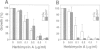Abstract
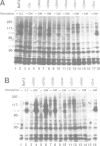
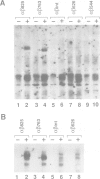
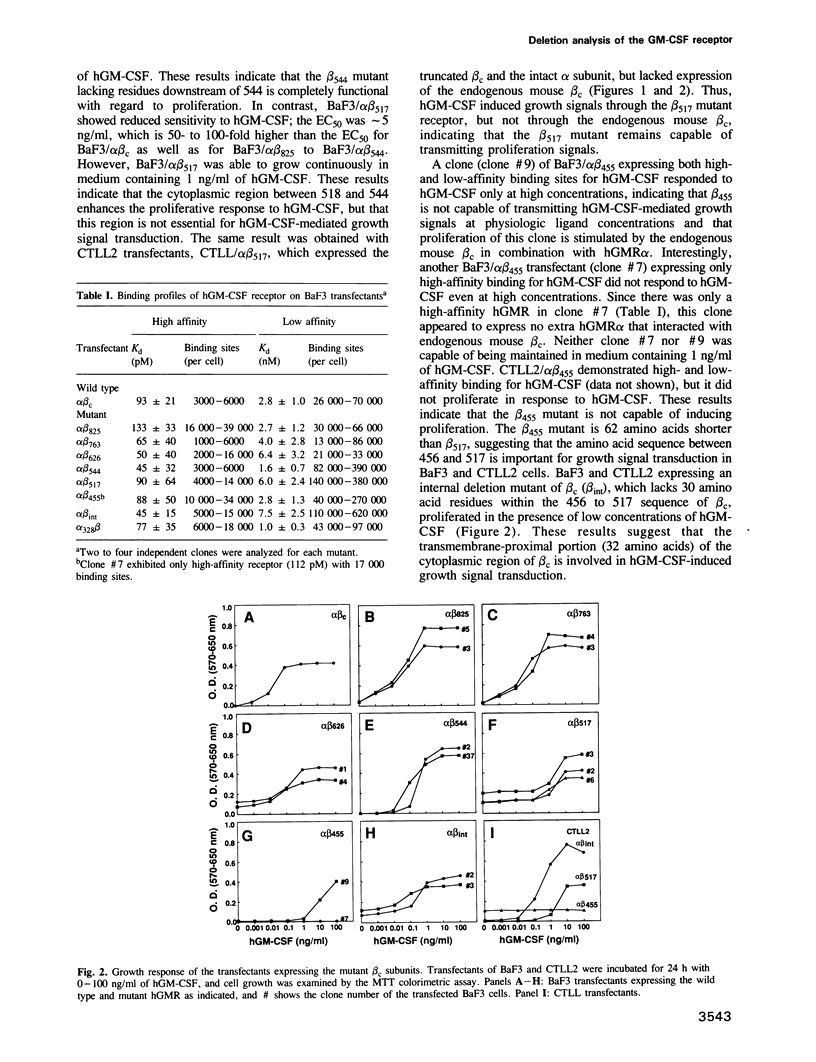
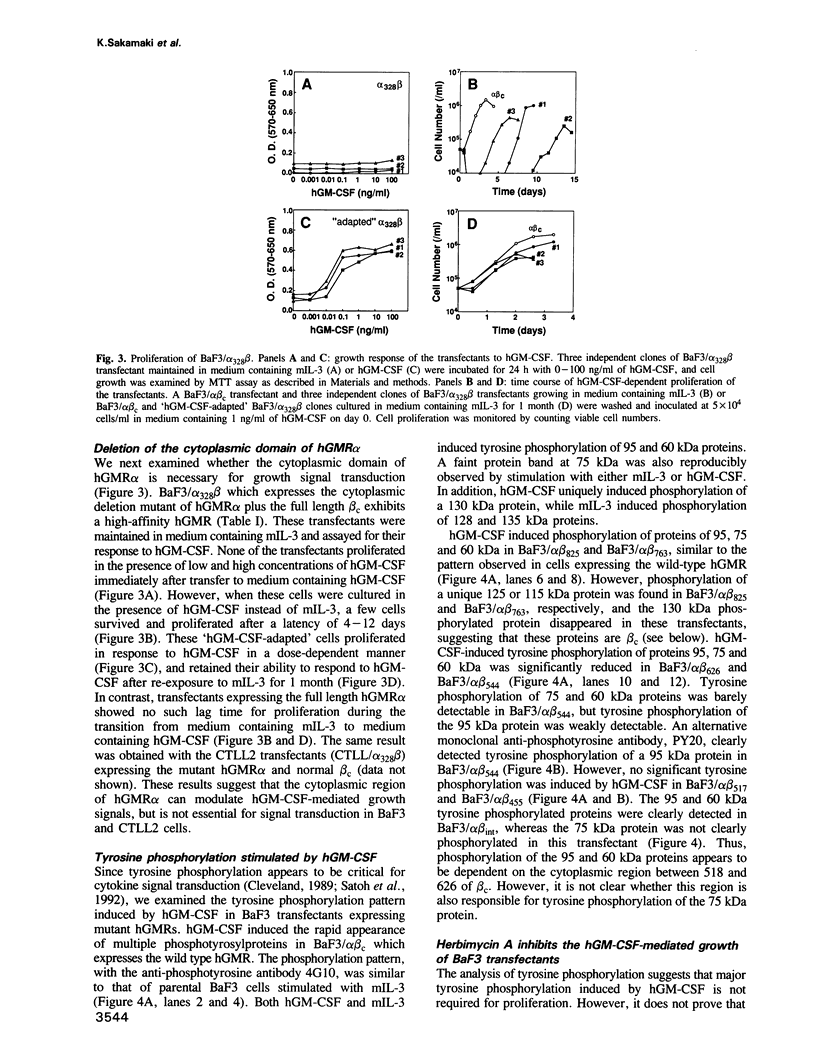
The high-affinity receptors for human granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3) and interleukin 5 (IL-5) are composed of two distinct subunits, alpha and beta c. The alpha subunits are specific for each cytokine, whereas the beta subunit (beta c) is shared by the three receptors and is an essential component of signal transduction. We have made a series of mutant beta c cDNAs that delete various regions of the cytoplasmic domain and examined the function of these mutants by coexpressing them with the alpha subunit of the human GM-CSF receptor (hGMR) in an IL-3-dependent mouse pro-B cell line BaF3. Two domains in the membrane-proximal portion of beta c were found to be important for transducing the hGM-CSF-mediated growth signals: one domain between Arg456 and Phe487 appears to be essential for proliferation, and the second domain between Val518 and Asp544 enhances the response to GM-CSF, but is not absolutely required for proliferation. The region between Val518 and Leu626 was responsible for major tyrosine phosphorylation of 95 and 60 kDa proteins. Thus, beta c-mediated major tyrosine phosphorylation of these proteins was apparently separated from proliferation. However, the beta 517 mutant lacking residues downstream of Val518 transmitted a herbimycin-sensitive proliferation signal, suggesting that beta 517 still activates a tyrosine kinase(s). We also evaluated the role of the cytoplasmic domain of the GMR alpha subunit and the results suggest that it is involved in the hGM-CSF-mediated signal transduction, but is not essential.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budel L. M., Elbaz O., Hoogerbrugge H., Delwel R., Mahmoud L. A., Löwenberg B., Touw I. P. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood. 1990 Apr 1;75(7):1439–1445. [PubMed] [Google Scholar]
- Carroll M. P., Clark-Lewis I., Rapp U. R., May W. S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Tojo A., Kitamura T., Urabe A., Miyazono K., Takaku F. Characterization and molecular features of the cell surface receptor for human granulocyte-macrophage colony-stimulating factor. Leukemia. 1990 Jan;4(1):29–36. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Dean M., Rosenberg N., Wang J. Y., Rapp U. R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989 Dec;9(12):5685–5695. doi: 10.1128/mcb.9.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosier K. E., Wong G. G., Mathey-Prevot B., Nathan D. G., Sieff C. A. A functional isoform of the human granulocyte/macrophage colony-stimulating factor receptor has an unusual cytoplasmic domain. