Abstract
Weight changes are accompanied by imbalances between calorie intake and expenditure. This fact is often misinterpreted to suggest that obesity is caused by gluttony and sloth and can be treated by simply advising people to eat less and move more. However, various components of energy balance are dynamically interrelated and weight loss is resisted by counterbalancing physiological processes. While low-carbohydrate diets have been suggested to partially subvert these processes by increasing energy expenditure and promoting fat loss, our meta-analysis of 32 controlled feeding studies with isocaloric substitution of carbohydrate for fat found that both energy expenditure (26 kcal/d; P <.0001) and fat loss (16 g/d; P <.0001) were greater with lower fat diets. We review the components of energy balance and the mechanisms acting to resist weight loss in the context of static, settling point, and set-point models of body weight regulation, with the set-point model being most commensurate with current data.
Keywords: Body Weight Regulation, Energy Intake, Energy Expenditure, Macronutrients
Obesity is often described as a disorder of energy balance arising from consuming calories in excess to the energy expended to maintain life and perform physical work. While this energy balance concept is a useful framework for investigating obesity, it does not provide a causal explanation for why some people have obesity or what to do about it.
In particular, obesity prevention is often erroneously portrayed as a simple matter of bookkeeping whereby calorie intake must be balanced by calorie expenditure.1 Under this “calories in, calories out” model, treating obesity amounts to advising people to simply eat less and move more, thereby tipping the scales of calorie balance and resulting in steady weight loss that accumulates according to the widely known, but erroneous, 3500 kcal per pound rule.2,3 Therefore, failure to experience substantial weight loss implies that an individual lacks the willpower to adhere to a modest lifestyle intervention over a sufficient period of time.
However, this naïve view is incorrect because it considers energy intake and expenditure to be independent parameters that can be adjusted at will and thereafter remain static without being influenced by homeostatic signals related to weight loss.3 We now understand that energy intake and expenditure are interdependent variables that are dynamically influenced by each other and body weight.4 Attempts to alter energy balance through diet or exercise are countered by physiological adaptations that resist weight loss.5
This review focuses on our current understanding of the components of human energy balance and the counterbalancing physiological processes that act to resist weight loss. Furthermore, we address the question of whether all diet calories are created equal regarding the effects of carbohydrate, fat, and protein on energy balance, body weight, and composition. Finally, we compare 3 conceptual models of energy balance regulation and examine the implications for human body weight dynamics and the treatment of obesity.
Components of Daily Energy Expenditure
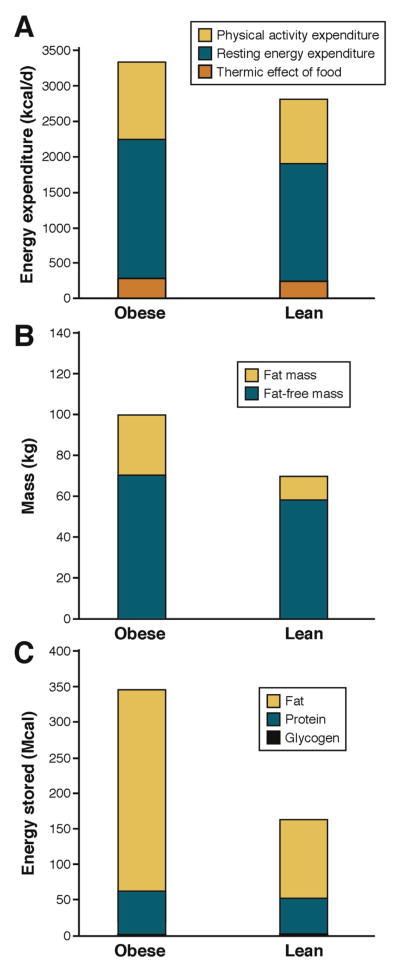
There are 3 components of daily energy expenditure: the thermic effect of food, physical activity expenditure, and resting energy expenditure (REE) (Figure 1A).
Figure 1.
Components of human energy expenditure and body composition in average 100-kg and 70-kg men. (A) Daily energy expenditure comprises the energy cost of digesting and processing food, called the thermic effect of food, the energy expended in physical activity, and the energy expended at rest to maintain life. People with obesity have a higher thermic effect of food because of greater food intake. Furthermore, people with obesity may expend more energy for physical activity despite typically moving around less than a lean individual because physical activity expenditure is proportional to body weight. Energy expended at rest is also greater in people with obesity because they have more metabolically active fat-free mass in addition to greater body fat as depicted in (B). (C) Body fat represents the vast majority of energy stores in the body compared with the energy content of body protein and glycogen. People with obesity can have vast quantities of stored energy in the form of body fat that represents several months of energy expenditure.
Thermic Effect of Food
The smallest component of daily energy expenditure in humans is the thermic effect of food (also called diet induced thermogenesis or specific dynamic action), which is defined as the increase of metabolic rate observed for several hours following the ingestion of a meal.6,7 The thermic effect of food is believed to represent the energy cost of digestion, absorption, storage, and metabolic fate of dietary macronutrients.7 While the precise mechanisms underlying the thermic effect of food are not fully understood, there is a clear macronutrient hierarchy, with protein causing a greater energy expenditure increment than carbohydrate. which is greater than that of fat.7 For typical diet compositions, the thermic effect of food is approximated to be about 10% of energy intake (Figure 1A).
