint-based culture system (“cage”) that physically confines osmotically induced tissue growth and swelling. Cages were designed to mechanically restrain construct growth within a fixed volume and shape, and provide sufficient nutrient access to the construct.
In Study 1, we test our hypothesis utilizing an agarose scaffold seeded with juvenile bovine chondrocytes, a system we have used successfully for more than a decade.41 Based on the encouraging results of Study 1, we performed a pilot test (Study 2) of translating the cage system to a scaffold derived from native cartilage extracellular matrix (cartilage-derived matrix hydrogel, CDMH), which contracts significantly in the initial culture period, before swelling upon the synthesis and deposition of extracellular matrix. For both studies, constructs cultured within cages employed our previously reported tissue culture strategies for optimizing growth, namely the introduction of an optimal arrangement of nutrient channels,42 which are perfused by orbital shaking of the culture system43 at a sufficient media volume:tissue ratio that prevents glucose depletion.44
Methods
CDMH preparation
Hyaline cartilage was harvested from Yorkshire pigs weighing 40–50 kg immediately after euthanasia under a tissue-sharing protocol approved by the Columbia University Institutional Animal Care and Use Committee. Hyaline cartilage was cleaned of all connective tissue and debris, rinsed in cold sterile normal saline, and frozen at −80°C for a minimum of 24 h and a maximum of 2 weeks before isolation and processing of CDMH.
Cartilage was sectioned to 1 mm thickness and serially washed in an orbital shaker in hypertonic (2× ) phosphate-buffered saline (PBS) for 15 min, 0.02% trypsin for 15 min, and 3% Tween-20 for 30 min. After each step, cartilage was washed in 2× PBS for 15 min. Cartilage sections were snap frozen in liquid nitrogen, milled into a fine powder, and lyophilized for 24 h. Powdered cartilage was washed in 4% sodium deoxycholate for 30 min, 2× PBS for 15 min, 0.2 mg/mL DNase I (Sigma D4527) for 24 h, 0.1% peracetic acid for 30 min, and sterile deionized water for 30 min. The cartilage matrix slurry was then snap frozen in liquid nitrogen, lyophilized for 24 h, and stored at room temperature.
Dry cartilage matrix powder was digested as previously described.45 In brief, 1 g of lyophilized cartilage matrix powder was mixed with 0.1 g pepsin (Sigma P7012) in 0.01 M hydrochloric acid and digested for 18 h at room temperature (25°C) under constant stirring. Matrix digests were aliquoted and stored at −80°C until use. Cells were encapsulated within hydrogels by reconstituting the cartilage matrix digest with 0.1 M sodium hydroxide, 10× PBS, and chondrocytes suspended in culture media to yield a cartilage matrix hydrogel with a final concentration of 6 mg/mL.
Construct casting and culture
Chondrocytes were isolated from juvenile (4–8 weeks old) bovine carpometacarpal joints and passaged twice in monolayer culture, as previously described.46 Cells were released with 0.05% trypsin and encapsulated in either agarose (Study 1) or CDMH (Study 2).
In Study 1, constructs were made as previously described43: chondrocytes (120 × 106 cells/mL) were mixed 1:1 with type VII-A agarose (Sigma) and poured into a glass and Teflon-backed mold with a pattern of prearranged pins, such that after punching ø10 mm × 2.34 mm constructs, each construct possessed 12 × ø1 mm nutrient channels.43 In Study 2, chondrocytes (120 × 106 cells/mL) were mixed with CDMH and gelled at 37°C and 5% CO2 before biopsy punching ø10 mm × 2.34 mm constructs. Final nominal cell densities were 60 × 106 cells/mL in the agarose constructs of Study 1 and 33 × 106 cells/mL in the CDMH constructs of Study 2 before scaffold contraction, as described hereunder.
CDMH constructs were cast and initially punched without nutrient channels. Cellular contraction of the CDMH47 reduced the ø10 mm × 2.34 mm constructs to approximately ø4 mm × 0.8 mm after 14 days of culture. In a preliminary study using CDMH, the scaffold contraction caused a large increase in cell density such that constructs exhibited severe matrix heterogeneities despite their small size (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Therefore, to enhance nutrient exchange in Study 2, a single ø1 mm channel was punched in the center of each ø4 mm × 0.8 mm CDMH construct on day 14; the large degree of scaffold contraction in the first 14 days of culture precluded our use of the channel molds from Study 1, as this contraction would have distorted any channel arrangement.
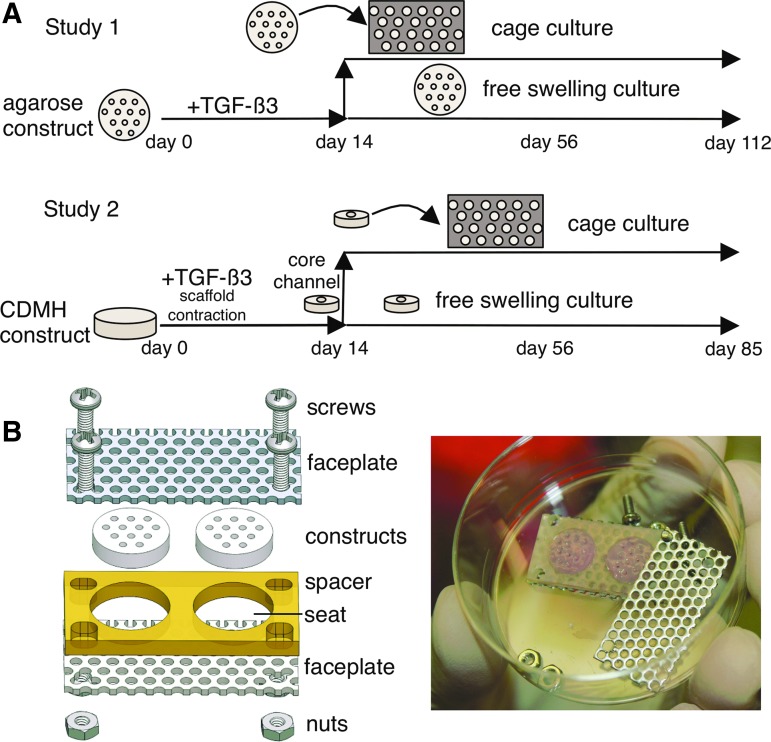
Constructs in both studies were cultured in standard chemically defined chondrogenic media under 0.8 Hz orbital shaking, and the large agarose constructs of Study 1 were placed vertically in culture racks to enhance nutrient access.43 Constructs received 10 ng/mL transforming growth factor (TGF)-β3 supplementation (R&D Systems) for the first 14 days of culture.48 As tissue growth and expansion typically become significant after the 14-day TGF-β priming, constructs were divided into free swelling (FS) and cage groups on day 14.9,49 Caged constructs were cultured for the remainder of the study within the cage, whereas FS constructs continued their culture unconstrained. Constructs were assessed in Study 1 on days 0 (n = 2), 14 (n = 4), 56 (n = 6), and 112 (n = 6). As little differences were observed between days 56 and 112 in Study 1, Study 2 constructs were assessed on days 14 (n = 4), 56 (n = 4–6), and 85 (n = 6) (Fig. 1A). Before scaffold contraction, CDMH constructs lacked mechanical integrity and were, therefore, not tested or sampled immediately after casting on day 0.