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7744–7748. doi: 10.1073/pnas.88.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Van der Heyden J., Cornelis S., Vandekerckhove J., Fiers W., Tavernier J. Molecular basis of a high affinity murine interleukin-5 receptor. EMBO J. 1991 Aug;10(8):2133–2137. doi: 10.1002/j.1460-2075.1991.tb07747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Gasson J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991 Mar 15;77(6):1131–1145. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesner T. G., Mufson R. A., Norton C. R., Turner K. J., Yang Y. C., Clark S. C. Specific binding, internalization, and degradation of human recombinant interleukin-3 by cells of the acute myelogenous, leukemia line, KG-1. J Cell Physiol. 1988 Sep;136(3):493–499. doi: 10.1002/jcp.1041360314. [DOI] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Doi T., Kono T., Maruyama M., Minamoto S., Mori H., Kobayashi M., Uchiyama T., Taniguchi T. Transmembrane signaling of interleukin 2 receptor. Conformation and function of human interleukin 2 receptor (p55)/insulin receptor chimeric molecules. J Exp Med. 1987 Aug 1;166(2):362–375. doi: 10.1084/jem.166.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Ihle J. N. Multiple hematopoietic growth factors signal through tyrosine phosphorylation. Growth Factors. 1990;2(2-3):213–220. doi: 10.3109/08977199009071507. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Wood K. W., Mamon H. J., Okuda K., Roberts T. M., Griffin J. D. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce rapid phosphorylation and activation of the proto-oncogene Raf-1 in a human factor-dependent myeloid cell line. Blood. 1991 Jan 15;77(2):243–248. [PubMed] [Google Scholar]
- Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Miyajima A. Functional reconstitution of the human interleukin-3 receptor. Blood. 1992 Jul 1;80(1):84–90. [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Vadas M. A., Woodcock J. M., Milton S. E., Lewis A., Elliott M. J., Gillis D., Ireland R., Olwell E., Park L. S. Interleukin-5, interleukin-3, and granulocyte-macrophage colony-stimulating factor cross-compete for binding to cell surface receptors on human eosinophils. J Biol Chem. 1991 Dec 25;266(36):24741–24747. [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Schreurs J., Otsu K., Kondo A., Arai K., Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58(2-3):273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Takaki S., Migita M., Kikuchi Y., Tominaga A., Takatsu K. Molecular cloning and expression of the human interleukin 5 receptor. J Exp Med. 1992 Feb 1;175(2):341–351. doi: 10.1084/jem.175.