Resting Energy Expenditure
REE corresponds to the energy expended when not performing physical work and is typically the largest contribution to daily energy expenditure. Contrary to popular belief, people with obesity generally have a higher absolute REE (Figure 1A).8 It has long been recognized that fat-free mass comprises the metabolically active tissues of the body and therefore contributes more to REE than body fat. Fat-free mass is elevated in obesity, along with body fat, resulting in increased REE compared with lean subjects (Figure 1B). Across a wide range of weights, REE is linearly related to both fat-free mass and body fat,8 such that the elevated REE with obesity is generally in line with what is expected for the body weight and composition.
While fat-free mass, and to a lesser extent fat mass, are good predictors of REE, these variables explain only about 70% of inter-individual REE variability, such that for a given body composition the REE residual standard deviation is about 300 kcal/d.8 Because the organs that contribute to the fat-free mass have a wide range of metabolic rates,9 some of the residual REE variability after accounting for body fat and fat-free mass may be caused by variations in organ masses. Magnetic resonance imaging methodologies have been used to quantify organ sizes and REE prediction equations that sum the individual metabolic rates of various organs explain about 80% of the REE variability.10,11
Another potentially important contributor to REE involves fluxes through various energy-requiring metabolic pathways. Major macronutrient fluxes such as gluconeogenesis, de novo lipogenesis, triglyceride synthesis, and protein turnover all require energy and these flux rates can be significantly influenced by both the energy content of the diet as well as its composition.12
Physical Activity Expenditure
Physical activity expenditure can be subdivided into volitional exercise and the activities of daily living, also called spontaneous physical activity or non-exercise activity thermogenesis. The energy expended in physical activities is determined by their duration and intensity in proportion to overall body weight.13 Thus, despite typically being less physically active, people with obesity often have similar daily energy costs for physical activity as those without obesity (Figure 1A)14 and physical activity energy expenditure declines with weight loss unless its quantity or intensity increases to compensate.
Influence of Exercise on Energy Expenditure and Body Weight
While often considered a first-line treatment option for obesity, large amounts of exercise are required to result in a modest degree of average weight loss.15 However, exercise results in preferential loss of body fat and maintenance of fat-free mass compared with diet-induced weight loss.16,17 but exercise does not appear to prevent the slowing of metabolic rate during weight loss.18 Exercise interventions typically result in less average weight loss than expected, based on the exercise calories expended, and individual weight changes are highly variable even when exercise is supervised to ensure adherence.19 A likely explanation for these observations is that the energy expended during exercise is variably compensated by changes in food intake and non-exercise physical activity behaviors.19
The recently proposed “constrained energy expenditure model” provides an alternative explanation for why exercise interventions often result in minimal weight loss.20 According to this model, daily energy expenditure is regulated and increments in physical activity expenditure are predicted to be offset by decreases in non-physical activity expenditure (ie, the thermic effect of food or REE) resulting in minimal energy imbalance. The experimental basis of the constrained energy expenditure model in humans includes cross-sectional data demonstrating that free-living daily energy expenditure adjusted for body composition is relatively constant for a wide range of physical activity levels measured using accelerometry.21,22 Furthermore, longitudinal data have found that progressive increases in the quantity and intensity of aerobic exercise training do not lead to corresponding increases in total daily energy expenditure in ad libitum-fed men and women.23
However, in contrast to the predictions of the constrained energy expenditure model, exercise training does not lead to decreased REE under conditions of weight stability24 and REE adjusted for body composition is not different between people with a wide range of physical activity levels.21 Furthermore, exercise may actually increase the thermic effect of food.6 Therefore, when physical activity increases via exercise, the non-physical activity components of daily energy expenditure do not decrease as predicted by the constrained model.
Finally, increments in daily energy expenditure shortly after starting an exercise program can be greater than the expected energy cost of the exercise.23 Subsequent failure to increase daily expenditure as training progresses, despite increasing exercise volume and intensity, may be because of improvements in biomechanical efficiency that decrease the energy cost of exercise.23
Influence of Energy Intake on Energy Expenditure
Reductions in energy intake lead to decreased energy expenditure to a degree that is often greater than expected based on changes in body composition or the thermic effect of food.25,26 This phenomenon has been called adaptive thermogenesis or metabolic adaptation and it may continue for years after energy balance is reestablished at a lower weight,27–29 although controversy remains regarding its persistence.30,31 The mechanistic basis of metabolic adaptation is unclear, but it may be related to reduced sympathetic drive or blunted thyroid activity, possibly as a result of decreased leptin.25,32–36
Experimental quantification of metabolic adaptation depends on calculating the residual difference between measured and expected values of energy expenditure.37,38 However, the expected energy expenditure is typically calculated based on observed body fat and fat-free mass changes and typically ignores factors like organ sizes or altered fluxes through energy-requiring metabolic pathways. Whether such considerations can explain the observed adaptations in energy expenditure is presently unclear.