FIG. 1.
(A) Study 1 (agarose) and Study 2 (CDMH) overview including channeling, transforming growth factor-β3 supplementation, caging, and construct time points. (B) Cage assembly diagram (left) and with construct (right).
Cage components and manufacturing
Cages consisted of two faceplates abutting a single spacer and the assembly was secured with four pairs of stainless steel screws and nuts (Fig. 1B). Faceplates consisted of perforated stainless steel sheets (316, ø0.625 mm holes, center-to-center spacing 0.094 mm; McMaster-Carr) to allow media to flow to the construct while preventing axial swelling. Spacers were made from an impermeable biocompatible material (Study 1: polysulfone; Study 2: polytetrafluoroethylene) and construct seats were sized to fit day 14 construct dimensions (Study 1: ø10.5 mm × 2.34 mm; Study 2: ø4 mm × 0.8 mm) and prevent radial swelling. All materials were autoclaved before assembly.
Cages were assembled by placing constructs into the seats of the spacer and fastening faceplates to opposing sides of the spacer. To promote nutrient transport, channels were aligned with faceplate perforations and a ø1 mm biopsy punch was passed through the faceplates and construct to ensure clear media passage. Channels of the cage constructs became progressively occluded in Study 1 and were recored once (day 58). The supplementation of TGF-β3 (all constructs in Study 1 and Study 2) and recoring of channels (cage constructs, where channels were occluded, in Study 1) served to enhance nutrient transport (primarily glucose and TGF-β3) where we had evidence of nutrient limitation (large agarose constructs46 and CDMH: Supplementary Fig. S1).
Mechanical testing
Mechanical testing included measurements of compressive unconfined equilibrium modulus at 10% compression (EY) and compressive unconfined dynamic modulus (G*) at 0.01 Hz and 1% strain amplitude, as previously described.43
Biochemical analyses
Biochemical analyses measured the matrix deposition of negatively charged GAG using the dimethylmethylene blue dye-binding assay50 and collagen using the acid hydrolysis–orthohydroxyproline (OHP) assay and a collagen:OHP conversion ratio of 7.6:1.51,52 Cell content was measured using the PicoGreen assay (Invitrogen) based on a ratio of 7.7 pg DNA/cell.53 Cell and matrix concentrations were normalized to the current day ww (%ww) to provide a measure of the current matrix content and to the day 0 ww (%D0-ww) to provide a measure of total matrix deposition.44
Soluble collagen content was measured following the protocol of Bank et al.13: subpunched ø3 mm construct cores were desorbed of unbound collagen for 48 h at 4°C under continuous rotation in a 4 M guanidine HCl buffer solution (0.1 M TrisHCl, 10 μg/mL pepstatin-A, 1 mM iodoacetamide, 1 mM ethylenediaminetetraacetic acid, pH 7.3). Constructs were rinsed for 8 h in buffer at 4°C before adding a 0.5 mg/mL solution (0.5 mL per sample) of α-chymotrypsin (Sigma) and incubating for 16 h at 37°C. α-chymotrypsin separates collagens into soluble and intact (bound) fractions corresponding to their stability within the tissue. Aspiration of the incubation buffer isolates the soluble collagen fraction, leaving the intact collagen fraction within the tissue pellet. The aspirant and pellet were assayed with the OHP assay, and collagen solubility was calculated as (soluble collagen mass)/(soluble collagen mass + intact collagen mass). The collagen crosslink pyridinoline (PYD) was measured with a PYD enzyme linked immunosorbent assay (Quidel) using the construct acid hydrolysis digests.49 PYD content is presented on a per tissue ww basis (nmol/mL) and a per collagen basis (mol PYD/mol COL), assuming a collagen molecular weight of 285 kDa.54
Histology
Samples were fixed for 24 h in acid–formalin–ethanol and placed into 70% ethanol before sectioning (5 μm slices).46 GAG was observed with 0.01% Safranin-O staining and collagen was observed with 0.1% Picrosirius Red staining.55
Statistics
Differences between treatments and time points of the mechanical and biochemical data were assessed with a two-way analysis of variance (ANOVA; α = 0.05, computed in R, r-project.org) to determine the statistical influence of culture duration (day), culture treatment (FS vs. cage), and their interaction (duration × treatment). Tukey corrected post hoc comparisons were made between groups exhibiting significant differences (p ≤ 0.05) according to the ANOVA. The significance of each factor (day, group, interaction) for each measure is listed in the Supplementary Table S1 and when differences between the specific factor levels (day or treatment) or individual groups (interaction) were significant (p < 0.05), the significance was displayed within the corresponding figure (Figs. 2–5). Data reported here are presented as mean ± standard deviation.
FIG. 2.
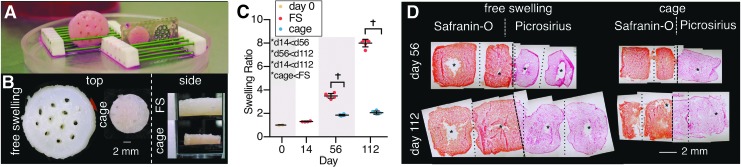
Study 1, agarose constructs: (A) Image of construct differences from FS (left) and cage (right; front faceplate removed for construct viewing) on day 112. (B) Morphological differences in cage growth from top and side perspectives (FS side view only shows middle of construct). (C) Swelling ratio differences between FS and caged constructs. *Denotes significant (p < 0.05) differences between factor levels. †Denotes significant (p < 0.05) differences between individual groups at the same time point. X-axis spacing is not temporally representative. (D) Histological images (side view) of constructs (Safranin-O displays GAG, Picrosirius displays collagen). Dashed lines represent construct midlines and separates Safranin-O and Picrosirius Red images; dotted lines represent construct channels or preexisting channels; * are voids or tears in construct. GAG, glycosaminoglycan; FS, free swelling.