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Yamaguchi N., Hitoshi Y., Tominaga A., Takatsu K. Interleukin 5 and interleukin 3 induce serine and tyrosine phosphorylations of several cellular proteins in an interleukin 5-dependent cell line. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1102–1108. doi: 10.1016/s0006-291x(05)80899-x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Uehara Y., Kaziro Y. Inhibition of interleukin 3 and granulocyte-macrophage colony-stimulating factor stimulated increase of active ras.GTP by herbimycin A, a specific inhibitor of tyrosine kinases. J Biol Chem. 1992 Feb 5;267(4):2537–2541. [PubMed] [Google Scholar]
- Schrader J. W. The panspecific hemopoietin of activated T lymphocytes (interleukin-3). Annu Rev Immunol. 1986;4:205–230. doi: 10.1146/annurev.iy.04.040186.001225. [DOI] [PubMed] [Google Scholar]
- Schreurs J., Hung P., May W. S., Arai K., Miyajima A. AIC2A is a component of the purified high affinity mouse IL-3 receptor: temperature-dependent modulation of AIC2A structure. Int Immunol. 1991 Dec;3(12):1231–1242. doi: 10.1093/intimm/3.12.1231. [DOI] [PubMed] [Google Scholar]
- Shanafelt A. B., Miyajima A., Kitamura T., Kastelein R. A. The amino-terminal helix of GM-CSF and IL-5 governs high affinity binding to their receptors. EMBO J. 1991 Dec;10(13):4105–4112. doi: 10.1002/j.1460-2075.1991.tb04987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki S., Mita S., Kitamura T., Yonehara S., Yamaguchi N., Tominaga A., Miyajima A., Takatsu K. Identification of the second subunit of the murine interleukin-5 receptor: interleukin-3 receptor-like protein, AIC2B is a component of the high affinity interleukin-5 receptor. EMBO J. 1991 Oct;10(10):2833–2838. doi: 10.1002/j.1460-2075.1991.tb07832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki S., Tominaga A., Hitoshi Y., Mita S., Sonoda E., Yamaguchi N., Takatsu K. Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 1990 Dec;9(13):4367–4374. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Harada N., Mita S., Matsumoto M., Takahashi T., Kikuchi Y., Yamaguchi N. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988 Feb;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Taketazu F., Chiba S., Shibuya K., Kuwaki T., Tsumura H., Miyazono K., Miyagawa K., Takaku F. IL-3 specifically inhibits GM-CSF binding to the higher affinity receptor. J Cell Physiol. 1991 Feb;146(2):251–257. doi: 10.1002/jcp.1041460209. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Collins M., Arai K., Miyajima A. EGF induces differentiation of an IL-3-dependent cell line expressing the EGF receptor. EMBO J. 1989 Dec 1;8(12):3677–3684. doi: 10.1002/j.1460-2075.1989.tb08542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]