Metabolic adaptation can be interpreted teleologically as the body’s response to a perceived state of starvation by decreasing the energy cost of living in an attempt to prolong life given the body’s finite energy stores. In this context, it is interesting that the robustness of metabolic adaptation does not appear to be attenuated by the quantity of stored energy such that people with obesity, who have very large energy reserves, experience decreases in energy expenditure that are similar in magnitude to those having dramatically fewer energy reserves (Figure 1C).39
In contrast to the growing consensus supporting the existence of important metabolic adaptations to underfeeding and weight loss, the adaptive response to over-feeding and weight gain is less clear.26 While some investigators have observed that overfeeding induces highly variable increases in spontaneous physical activity expenditure that may be greater than expected based on the observed weight changes,39–41 others have found that the increased energy expenditure associated with overfeeding is in accord with the expected increased thermic effect of food, along with increased REE and physical activity expenditure based on the observed weight gain.14,26
The effect of energy intake changes on energy expenditure and body weight have recently been incorporated within dynamic mathematical models in both adults42 and children43 to replace the static “calories in, calories out” model that erroneously assumed independence of energy intake and expenditure.44 Mathematical models have been used to calculate the so-called “energy gap” required to reverse obesity, which amounts to about 200–250 kcal/d for both adults and children at the population level,44 and have been used to estimate the potential effects of policy changes on overweight and obesity prevalence.45
Influence of Dietary Macronutrients on Energy Balance and Body Composition
The energy released during carbohydrate, fat, and protein oxidation within the body can be equated to the energy derived from their combustion in a bomb calorimeter, with suitable corrections for the differing thermodynamic constraints and the end products of the reactions. In other words, “a calorie is a calorie” when macronutrients are oxidized either in the bomb calorimeter through combustion or via the intricate biochemical pathways of oxidative phosphorylation inside cells.
However, thermodynamic arguments alone do not necessarily imply that “a calorie is a calorie” when it comes to the effects of diet composition on body weight or composition. Isocaloric diets differing in macronutrient composition may result in preferential partitioning of energy storage toward body fat and away from body protein. Such energy partitioning differences over the long term will alter the proportions of body fat and fat-free mass and thereby influence energy expenditure. Dietary protein in particular is known to positively influence fat-free mass during weight loss46,47 and weight gain.48 A recent meta-analysis found that dietary protein resulted in a small positive influence on REE during reduced-calorie, low-fat diets amounting to about 150 kcal/d.49 Overfeeding with higher protein diets also led to small increases in REE and increases in daily energy expenditure,48 as did weight loss maintenance diets with higher protein.50
While higher protein diets appear to offer advantages regarding energy expenditure and body composition, the relative effects of dietary carbohydrate and fat are debated. According to the popular “carbohydrate-insulin model” of obesity, diets high in carbohydrate are conjectured to be particularly fattening because of their propensity to elevate insulin secretion, thereby directing fat toward storage in adipose tissue and away from oxidation by metabolically active tissues, leading to an adaptive decrease in metabolic rate.51,52 In contrast, because dietary fat does not stimulate insulin secretion, isocaloric diets lower in carbohydrate but higher in fat reduce insulin secretion, thereby promoting fat loss from adipose tissue, making free-fatty acids available for use by metabolically active tissues. The increased fuel availability theoretically leads to increased metabolic rate with a net “metabolic advantage” of very low carbohydrate diets amounting to as much as 400–600 kcal/d of additional energy expenditure.53
Such large increases in daily energy expenditure could explain why low carbohydrate diets that are unrestricted in calories typically result in greater short-term weight loss compared with reduced energy, low-fat diets.54–56 Indeed, the original Atkins diet that restricted dietary carbohydrates, but not calories, promised its followers a “high calorie way to stay thin forever” as a result of increased energy expenditure.57 Unfortunately, the experimental evidence does not support such a metabolic advantage.