FIG. 3.
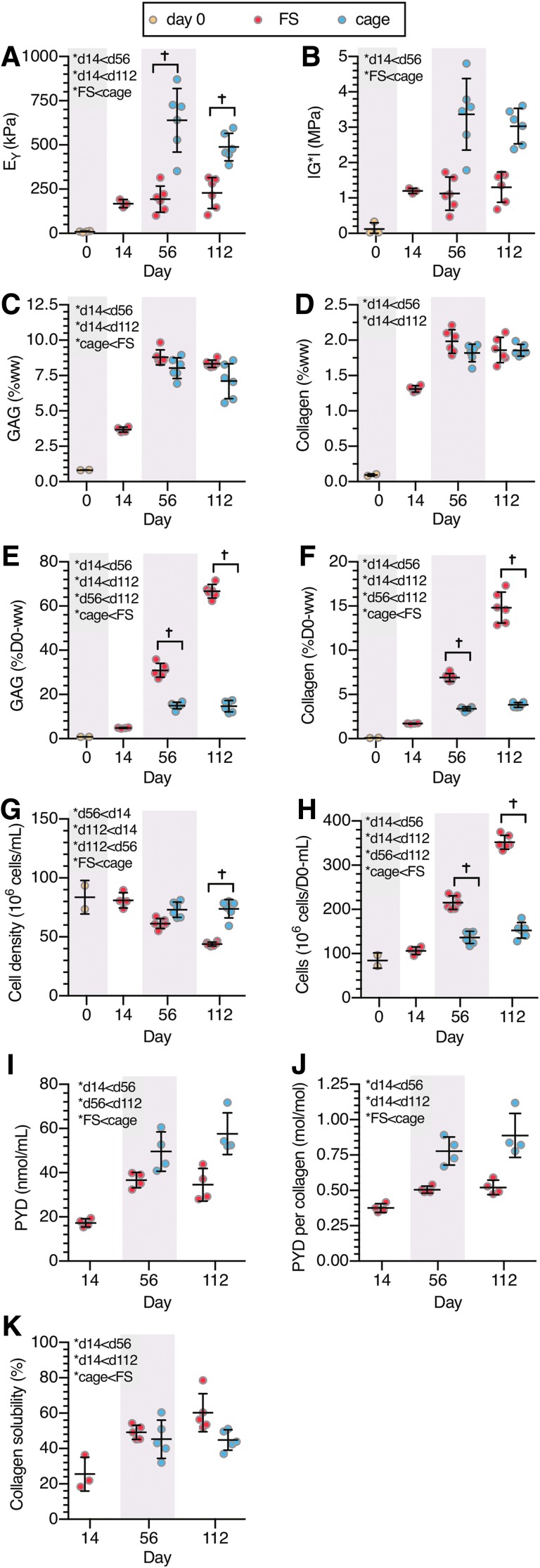
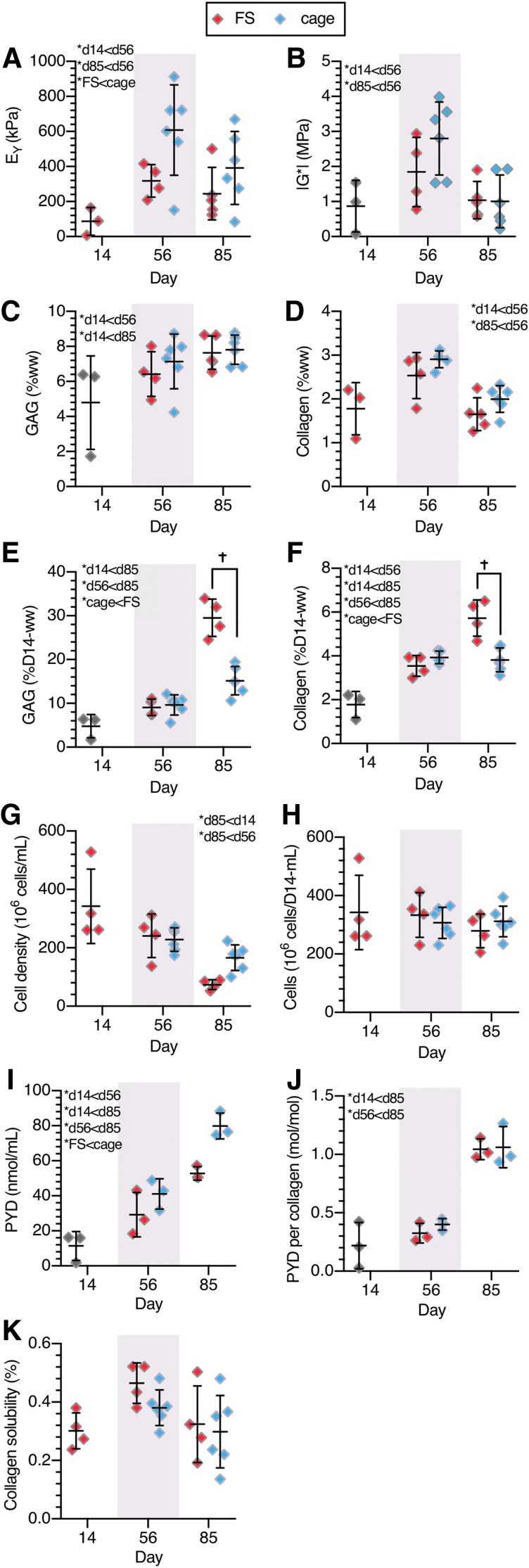
Study 1, agarose constructs: (A) equilibrium compressive modulus, EY, (B) dynamic modulus, G*, (C) GAG (%ww), (D) collagen (%ww), (E) GAG (%D0-ww), (F) collagen (%D0-ww), (G) cell density, (H) total cell content, (I) PYD (nmol/mL), (J) PYD/collagen (mol/mol), (K) collagen solubility. *Denotes significant (p < 0.05) differences between factor levels. †Denotes significant (p < 0.05) differences in individual groups at the same time point. X-axis spacing is not temporally representative. PYD, pyridinoline; ww, wet weight.
FIG. 4.
