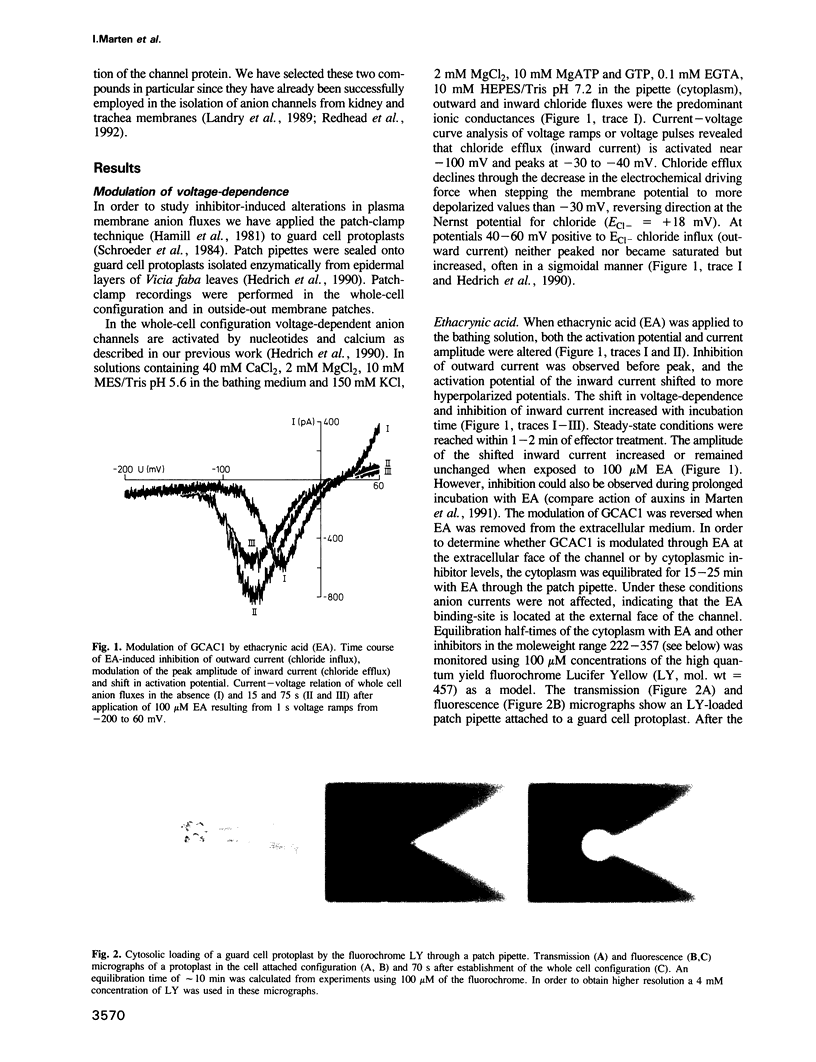
Abstract
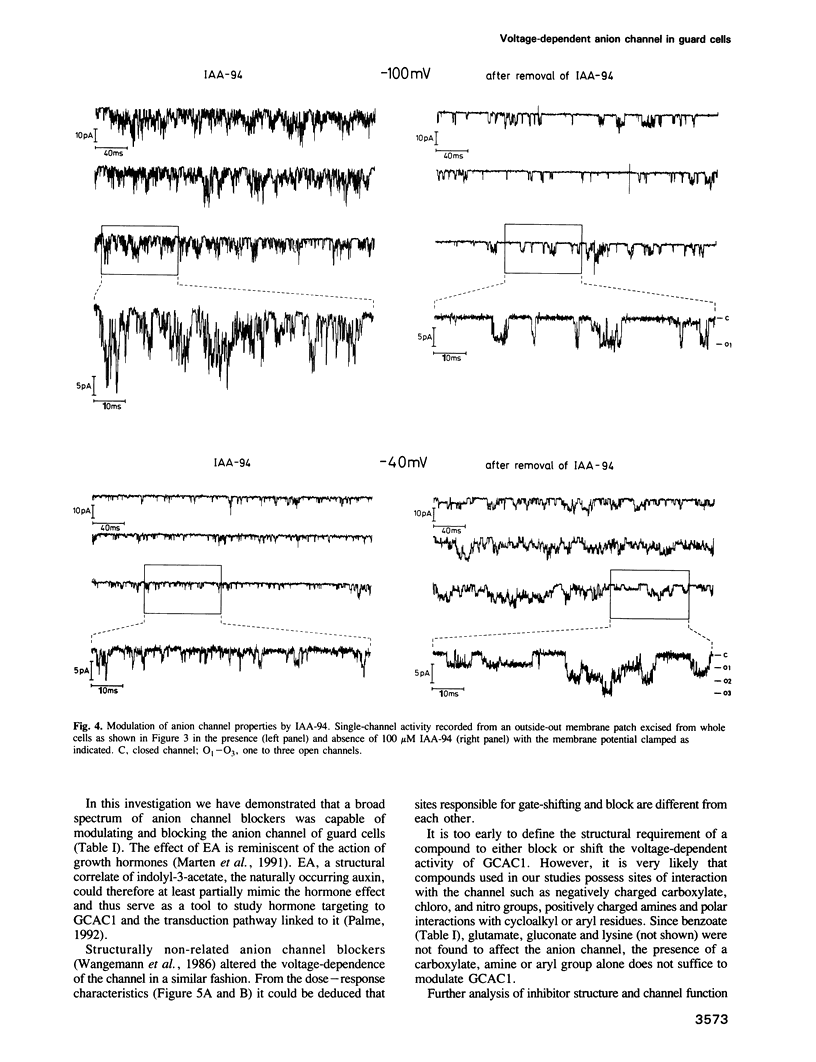
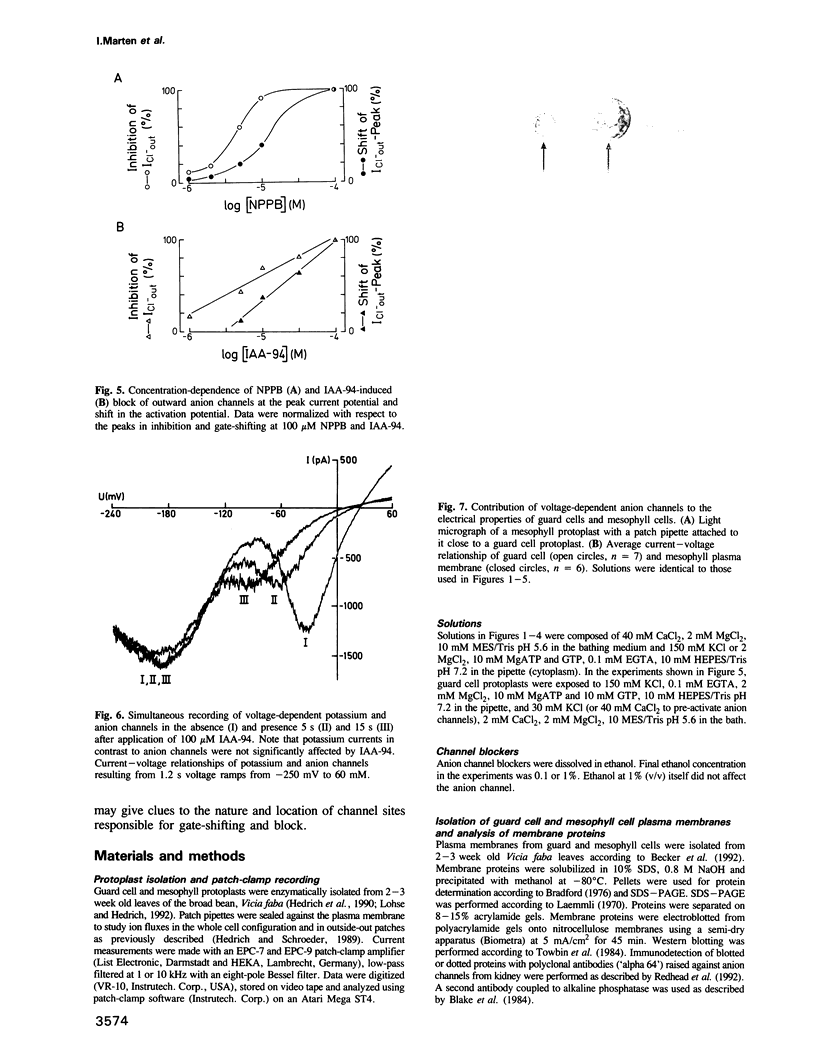
Guard cell anion channels (GCAC1) catalyze the release of anions across the plasma membrane during regulated volume decrease and also seem to be involved in the targeting of the plant growth hormones auxins. We have analyzed the modulation and inhibition of these voltage-dependent anion channels by different anion channel blockers. Ethacrynic acid, a structural correlate of an auxin, caused a shift in activation potential and simultaneously a transient increase in the peak current amplitude, whereas other blockers shifted and blocked the voltage-dependent activity of the channel. Comparison of dose-response curves for shift and block imposed by the inhibitor, indicate two different sites within the channel which interact with the ligand. The capability to inhibit GCAC1 increases in a dose-dependent manner in the sequence: probenecid less than A-9-C less than ethacrynic acid less than niflumic acid less than IAA-94 less than NPPB. All inhibitors reversibly blocked the anion channel from the extracellular side. Channel block on the level of single anion channels is characterized by a reduction of long open transitions into flickering bursts, indicating an interaction with the open mouth of the channel. IAA-23, a structural analog of IAA-94, was used to enrich ligand-binding polypeptides from the plasma membrane of guard cells by IAA-23 affinity chromatography. From this protein fraction a 60 kDa polypeptide crossreacted specifically with polyclonal antibodies raised against anion channels isolated from kidney membranes. In contrast to guard cells, mesophyll plasma membranes were deficient in voltage-dependent anion channels and lacked crossreactivity with the antibody.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Cota G. Calcium ion as a cofactor in Na channel gating. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6528–6531. doi: 10.1073/pnas.88.15.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Busch H., Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990 Dec;9(12):3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988 Dec 1;7(12):3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry D. W., Akabas M. H., Redhead C., Edelman A., Cragoe E. J., Jr, Al-Awqati Q. Purification and reconstitution of chloride channels from kidney and trachea. Science. 1989 Jun 23;244(4911):1469–1472. doi: 10.1126/science.2472007. [DOI] [PubMed] [Google Scholar]
- Lew R. R. Substrate regulation of single potassium and chloride ion channels in Arabidopsis plasma membrane. Plant Physiol. 1991 Feb;95(2):642–647. doi: 10.1104/pp.95.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K. Molecular analysis of plant signaling elements: relevance of eukaryotic signal transduction models. Int Rev Cytol. 1992;132:223–283. doi: 10.1016/s0074-7696(08)62457-2. [DOI] [PubMed] [Google Scholar]
- Redhead C. R., Edelman A. E., Brown D., Landry D. W., al-Awqati Q. A ubiquitous 64-kDa protein is a component of a chloride channel of plasma and intracellular membranes. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3716–3720. doi: 10.1073/pnas.89.9.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Keller B. U., Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987 Dec 3;330(6147):498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Klocke R., Ortland C., Gronemeier M., Jockusch H., Gründer S., Jentsch T. J. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991 Nov 28;354(6351):304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., Jentsch T. J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991 Nov 28;354(6351):301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]