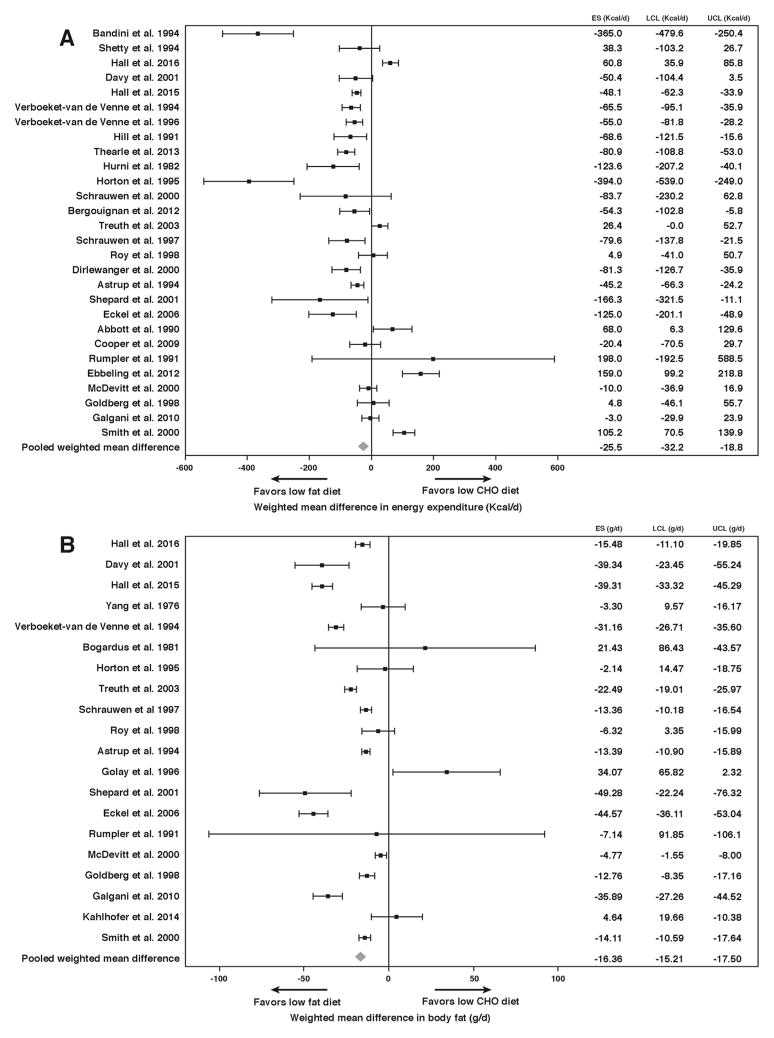
We conducted a systematic review and meta-analysis of the effects on daily energy expenditure and body fat of isocaloric diets differing in their fraction of carbohydrate to fat but with equal protein. To minimize confounding by dietary nonadherence, we included only controlled feeding studies where all food was provided to the subjects. We found 32 studies representing 563 subjects matching our inclusion criteria with dietary carbohydrate ranging from 1%–83% and dietary fat ranging from 4%–84% of total calories (see Supplementary Materials).
Figure 2A shows the daily energy expenditure differences between isocaloric diets with equal protein but differing in the ratio of carbohydrate to fat. The pooled weighted mean difference in energy expenditure was 26 kcal/d (P <.0001) greater with lower fat diets. Figure 2B shows differences in the rate of body fat change between diets with the pooled weighted mean difference of 16 g/d (P <.0001) greater body fat loss in favor of the lower fat diets. These results are in the opposite direction to the predictions of the carbohydrate-insulin model, but the effect sizes are so small as to be physiologically meaningless. In other words, for all practical purposes “a calorie is a calorie” when it comes to body fat and energy expenditure differences between controlled isocaloric diets varying in the ratio of carbohydrate to fat.
Figure 2.
Meta-analysis of controlled isocaloric feeding studies with constant dietary protein and varying ratios of carbohydrate to fat. Studies are ordered from top to bottom according to the largest difference in carbohydrate between diet comparisons. Effect size (ES), upper and lower 95% confidence limits (UCL and LCL, respectively) are indicated for the differences in daily energy expenditure (A) and rate of body fat change (B). The pooled weighted mean difference across studies demonstrated small differences in daily energy expenditure (26 kcal/d, P <.0001) and body fat change (16 g/d, P <.0001) favoring lower fat diets
Nevertheless, it is possible that isocaloric diets differing in carbohydrate and fat may have overall health effects unrelated to total body fat or energy expenditure.58 For example, dietary carbohydrates may play an important role in determining the anatomic location of body fat stores, with diets higher in refined carbohydrate possibly leading to increased visceral and liver fat.59,60
Diet composition may also influence energy intake when the amount of the diet consumed is not controlled. For example, increasing dietary fat results in greater energy intake61,62 and decreasing dietary fat has the opposite effect.63,64 However, very low carbohydrate, high-fat diets may reduce appetite by promoting an increase in circulating ketones,65 although the mechanism for this effect is unclear.66 Furthermore, low carbohydrate diets often increase protein intake, which may independently increase satiety and decrease overall energy intake.46,47 These effects may help explain the short-term benefits for weight loss of lower carbohydrate, higher protein diets.54–56
However, long-term weight loss diet studies targeting different macronutrients demonstrate similar mean body weight trajectories corresponding to a similar exponential decay of diet adherence with all diets.67 The reasons for the long-term erosion of diet adherence are not well understood. Likely factors include practical challenges in sustaining diet changes in the face of engrained food habits developed over years of living in an obesogenic environment,68 as well as the likelihood that feedback control of energy intake makes it difficult to persistently resist an ever-increasing appetite as weight is lost.