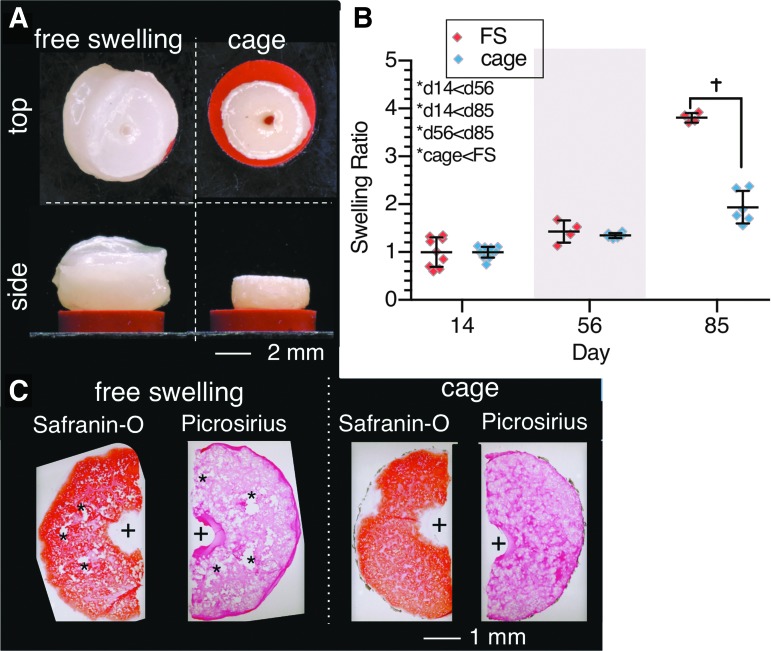
Study 2, CDMH constructs: (A) Morphological differences (top and side profiles) of FS and cage groups on day 85. (B) Swelling ratio differences between FS and caged constructs. *Denotes significant (p < 0.05) differences between factor levels. †Denotes significant (p < 0.05) differences between individual groups at the same time point. X-axis spacing is not temporally representative. (C) Histological (top view) images of constructs (Safranin-O displays GAG, Picrosirius displays collagen) on day 56. + represents channel location, * represent voids or tears in construct. CDMH, cartilage-derived matrix hydrogel.
FIG. 5.
Study 2, CDMH constructs: (A) equilibrium compressive modulus, EY, (B) dynamic modulus, G*, (C) GAG (%ww), (D) collagen (%ww), (E) GAG (%D0-ww), (F) collagen (%D0-ww), (G) cell density, (H) total cell content, (I) PYD (nmol/mL), (J) PYD/collagen (mol/mol), (K) collagen solubility. *Denotes significant (p < 0.05) differences between factor levels. †Denotes significant (p < 0.05) differences between individual groups at same time point. X-axis spacing is not temporally representative.
Results
Study 1: agarose constructs
FS agarose constructs grew to 8 × their original size after 112 days of culture, whereas caged constructs swelled significantly less (2 × , p < 0.001), essentially maintaining the size they had achieved before being placed in the cage on day 14 (Fig. 2). FS constructs had dense Safranin-O and Picrosirius Red staining in the periphery, but interior voids were present on days 56 and 112 (Fig. 2). Caged constructs also exhibited rich Safranin-O and Picrosirius Red staining and maintained tissue integrity on day 56, but by day 112, they appeared less organized and interior tears were evident.
Polarized light microscopy images showed no directed tissue ultrastructure in caged constructs, but showed highly aligned collagen ultrastructure between the interior tissue void and construct periphery of FS tissues, reminiscent of native cartilage (Supplementary Fig. S2).
Construct functional properties (Fig. 3A, B) improved with culture duration before plateauing after day 56 (p > 0.40 for EY and G* between days 56 and 112), with both EY and G* higher in caged constructs than in FS constructs (p < 0.001). GAG and collagen %ww contents followed similar temporal trends as the mechanical properties and plateaued after day 56 (p < 0.11 vs. day 112, Fig. 3C, D). GAG content was significantly lower in caged constructs (p < 0.004); meanwhile collagen content was similar between the groups (p = 0.17). In contrast, both GAG and collagen matrix deposition (%D0-ww) increased throughout culture and was significantly higher in FS constructs (p < 0.001, Fig. 3E, F). By day 14, constructs had deposited 4.8 ± 0.2%D0-ww GAG and 1.7 ± 0.05%D0-ww collagen, after which time FS constructs continued to deposit matrix, amounting to 66.6 ± 3.1%D0-ww GAG and 14.8 ± 1.7%D0-ww collagen by day 112 (p < 0.001). Caged constructs deposited less matrix once constrained, and matrix deposition did not change after day 56 (p > 0.98), holding at 14.7 ± 2.6%D0-ww GAG and 3.4 ± 0.3%D0-ww collagen.
By day 112, cell densities did not change significantly from day 0 levels (84 ± 14 million cells/mL) in caged constructs (74 ± 8 million cells/mL, p = 0.56), but significantly decreased in FS constructs (44 ± 2 million cells/mL, p < 0.001) (Fig. 3G). Conversely, total cell content (Fig. 3H) was higher in FS constructs (p < 0.001) and increased throughout culture (p = 0.002). Total cell content in caged constructs did not change with time (p = 0.52).
PYD content was higher in cage culture than in FS for both measures of PYD content (p < 0.001) and PYD normalized to collagen (p < 0.001) (Fig. 3I, J). PYD content did not significantly change after day 56 (p > 0.34), similar to collagen content. In particular, cage constructs exhibited 70% more PYD normalized to collagen and 67% more PYD content than FS constructs on day 112. Collagen solubility (Fig. 3K) increased between days 14 and 56 (p = 0.003) and was higher in FS constructs (p = 0.03), suggesting that collagen was more stable in cage constructs.
Fluid volume fractions (Supplementary Fig. S3) changed throughout culture and were higher in cage constructs (p = 0.011).
Study 2: CDMH constructs
CDMH constructs grew significantly over the 85-day culture (Fig. 4A), with FS constructs swelling significantly more (3.8 × , p < 0.001) than cage constructs (1.9 × swelling) when normalized to their day 14 (postcontraction) ww (Fig. 4B). Adding a channel aided in nutrient availability and the large interior voids seen in our preliminary work (Supplementary Fig. S1) were not present in Study 2; with channels, robust Picrosirius Red and Safranin-O staining was observed throughout the CDMH constructs (Fig. 4C). In particular, constructs grown in cages exhibited more uniform staining than FS CDMH, and caged CDMH constructs displayed richer Picrosirius Red staining than FS CDMH.
Caged growth enhanced EY over FS culture (p = 0.007) (Fig. 5A), whereas G* was similar between the groups, peaking on day 56 (p = 0.001) before returning to day 14 levels by day 85 (p = 0.89) (Fig. 5B). GAG content was also similar between the culture conditions (p = 0.52) and increased with culture duration (p = 0.005) (Fig. 5C). Collagen content trended higher in caged constructs, although not significantly so (p = 0.07) (Fig. 5D).
GAG deposition in caged CDMH was lower than in FS CDMH constructs (p < 0.006), as was collagen deposition (p = 0.01). Overall, deposition of GAG and collagen increased from days 14 to 85 for both groups (cage p < 0.001, FS p < 0.001) (Fig. 5E, F).
Owing to the initial scaffold contraction over the first 14 days of culture, cell density in CDMH constructs (342 ± 150 million cells/mL; Fig. 5G) was markedly higher than the seeding density used in Study 1 (84 million cells/mL). Total cell content (cells normalized to day 14 ww) did not change over the culture duration in CDMH constructs (p = 0.15) and was not influenced by the cage treatment (p = 0.25; Fig. 5H). However, when accounting for construct swelling, cell density within CDMH constructs decreased from day 14 to day 85 in both cage and FS groups (p < 0.03; Fig. 5G).
Similar to the agarose constructs of Study 1, cages produced increased PYD content within CDMH constructs (Fig. 5I), although when normalized to collagen content, cage and FS had a similarly elevated level by day 85 (Fig. 5K; ∼1 mol PYD/mol collagen). The collagen solubility assay did not detect any differences with treatment or culture duration in CDMH constructs (Fig. 5K).
Discussion
Constrained cage growth of engineered cartilage reduced swelling (Figs. 2C and 4B) and improved construct functional properties in both the agarose and CDMH constructs (Figs. 3A, B and 5A, B), supporting our hypothesis that osmotically induced swelling from rapid GAG deposition can hinder the properties of developing engineered cartilage constructs. We developed this strategy based on our prior observation that although cartilage constructs can synthesize GAG and collagen to match native levels, chondrocytes synthesize GAG more rapidly than collagen.12
Here, the mismatch in matrix deposition in FS constructs resulted in extreme tissue swelling and growth [8 × in agarose constructs (Fig. 2C) and 3.8 × in CDMH (Fig. 4B)] and disrupted maturation of an intact collagen network (Fig. 3I–K for agarose), thereby weakening the tissue56 (Fig. 3A, B for agarose; Fig. 5A, B for CDMH). Cage culture constrained tissue swelling and excessive deposition of GAG, improving functional properties in constructs with both agarose (EY and G*, Fig. 3A, B) and CDMH (EY, Fig. 5A) scaffolds.
Therefore, mechanical constraints against swelling can enhance functional properties by preventing excessive proteoglycan deposition (agarose: Fig. 3E; CDMH: Fig. 5E), a widespread issue across engineered cartilage tissues (Table 1). However, cages also constrained total collagen deposition (agarose: Fig. 3F; CDMH: Fig. 5F), so that concentrations of GAG and collagen were similar in FS and caged constructs when accounting for the tissue swelling disparities (agarose: Fig. 3C, D; CDMH: Fig. 5C, D).
The best mechanical properties achieved in this study were observed in caged agarose (Fig. 3A, B) and caged CDMH (Fig. 5A, B) constructs on day 56. The equilibrium Young's modulus in unconfined compression was EY = 639 ± 179 kPa for agarose constructs and EY = 608 ± 257 kPa for CDMH constructs and the dynamic unconfined compression modulus at 0.01 Hz was G* = 3.4 ± 1.0 MPa for agarose constructs and G* = 2.8 ± 1.0 MPa for CDMH constructs. For comparison purposes, we have previously measured EY = 660 ± 230 kPa and G* = 6.5 ± 2.1 MPa in native immature bovine cartilage explants.57 Therefore, EY in the best constructs matches the native level, whereas G* is half the native value. These findings are consistent with the observation that GAG levels in this group of constructs (agarose: 8.0 ± 0.7%ww; CDMH: 7.7 ± 0.8%ww) are higher than native cartilage levels (3.3 ± 0.8%ww), whereas collagen content (agarose: 1.8 ± 0.1%ww; CDMH: 2.9 ± 0.2%ww) is lower than native levels (9.5 ± 2.1%ww).58 Proteoglycans are known to enhance the compressive properties,59,60 as manifested in EY, whereas collagen enhances the tensile properties61 and thus the dynamic unconfined compression modulus G*.57
Despite the increase in GAG content from days 56 to 85, the functional properties of all CDMH groups declined between these time points as did collagen content (Fig. 5A–D), whereas the functional properties in agarose constructs were similar on days 56 and 112. Unlike the cage agarose constructs, cage CDMH swelling ratio increased between days 56 and 85, suggesting that swelling was still possible within the cages likely because of heterogeneous construct sizing after cellular contraction, resulting in an imperfect fit within the confining cage (Fig. 4B). Despite the variability in CDMH cell density on day 14, 342 ± 150 million cells/mL (Fig. 5G), constructs had similar total cell contents, 2.44 ± 0.51 million cells per construct, suggesting that scaffolds were still transiently contracting at this time point. The low variance of cell density at later time points supports this idea: as initial scaffold contraction subsides, a reversal occurs and the tissue swells from matrix deposition, leading to more consistent biochemical measures. Future studies will characterize the role of cell seeding density on scaffold contraction magnitude and duration.
Although matrix deposition was nearly constant in FS constructs throughout culture (nearly linear rise in %D0-ww for FS agarose constructs shown in Fig. 3E, F starting on day 14 and CDMH constructs shown in Fig. 5E, F), net deposition ceased in caged constructs after day 56. However, despite this dramatic difference in net matrix deposition, both FS and caged constructs had similar matrix concentrations (agarose: ∼8.0%ww GAG and ∼1.9%ww collagen; CDMH ∼7.3%ww GAG and ∼2.4%ww collagen). These two observations suggest a signaling role of the extracellular matrix to control chondrocyte matrix synthesis. In FS constructs, negative feedback cues may be continually diluted by the swelling effect, promoting continuous matrix synthesis. Conversely, when construct swelling is prevented, these signals concentrate and inhibit matrix synthesis. Although the nature of this signaling mechanism was not identified here, recent studies have implicated TRPV-4 channels in the transduction of extracellular environment signals to matrix synthesis modulation.62 TRPV-4 is responsive to the osmotic environment that is regulated by the GAG %ww concentration; thus, the observed plateau in GAG %ww for both groups (Fig. 3C) would be consistent with this type of signaling mechanism.