Feedback Control of Energy Intake
While there is a growing consensus that human energy expenditure is actively controlled to resist weight loss,26 the evidence for active control of energy intake is less clear. Observations regarding long-term precision of calorie balance and relative weight stability have been offered in support of energy intake feedback control, but these observations also have alternative explanations69–71 relying solely on energy expenditure adaptations and correspond to a “settling point” model of body weight regulation.72
However, the molecular mechanisms of appetite regulation strongly suggest that feedback control of energy intake acts as part of a complex neurobiological system integrating social and environmental influences with homeostatic signals related to body weight, such as leptin, to control both short-term feeding behavior and long-term energy intake.73,74
Establishing that energy intake is under active control in humans is challenging under laboratory conditions and extremely difficult under free-living conditions. Accurate measurements of energy intake in the laboratory have demonstrated that diet manipulations can lead to short-term compensatory changes in energy intake.61,62,75,76 and hyperphagia has been demonstrated following experimental semi-starvation77 and short-term underfeeding,78–80 possibly resulting from homeostatic signals arising from loss of both body fat and lean tissues.81,82 However, these results cannot be readily extrapolated to the long time scales associated with regulation of human energy balance in the real world.83 Indeed, free-living energy intake fluctuates widely from day to day and exhibits little short-term correlation with energy expenditure or body weight.69,84
Therefore, investigating the control of energy intake requires obtaining accurate food intake measurements in free-living people over long time scales, which has been a major challenge.85–87 Furthermore, measuring the strength of the energy intake feedback circuit requires a means of covertly perturbing energy expenditure over extended time periods and measuring the resulting energy intake response. These problems were recently addressed and the strength of the long-term energy intake feedback control circuit was quantified for the first time in humans.88
A validated mathematical method89 using repeated body weight measurements calculated the long-term changes in energy intake in patients with type 2 diabetes who were treated for 1 year with an inhibitor of the renal sodium glucose transporter type-2, causing a substantial increase in the amount of glucose excreted in the urine.88 The loss of glucose calories occurred without the subjects being directly aware of the calorie deficit and resulted in gradual weight loss. The subjects were found to progressively increase average energy intake by about 100 kcal/d per kilogram of lost weight – an effect more than 3-fold larger than the corresponding energy expenditure adaptations to weight loss.88
Static, Settling Point, and Set-Point Models of Human Body Weight Regulation
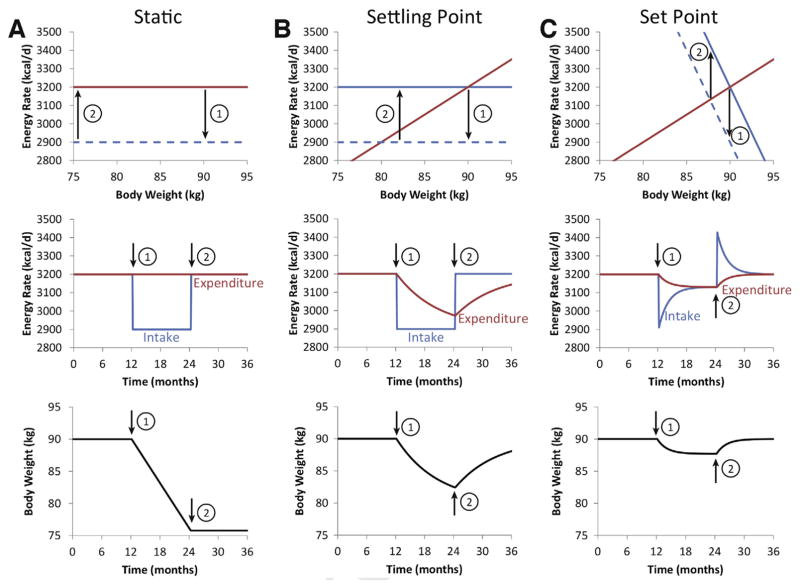
Long-term feedback control of energy intake and expenditure has profound implications for body weight regulation and our understanding of obesity treatment. Without such feedback control mechanisms, body weight dynamics correspond to the static “calories in, calories out” model, where energy intake and expenditure are independent variables and weight changes, or related homeostatic signals like leptin, are assumed to have negligible effects on energy intake or expenditure.
Figure 3A illustrates the body weight dynamics of the static model simulating a 90-kg man eating and expending 3200 kcal/d for 1 year, followed by a year of cutting 300 kcal from his daily diet and then a final year consuming the original 3200 kcal/d diet. The horizontal lines depicted in the top panel of Figure 3A indicate that energy intake and expenditure have no relationship to weight, and the solid lines overlap in energy balance. When intake is reduced (dashed line, top panel) energy imbalance is determined by the distance to the expenditure line and is equal at all weights (top panel). The constant energy imbalance (middle panel) results in a constant rate of weight loss (bottom panel) until resumption of the original 3200 kcal/d diet reestablishes energy balance at a stable lower body weight for the final year.
Figure 3.
Comparison of static, settling point, and set point models of body weight regulation in a simulated 90-kg man. (A) The static model assumes that energy intake (blue) and expenditure (red) are independent quantities that do not depend on body weight, as shown by the horizontal lines in the top panel. For the first year, energy intake and expenditure are assumed to be balanced and correspond to overlapping solid lines. At the end of the first year, the energy intake line is shifted downward by 300 kcal/d to the dashed blue line corresponding with the start of a weight loss intervention indicated by (1). Because energy expenditure is assumed to be constant, a constant energy deficit is achieved (top and middle panels) resulting in a linear rate of weight loss (bottom panel). At the start of the second year (2), the intervention is stopped and the dashed energy intake curve shifts back to the solid baseline intake line and weight loss is maintained. (B) The settling point model assumes that energy expenditure is an increasing function of body weight whereas energy intake is independent of weight (top panel). Shifting the intake curve down by the same 300 kcal/d after the first year (1) results in an energy deficit that decreases in proportion to weight lost (top panel) resulting in an exponential decay of the energy deficit over time (middle panel). Body weight falls according to a parallel exponential pattern and it takes years to reach a new equilibrium weight (bottom panel). At the beginning of the second year (2), the energy intake curve is shifted upward (top panel) and energy intake increases (middle panel), generating an energy surplus that results in an exponential pattern of weight regain mirroring prior weight loss. (C) The set point model assumes that both energy intake and expenditure are functions of body weight (top panel) and the same 300 kcal/d shift in the energy intake curve (top panel) results in an initial decrease in energy intake that subsequently exponentially increases (middle panel) as weight is lost and energy expenditure decreases. Body weight is lost in an abbreviated exponential pattern and achieves a new equilibrium after about 6 months and no further loss occurs despite the continued intervention (bottom panel). At the start of the second year (2), the energy intake curve shifts to baseline (top panel) and leads to transient hyperphagia (middle panel) and rapid weight regain (bottom panel).