Despite cessation of net matrix deposition, caged constructs developed higher PYD content and better functional properties. In effect, the 54% increase in PYD between FS and caged agarose constructs (Fig. 3I, J) correlated with a threefold increase in EY and G* on day 56 (Fig. 3A, B) and a 10% decrease in solubility (Fig. 3K). This finding suggests that enhancing collagen maturity is critical to developing functional engineered cartilage.63,64 PYD and other intermolecular collagen crosslinks form spontaneously between enzymatically (lysyl oxidase, LOX) formed residues on adjacent collagen molecules.65,66 The finding that collagen maturation is promoted by constrained growth is strong evidence that the physical expansion of a growing engineered tissue hinders the formation of PYD and prevents collagen maturation because of collagen content dilution.
Makris et al. demonstrated that mechanical properties can be increased with increase in PYD content by treating self-assembled cartilage constructs with exogenous LOX.30 However, even the highest LOX dose did not develop tissue matching the functionality of native cartilage. In comparison with their study, the constrained culture approach of this study increased PYD content more than exogenous LOX supplementation alone. These results suggest that a proximity between collagen molecules needs to be maintained to facilitate PYD formation in engineered cartilage. Furthermore, the considerable enhancement in EY and G* between FS and caged groups may not have resulted entirely from the increased PYD levels, because other types of crosslinks may also exist in cartilage67 that are not characterized here.
The constraint strategy employed here increased collagen stability in agarose constructs and maintained cellular content but did not improve collagen levels, even though PYD of caged constructs matched native levels in adult bovine cartilage (0.74–0.97 mol PYD/mol COL)33 when normalized to collagen. This deficiency in collagen content likely explains why tissue construct G* remains less than that of native cartilage. In previous work, our laboratory and others digested existing GAG using the enzyme chondroitinase ABC to temporarily increase collagen content relative to GAG. Although initially promising, these treatments proved deleterious to cellular health and viability, stymieing further matrix synthesis.68–70 Therefore, although the constrained culture presented here will likely prove critical in enhancing tissue functionality and collagen stability, the field of cartilage tissue engineering still requires strategies to enhance collagen content.
The nonintuitive strategy of constrained growth became evident after our extensive work to enhance and optimize growth conditions utilizing native cell densities in young cartilage with sufficient nutrient availability.12,52 Elevated cell densities, approaching those found in developing cartilage, were seeded in both agarose (89 million cells/mL) and collagen (342 million cells/mL) scaffolds.71 Physiological cell densities, effective for engineered cartilage growth with juvenile bovine and adult human chondrocytes, require adequate nutrition to the developing tissue. Tissue constructs of either high cell density or anatomical dimensions, or both, are prone to nutrient scarcities and subsequent heterogeneous matrix deposition and growth.3,72,73 Supplementing nutrient channels within engineered cartilage constructs has provided a simple means of ameliorating nutrient scarcities.74–77 Although the need for channels was previously demonstrated in an agarose scaffold system, this study suggests that channels can also improve contracted collagen-based scaffold culture where the cell densities reach very high levels and may prove beneficial across other tissue engineering systems. In particular, scaffoldless systems, which exhibit high cell densities, often display heterogeneous matrix deposition and growth despite their small size, and may benefit from nutrient channel placement and a caging strategy, similar to the CDMH constructs here.31,73,78
Cages were specifically designed to allow media transport through the nutrient channels. The steel faceplates used here were adequate for this study, but the selection of cage materials will be dependent on the tissue culture system. In this study, we placed the constructs in cages only after 14 days of FS culture, coinciding with the termination of TGF-β supplementation. We have previously shown that steel has a high binding affinity for active TGF-β, suggesting that culture systems requiring continuous TGF-β supplementation will require different materials.79,80
The species of cells and CDMH scaffold presented here (bovine and porcine, respectively) were selected based on availability and past reports and experience. Future studies will involve validating the cage strategy using mature chondrocytes (canine and human) with agarose and CDMH scaffolds. With translation in mind, we will continue to use the porcine CDMH scaffolds, because sourcing canine and human cartilage matrix for the scaffolds would be limited because of availability. Mature canine and human chondrocytes exhibit the GAG–collagen matrix deposition mismatch as previously reported in immature chondrocytes and mesenchymal stem cells.27,28,52,81 Our recent findings demonstrate that after 56 days, mature human chondrocytes can create cartilage tissue with biochemical and mechanical properties matching tissue grown using immature bovine cells.52 The results of this work show promise for the translation of the caging strategy across multiple scaffold platforms and, in conjunction with our recent study,52 demonstrate the potential for translation to improve tissues grown from adult human chondrocytes.
This study demonstrated that cages may be used effectively for constraining the swelling of engineered constructs resulting from high levels of matrix deposition. The primary benefits of this cage system were to enhance mature crosslink formation and to maintain the desired construct dimensions, leading to better functional properties than FS controls. This approach was shown to be effective using agarose as well as cartilage-derived collagen scaffolds. Therefore, this novel constrained culture approach holds promise for improving functional properties of large anatomically sized constructs in a variety of tissue engineering systems.46
Supplementary Material
Acknowledgments
We thank Dr. Sevan R. Oungoulian and Mr. Mohamed Haroun for their assistance in developing and constructing culture cages. Research reported in this publication was supported by the National Institutes of Health under Award Numbers R01AR060361, R01AR046568, T32AR059038, R01DE016525, and P41EB002520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interest exist.
References
- 1.Butler D.L., Goldstein S.A., and Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng 122, 570, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Moisio K., Eckstein F., Chmiel J.S., Guermazi A., Prasad P., Almagor O., Song J., Dunlop D., Hudelmaier M., and Kothari A. Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis Rheum 60, 3703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung C.T., Lima E.G., Mauck R.L., Taki E., LeRoux M.A., Lu H.H., Stark R.G., Guo X.E., and Ateshian G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech 36, 1853, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Setton L., Tohyama H., and Mow V. Swelling and curling behaviors of articular cartilage. J Biomech Eng 120, 355, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Asanbaeva A., Masuda K., Thonar E.J., Klisch S.M., and Sah R.L. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum 56, 188, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Hosseini S.M., Wu Y., Ito K., and van Donkelaar C.C. The importance of superficial collagen fibrils for the function of articular cartilage. Biomech Model Mechanobiol 13, 41, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Ateshian G.A., Soltz M.A., Mauck R.L., Basalo I.M., Hung C.T., and Lai W.M. The role of osmotic pressure and tension-compression nonlinearity in the frictional response of articular cartilage. Transport Porous Med 50, 5, 2003 [Google Scholar]
- 8.Soltz M.A., and Ateshian G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech 31, 927, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Byers B.A., Mauck R.L., Chiang I.E., and Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14, 1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang A.H., Yeger-McKeever M., Stein A., and Mauck R.L. Tensile properties of engineered cartilage formed from chondrocyte-and MSC-laden hydrogels. Osteoarthritis Cartilage 16, 1074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeGroot J., Verzijl N., Bank R.A., Lafeber F., Bijlsma J.W., and TeKoppele J.M. Age-related decrease in proteoglycan synthesis of human articular chondrocytes. Arthritis Rheum 42, 1003, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Nims R.J., Durney K.M., Cigan A.D., Dusséaux A., Hung C.T., and Ateshian G.A. Continuum theory of fibrous tissue damage mechanics using bond kinetics: application to cartilage tissue engineering. Interface Focus 6, 20150063, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bank R.A., Soudry M., Maroudas A., Mizrahi J., and TeKoppele J.M. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum 43, 2202, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Thibault M., Robin Poole A., and Buschmann M.D. Cyclic compression of cartilage/bone explants in vitro leads to physical weakening, mechanical breakdown of collagen and release of matrix fragments. J Orthopaed Res 20, 1265, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Temple M.M., Xue Y., Chen M.Q., and Sah R.L. Interleukin-1α induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum 54, 3267, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Buschmann M.D., Gluzband Y.A., Grodzinsky A.J., and Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 108, 1497, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Knight M., Lee D., and Bader D. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim Biophys Acta 1405, 67, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Buckley C.T., Thorpe S.D., O'Brien F.J., Robinson A.J., and Kelly D.J. The effect of concentration, thermal history and cell seeding density on the initial mechanical properties of agarose hydrogels. J Mech Behav Biomed Mater 2, 512, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Mauck R., Yuan X., and Tuan R. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14, 179, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Erickson I.E., Huang A.H., Chung C., Li R.T., Burdick J.A., and Mauck R.L. Differential maturation and structure–function relationships in mesenchymal stem cell-and chondrocyte-seeded hydrogels. Tissue Eng Part A 15, 1041, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J.C., and Athanasiou K.A. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials 26, 2001, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Awad H.A., Wickham M.Q., Leddy H.A., Gimble J.M., and Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25, 3211, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mouw J., Case N.D., Guldberg R., Plaas A., and Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage 13, 828, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Quinn T., Schmid P., Hunziker E., and Grodzinsky A. Proteoglycan deposition around chondrocytes in agarose culture: construction of a physical and biological interface for mechanotransduction in cartilage. Biorheology 39, 27, 2002 [PubMed] [Google Scholar]
- 25.Vunjak-Novakovic G., Martin I., Obradovic B., Treppo S., Grodzinsky A., Langer R., and Freed L. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthopaed Res 17, 130, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Mauck R.L., Seyhan S.L., Ateshian G.A., and Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng 30, 1046, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Chung C., Beecham M., Mauck R.L., and Burdick J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials 30, 4287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng K.W., Lima E.G., Bian L., O'Conor C.J., Jayabalan P.S., Stoker A.M., Kuroki K., Cook C.R., Ateshian G.A., and Cook J.L. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A 16, 1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erickson I.E., Kestle S.R., Zellars K.H., Farrell M.J., Kim M., Burdick J.A., and Mauck R.L. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater 8, 3027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makris E.A., Responte D.J., Paschos N.K., Hu J.C., and Athanasiou K.A. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A 111, E4832, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhumiratana S., Eton R.E., Oungoulian S.R., Wan L.Q., Ateshian G.A., and Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A 111, 6940, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesallati T., Buckley C.T., and Kelly D.J. Engineering cartilaginous grafts using chondrocyte-laden hydrogels supported by a superficial layer of stem cells. J Tissue Eng Regen Med 2015. [Epub ahead of print]; DOI: 10.1002/term.2033 [DOI] [PubMed] [Google Scholar]
- 33.Williamson A.K., Chen A.C., Masuda K., Thonar E.J., and Sah R.L. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthopaed Res 21, 872, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Burdick J.A., and Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23, H41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H.D., Heo J., Hwang Y., Kwak S.-Y., Park O.K., Kim H., Varghese S., and Hwang N.S. Extracellular-matrix-based and Arg-Gly-Asp–modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng Part A 21, 757, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowland C.R., Colucci L.A., and Guilak F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials 91, 57, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck E.C., Barragan M., Libeer T.B., Kieweg S.L., Converse G.L., Hopkins R.A., Berkland C.J., and Detamore M.S. Chondroinduction from naturally derived cartilage matrix: a comparison between devitalized and decellularized cartilage encapsulated in hydrogel pastes. Tissue Eng Part A 22, 665, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisiday J., Jin M., Kurz B., Hung H., Semino C., Zhang S., and Grodzinsky A. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A 99, 9996, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novak T., Seelbinder B., Twitchell C.M., van Donkelaar C.C., Voytik-Harbin S.L., and Neu C.P. Mechanisms and microenvironment investigation of cellularized high density gradient collagen matrices via densification. Adv Funct Mater 26, 2617, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang B.J., Hu J.C., and Athanasiou K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauck R.L., Soltz M.A., Wang C.C., Wong D.D., Chao P.