Because expenditure is erroneously considered to be constant, the static model provides unrealistic predictions for the expected weight loss for a given diet intervention. Furthermore, the static model ignores the difficulties of maintaining lost weight because the same baseline energy intake allows for maintenance of the reduced weight. Despite such obvious errors, the static model continues to be used both individuals2 and populations44 to predict weight changes.
Figure 3B illustrates the negative feedback effect of increasing energy expenditure with body weight (with a slope of about 20–30 kcal/d per kg39,42 as depicted in the top panel) corresponding to a “settling point” model.72 The stable body weight is determined by the intersection between the increasing expenditure line and the horizontal energy intake line assumed to be weight independent. When the same 300 kcal/d is cut from the diet, corresponding to a shift of the energy intake line downwards (dashed line, top panel), energy imbalance decreases (middle panel) along with weight (bottom panel) in an exponential fashion and it takes years to equilibrate at a stable lower weight.42 Once the energy intake line is shifted upwards to baseline, weight regain ensues with an exponential time course mirroring the weight loss phase.
Finally, Figure 3C illustrates the “set point” model that includes feedback control of both energy intake and expenditure,72 with energy intake being a decreasing function of weight (with a slope of about -100 kcal/d per kg88 as shown in the top panel). An intervention that shifts the energy intake line downwards by the same 300 kcal/d (dashed line, top panel) now results in a transient decrease in energy intake that subsequently increases exponentially because of escalating appetite as weight decreases along with a parallel decline in energy expenditure (middle panel).
The time scale to equilibrate at the lower weight (bottom panel) is greatly abbreviated compared with the settling point model, resulting in a weight loss plateau after about 6 months with no further weight loss despite continued intervention. This aligns closely to the typical weight plateau after about 6 months of starting a weight loss intervention.90 After the intervention is stopped, energy intake increases transiently above baseline, similar to what has been observed after periods of enforced calorie restriction,77–80 and weight is promptly regained.
In addition to the long-term feedback control of energy intake mediated by homeostatic signals related to body weight and composition, eating behavior is also strongly influenced by social and environmental influences in conjunction with learned eating habits.73,74 While previous conceptions of the set point model were thought to be incompatible with non-homeostatic influences on food intake and body weight,72 such effects can be naturally incorporated by altering the position or slope of the energy intake line depicted in Figure 3A and the defended body weight will be adjusted accordingly.
Unfortunately, we do not yet know the quantitative effects of non-homeostatic influences on the set point model, but there is likely to be a wide degree of individual variation. Some people may experience substantial changes in the energy intake, along with correspondingly large weight changes, whereas others will be more resistant. Re-engineering the social and food environments may facilitate shifts in the energy intake line, but losing weight and keeping it off using willpower alone to reduce energy intake is difficult because considerable effort is required to persistently resist the physiological adaptations that act to increase appetite and suppress energy expenditure.
Supplementary Material
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes & Digestive & Kidney Diseases.
Abbreviations used in this paper
- REE
resting energy expenditure
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2017.01.052.
Conflicts of interest
K.D.H. has received funding from the Nutrition Science Initiative to investigate the effects of ketogenic diets on human energy expenditure. K.D.H. also has a patent pending on a method of personalized dynamic feedback control of body weight (US Patent Application No. 13/754,058; assigned to the National Institutes of Health). J.G. has no conflicts of interest to disclose.