-H.G., Valhmu W.B., Hung C.T., and Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122, 252, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Cigan A.D., Nims R.J., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. Optimizing nutrient channel spacing and revisiting TGF-beta in large engineered cartilage constructs. J Biomech 49, 2089, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cigan A.D., Nims R.J., Albro M.B., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. Nutrient channels and stirring enhanced the composition and stiffness of large cartilage constructs. J Biomech 47, 3847, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nims R.J., Cigan A.D., Albro M.B., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. Matrix production in large engineered cartilage constructs is enhanced by nutrient channels and excess media supply. Tissue Eng Part C Methods 21, 747, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Neill J.D., Freytes D.O., Anandappa A.J., Oliver J.A., and Vunjak-Novakovic G.V. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials 34, 9830, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cigan A.D., Durney K.M., Nims R.J., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. Nutrient channels aid the growth of articular surface-sized engineered cartilage constructs. Tissue Eng Part A 22, 1063, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awad H.A., Butler D.L., Harris M.T., Ibrahim R.E., Wu Y., Young R.G., Kadiyala S., and Boivin G.P. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res 51, 233, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Cigan A.D., Nims R.J., Albro M.B., Esau J.D., Dreyer M.P., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue Eng Part A 19, 1941, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nims R.J., Cigan A.D., Albro M.B., Hung C.T., and Ateshian G.A. Synthesis rates and binding kinetics of matrix products in engineered cartilage constructs using chondrocyte-seeded agarose gels. J Biomech 47, 2165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farndale R.W., Buttle D.J., and Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 51.Hollander A.P., Heathfield T.F., Webber C., Iwata Y., Bourne R., Rorabeck C., and Poole A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest 93, 1722, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cigan A.D., Roach B.L., Nims R.J., Tan A.R., Albro M.B., Stoker A.M., Cook J.L., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J Biomech 49, 1909, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGowan K., Kurtis M., Lottman L., Watson D., and Sah R. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen® and Hoechst 33258. Osteoarthritis Cartilage 10, 580, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Grant M.E., and Prockop D.J. The biosynthesis of collagen. N Engl J Med 286, 242, 1972 [DOI] [PubMed] [Google Scholar]
- 55.Kelly T.-A.N., Ng K.W., Wang C.C.-B., Ateshian G.A., and Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech 39, 1489, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Williamson A.K., Masuda K., Thonar E.J.-M., and Sah R.L. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng 9, 625, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Park S., and Ateshian G.A. Dynamic response of immature bovine articular cartilage in tension and compression, and nonlinear viscoelastic modeling of the tensile response. J Biomech Eng 128, 623, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basalo I.M., Raj D., Krishnan R., Chen F.H., Hung C.T., and Ateshian G.A. Effects of enzymatic degradation on the frictional response of articular cartilage in stress relaxation. J Biomech 38, 1343, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S., Falcovitz Y., Schneiderman R., Maroudas A., and Sah R. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis Cartilage 9, 561, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Guterl C.C., Hung C.T., and Ateshian G.A. Electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage. J Biomech 43, 1343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kempson G., Muir H., Pollard C., and Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta 297, 456, 1973 [DOI] [PubMed] [Google Scholar]
- 62.O'Conor C.J., Leddy H.A., Benefield H.C., Liedtke W.B., and Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A 111, 1316, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riesle J., Hollander A., Langer R., Freed L., and Vunjak-Novakovic G. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem 71, 313, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Asanbaeva A., Masuda K., Thonar E.-M., Klisch S.M., and Sah R.L. Cartilage growth and remodeling: modulation of balance between proteoglycan and collagen network in vitro with β-aminopropionitrile. Osteoarthritis Cartilage 16, 1, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Siegel R.C. Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Natl Acad Sci U S A 71, 4826, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyre D.R., Paz M.A., and Gallop P.M. Cross-linking in collagen and elastin. Annu Rev Biochem 53, 717, 1984 [DOI] [PubMed] [Google Scholar]
- 67.Eyre D.R., Weis M.A., and Wu J.-J. Maturation of collagen ketoimine cross-links by an alternative mechanism to pyridinoline formation in cartilage. J Biol Chem 285, 16675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bian L., Crivello K.M., Ng K.W., Xu D., Williams D.Y., Ateshian G.A., and Hung C.T. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A 15, 2065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Natoli R.M., Revell C.M., and Athanasiou K.A. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A 15, 3119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connell G., Nims R., Green J., Cigan A., Ateshian G., and Hung C. Time and dose-dependent effects of chondroitinase ABC on growth of engineered cartilage. Eur Cells Mater 27, 312, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stockwell R.A. Biology of Cartilage Cells. Cambridge: CUP Archive, 1979, 329 pp [Google Scholar]
- 72.Saxena V., Kim M., Keah N.M., Neuwirth A.L., Stoeckl B.D., Bickard K., Restle D.J., Salowe R., Wang M.Y., and Steinberg D.R. Anatomic mesenchymal stem cell-based engineered cartilage constructs for biologic total joint replacement. Tissue Eng Part A 22, 386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang B.J., Huey D.J., Hu J.C., and Athanasiou K.A. Engineering biomechanically functional neocartilage derived from expanded articular chondrocytes through the manipulation of cell-seeding density and dexamethasone concentration. J Tissue Eng Regen Med 2016. [Epub ahead of print]; DOI: 10.1002/term.2132 [DOI] [PubMed] [Google Scholar]
- 74.Bian L., Angione S., Ng K., Lima E., Williams D., Mao D., Ateshian G., and Hung C. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage 17, 677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheehy E.J., Vinardell T., Toner M.E., Buckley C.T., and Kelly D.J. Altering the architecture of tissue engineered hypertrophic cartilaginous grafts facilitates vascularisation and accelerates mineralisation. PLoS One 9, e90716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckley C.T., Thorpe S.D., and Kelly D.J. Engineering of large cartilaginous tissues through the use of microchanneled hydrogels and rotational culture. Tissue Eng Part A 15, 3213, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Sheehy E.J., Buckley C.T., and Kelly D.J. Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J Tissue Eng Regen Med 5, 747, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Hadidi P., Yeh T.C., Hu J.C., and Athanasiou K.A. Critical seeding density improves the properties and translatability of self-assembling anatomically shaped knee menisci. Acta Biomater 11, 173, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albro M.B., Nims R.J., Durney K.M., Cigan A.D., Shim J.J., Vunjak-Novakovic G., Hung C.T., and Ateshian G.A. Heterogeneous engineered cartilage growth results from gradients of media-supplemented active TGF-β and is ameliorated by the alternative supplementation of latent TGF-β. Biomaterials 77, 173, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albro M.B., Nims R.J., Cigan A.D., Yeroushalmi K.J., Shim J.J., Hung C.T., and Ateshian G.A. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-β. J Biomech 46, 1433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bian L., Fong J.V., Lima E.G., Stoker A.M., Ateshian G.A., Cook J.L., and Hung C.T. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A 16, 1781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.