References
- 1.Levine DI. The curious history of the calorie in U.S. policy: a tradition of unfulfilled promises. Am J Prev Med. 2017;52:125–129. doi: 10.1016/j.amepre.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Guth E. JAMA patient page. Healthy weight loss. JAMA. 2014;312:974. doi: 10.1001/jama.2014.10929. [DOI] [PubMed] [Google Scholar]
- 3.Hall KD, Chow CC. Why is the 3500 kcal per pound weight loss rule wrong? Int J Obes (Lond) 2013;37:1614. doi: 10.1038/ijo.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall KD, Heymsfield SB, Kemnitz JW, et al. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochner CN, Tsai AG, Kushner RF, et al. Treating obesity seriously: when recommendations for lifestyle change confront biological adaptations. Lancet Diabetes Endocrinol. 2015;3:232–234. doi: 10.1016/S2213-8587(15)00009-1. [DOI] [PubMed] [Google Scholar]
- 6.de Jonge L, Bray GA. The thermic effect of food and obesity: a critical review. Obes Res. 1997;5:622–631. doi: 10.1002/j.1550-8528.1997.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 7.Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1:5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson KM, Weinsier RL, Long CL, et al. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56:848–856. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 9.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, editors. Energy metabolism: tissue determinants and cellular corollaries. New York: Raven Press; 1992. pp. 61–79. [Google Scholar]
- 10.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 11.Muller MJ, Bosy-Westphal A, Kutzner D, et al. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3:113–122. doi: 10.1046/j.1467-789x.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 12.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab. 2010;298:E449–E466. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoeller DA, Jefford G. Determinants of the energy costs of light activities: inferences for interpreting doubly labeled water data. Int J Obes (Lond) 2002;26:97–101. doi: 10.1038/sj.ijo.0801851. [DOI] [PubMed] [Google Scholar]
- 14.Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev. 2010;68:148–154. doi: 10.1111/j.1753-4887.2010.00270.x. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 16.Washburn RA, Szabo AN, Lambourne K, et al. Does the method of weight loss effect long-term changes in weight, body composition or chronic disease risk factors in overweight or obese adults?. A systematic review. PLoS One. 2014;9:e109849. doi: 10.1371/journal.pone.0109849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 18.Johanssen DL, Knuth ND, Huizenga R, et al. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melanson EL, Keadle SK, Donnelly JE, et al. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc. 2013;45:1600–1609. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontzer H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc Sport Sci Rev. 2015;43:110–116. doi: 10.1249/JES.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 21.Pontzer H, Durazo-Arvizu R, Dugas LR, et al. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr Biol. 2016;26:410–417. doi: 10.1016/j.cub.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontzer H, Raichlen DA, Wood BM, et al. Hunter-gatherer energetics and human obesity. PLoS One. 2012;7:e40503. doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerterp KR. Alterations in energy balance with exercise. Am J Clin Nutr. 1998;68:970S–974S. doi: 10.1093/ajcn/68.4.970S. [DOI] [PubMed] [Google Scholar]
- 24.Wilmore JH, Stanforth PR, Hudspeth LA, et al. Alterations in resting metabolic rate as a consequence of 20 wk of endurance training: the HERITAGE Family Study. Am J Clin Nutr. 1998;68:66–71. doi: 10.1093/ajcn/68.1.66. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerterp KR. Metabolic adaptations to over–and underfeeding–still a matter of debate? Eur J Clin Nutr. 2013;67:443–445. doi: 10.1038/ejcn.2012.187. [DOI] [PubMed] [Google Scholar]
- 27.Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97:990–994. doi: 10.3945/ajcn.112.050310. [DOI] [PubMed] [Google Scholar]
- 28.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016;24:1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum M, Hirsch J, Gallagher DA, et al. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Foster GD, Letizia KA, et al. Long-term effects of dieting on resting metabolic rate in obese out-patients. JAMA. 1990;264:707–711. [PubMed] [Google Scholar]
- 31.Weinsier RL, Nagy TR, Hunter GR, et al. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72:1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 32.Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011;96:E1512–E1516. doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Murphy EM, Heymsfield SB, et al. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum M, Nicolson M, Hirsch J, et al. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- 36.Weinsier RL, Hunter GR, Zuckerman PA, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000;71:1138–1146. doi: 10.1093/ajcn/71.5.1138. [DOI] [PubMed] [Google Scholar]
- 37.Galgani JE, Santos JL. Insights about weight loss-induced metabolic adaptation. Obesity (Silver Spring) 2016;24:277–278. doi: 10.1002/oby.21408. [DOI] [PubMed] [Google Scholar]
- 38.Muller MJ, Bosy-Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring) 2013;21:218–228. doi: 10.1002/oby.20027. [DOI] [PubMed] [Google Scholar]
- 39.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 40.Levine JA, Eberhardt NL, Jensen MD. Role of non-exercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 41.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 42.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall KD, Butte NF, Swinburn BA, et al. Dynamics of childhood growth and obesity: development and validation of a quantitative mathematical model. Lancet Diabetes Endocrinol. 2013;1:97–105. doi: 10.1016/s2213-8587(13)70051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall KD, Gortmaker SL, Lott M, et al. From calories to weight change in children and adults: the state of the science. issue brief. Durham, NC: Healthy Eating Research; 2016. [Google Scholar]
- 45.Lin BH, Smith TA, Lee JY, et al. Measuring weight outcomes for obesity intervention strategies: the case of a sugar-sweetened beverage tax. Econ Hum Biol. 2011;9:329–341. doi: 10.1016/j.ehb.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S–1329S. doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 47.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, et al. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 48.Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307:47–55. doi: 10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wycherley TP, Moran LJ, Clifton PM, et al. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:1281–1298. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 50.Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall KD. A review of the carbohydrate-insulin model of obesity. Eur J Clin Invest. 2017 doi: 10.1038/ejcn.2017.21. In press. [DOI] [PubMed] [Google Scholar]
- 52.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311:2167–2168. doi: 10.1001/jama.2014.4133. [DOI] [PubMed] [Google Scholar]
- 53.Fine EJ, Feinman RD. Thermodynamics of weight loss diets. Nutr Metab (Lond) 2004;1:15. doi: 10.1186/1743-7075-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 55.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight pre-menopausal women: the A to Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 56.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 57.Atkins RC., Dr . Atkins’ Diet revolution: the high calorie way to stay thin forever. Bantam Books; 1973. [Google Scholar]
- 58.Westman EC, Feinman RD, Mavropoulos JC, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- 59.Lim JS, Mietus-Snyder M, Valente A, et al. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz JM, Noworolski SM, Wen MJ, et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab. 2015;100:2434–2442. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stubbs RJ, Harbron CG, Murgatroyd PR, et al. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am J Clin Nutr. 1995;62:316–329. doi: 10.1093/ajcn/62.2.316. [DOI] [PubMed] [Google Scholar]
- 62.Stubbs RJ, Ritz P, Coward WA, et al. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr. 1995;62:330–337. doi: 10.1093/ajcn/62.2.330. [DOI] [PubMed] [Google Scholar]
- 63.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams RA, Roe LS, Rolls BJ. Comparison of three methods to reduce energy density. Effects on daily energy intake. Appetite. 2013;66:75–83. doi: 10.1016/j.appet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibson AA, Seimon RV, Lee CM, et al. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev. 2015;16:64–76. doi: 10.1111/obr.12230. [DOI] [PubMed] [Google Scholar]
- 66.Paoli A, Bosco G, Camporesi EM, et al. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6:27. doi: 10.3389/fpsyg.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freedhoff Y, Hall KD. Weight loss diet studies: we need help not hype. Lancet. 2016;388:849–851. doi: 10.1016/S0140-6736(16)31338-1. [DOI] [PubMed] [Google Scholar]
- 68.Guo J, Simmons WK, Herscovitch P, et al. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry. 2014;19:1078–1084. doi: 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chow CC, Hall KD. Short and long-term energy intake patterns and their implications for human body weight regulation. Physiol Behav. 2014;134:60–65. doi: 10.1016/j.physbeh.2014.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levitsky DA. The non-regulation of food intake in humans: hope for reversing the epidemic of obesity. Physiol Behav. 2005;86:623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 71.Speakman JR, Stubbs RJ, Mercer JG. Does body mass play a role in the regulation of food intake? Proc Nutr Soc. 2002;61:473–487. doi: 10.1079/pns2002194. [DOI] [PubMed] [Google Scholar]
- 72.Speakman JR, Levitsky DA, Allison DB, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9:584–597. doi: 10.1038/nrendo.2013.136. [DOI] [PubMed] [Google Scholar]
- 74.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71:478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almiron-Roig E, Palla L, Guest K, et al. Factors that determine energy compensation: a systematic review of preload studies. Nutr Rev. 2013;71:458–473. doi: 10.1111/nure.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bellisle F, Perez C. Low-energy substitutes for sugars and fats in the human diet: impact on nutritional regulation. Neurosci Biobehav Rev. 1994;18:197–205. doi: 10.1016/0149-7634(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 77.Keys A. The biology of human starvation. Minneapolis, MN: University of Minnesota Press; 1950. [Google Scholar]
- 78.Heyman MB, Young VR, Fuss P, et al. Underfeeding and body weight regulation in normal-weight young men. Am J Physiol. 1992;263:R250–R257. doi: 10.1152/ajpregu.1992.263.2.R250. [DOI] [PubMed] [Google Scholar]
- 79.Roberts SB, Fuss P, Heyman MB, et al. Control of food intake in older men. JAMA. 1994;272:1601–1606. doi: 10.1001/jama.1994.03520200057036. [DOI] [PubMed] [Google Scholar]
- 80.Winkels RM, Jolink-Stoppelenburg A, de Graaf K, et al. Energy intake compensation after 3 weeks of restricted energy intake in young and elderly men. J Am Med Dir Assoc. 2011;12:277–286. doi: 10.1016/j.jamda.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Blundell JE, Finlayson G, Gibbons C, et al. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiol Behav. 2015;152:473–478. doi: 10.1016/j.physbeh.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 82.Dulloo AG, Jacquet J, Girardier L. Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr. 1997;65:717–723. doi: 10.1093/ajcn/65.3.717. [DOI] [PubMed] [Google Scholar]
- 83.Gibbons C, Finlayson G, Dalton M, et al. Metabolic Phenotyping Guidelines: studying eating behaviour in humans. J Endocrinol. 2014;222:G1–G12. doi: 10.1530/JOE-14-0020. [DOI] [PubMed] [Google Scholar]
- 84.Edholm OG, Adam JM, Healy MJ, et al. Food intake and energy expenditure of army recruits. Br J Nutr. 1970;24:1091–1107. doi: 10.1079/bjn19700112. [DOI] [PubMed] [Google Scholar]
- 85.Dhurandhar NV, Schoeller DA, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109–1113. doi: 10.1038/ijo.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48:373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 87.Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6:199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 88.Polidori D, Sanghvi A, Seeley RJ, et al. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity (Silver Spring) 2016;24:2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanghvi A, Redman LA, Martin CK, et al. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am J Clin Nutr. 2015;102:353–358. doi: 10.3945/ajcn.115.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.