Abstract
The study of the Nuclear Pore Complex (NPC), the proteins that compose it (nucleoporins), and the nucleocytoplasmic transport that it controls has revealed an unexpected layer to pathogenic disease onset and progression. Recent advances in the study of the regulation of NPC composition and function suggest that the precise control of this structure is necessary to prevent diseases from arising or progressing. Here we discuss the role of nucleoporins in a diverse set of diseases, many of which directly or indirectly increase in occurrence and severity as we age, and often shorten the human lifespan. NPC biology has been shown to play a direct role in these diseases and therefore in the process of healthy aging.
Keywords: Nuclear pore complex, nucleoporin, nucleocytoplasmic transport, aging, neurological, cancer
1. Introduction – The dynamic nuclear pore complex
Nuclear Pore Complexes (NPCs) are large multi-protein complexes that form aqueous channels bridging the cytoplasm and nucleus of all eukaryotic cells. NPCs were first visually observed by electron microscopy in 1949 in Xenopus laevis oocytes [1,2] and, although it is assumed that the evolution of the nucleus progressed through a protonucleus stage that was freely permeable with the cytoplasm, all extant eukaryotes possess a nucleus with NPCs [3].
After the initial discovery of the complex many experiments were done to determine the physical structure of the NPC, which was found to be a ~110 MDa structure (~60 MDa in yeast), with a central channel that allows the free diffusion of molecules less than ~90–110 Angstrom and the active transport of molecules up to ~390 Angstrom [4]. The NPC is organized into 3 basic subunits: the cytoplasmic filaments and ring, the membrane-embedded scaffold and central channel, and the nuclear ring and basket. Each of these components is composed of multiples of 8 copies of ~30 proteins called nucleoporins, which are arranged into a highly organized structure with 8-fold rotational symmetry. Determining the structure/number of copies of nucleoporins per NPC is still a field of active research [5].
Previously it was thought that the nucleoporin composition of all NPCs was constant and the NPC was a passive structure, which served to allow diffusion of molecules between the cytoplasm and nucleus [2,6,7]. This view was supported by the high evolutionary conservation of the NPC architecture and of the fold type, domain organization, composition, and modularity of the nucleoporins [8]. Research over the last 25 years has demonstrated that not only does the protein composition of NPCs differ between different organisms, but it also differs between cells in a single organism [9].
Tissue specific differences in NPC composition were first described with the example of Nup210. This transmembrane nucleoporin is expressed at different levels in various tissues and during different stages of development [10,11]. Depleting Nup210 in mouse cell culture models was sufficient to prevent myogenic and neuronal differentiation [12]. Since Nup210, several other nucleoporins have been shown to have differential expression between different cells and tissues, and a few other pore components have also been found to be critical for differentiation in vitro and in vivo [13–16].
In addition to the main function of NPCs as mediators of nucleocytoplasmic transport, NPCs have been shown to regulate many cellular processes in a transport-independent manner including gene expression, chromatin organization, and cell cycle regulation. The transport-dependent and transport-independent roles of NPCs were reviewed recently [17,18].
Much of the seminal work in NPCs has been carried out in yeast, which, despite having a closed mitosis (where the nuclear envelope does not break down during cell division), has been used as a model organism to demonstrate many fundamental aspects about the assembly and structure of the NPC that also hold true in metazoans. This review will focus on the role of NPCs in development, aging, and disease with an emphasis on the effect on human health. Although yeast and other single-celled eukaryotes possess characteristics that allow them to be studied to answer questions about aging and disease [19], they are evolutionarily divergent from mammals and findings in these organisms many times do not translate to humans, so we will focus our analysis on more closely related eukaryotic model organisms and studies from humans.
Most of the nucleoporin homozygous knockout mice that have been reported so far die in embryogenesis or shortly after birth. Of the few specific nucleoporin null mice that survive early development some are sterile and others have phenotypes that shorten their lifespan. Even mice with reduced levels of nuclear pore components often have serious health problems (Table 1) [13,20–37]. These studies and other examples of NPC biology will be discussed below demonstrating the importance of nucleocytoplasmic transport, nucleoporins, and NPCs in disease and healthy aging.
Table 1.
Published phenotypes of mice possessing mutations in nucleoporins
| Nucleoporin | Mutation | Phenotype | References |
|---|---|---|---|
| Aladin/AAAS | Homozygous null | Sterility | [36] |
| ELYS | Homozygous null | Embryonic lethality | [35] |
| ELYS | Intestinal epithelium null | Juvenile growth delay | [37] |
| NDC1 | Homozygous null | Sterility and developmental defects | [34] |
| Nup35 | F192L Mutant | Degenerative colonic smooth muscle myopathy | [33] |
| Nup50 | Homozygous null | Embryonic lethality | [32] |
| Nup88 | Overexpression | Increased genetic tumor model tumorigenesis | [31] |
| Nup88 | Homozygous null | Not Viable | [31] |
| Nup96 | Homozygous null | Embryonic lethality | [30] |
| Nup96 | Heterozygous null | Reduced antigen presentation and immunodeficiency | [30] |
| Nup98 | Homozygous null | Embryonic lethality | [29] |
| Nup98 | HoxA9/HoxD13 fusion | Spontaneous leukemia | [26,27] |
| Nup133 | Homozygous null | Embryonic lethality | [13] |
| Nup155 | Heterozygous null | Atrial fibrillation and early sudden cardiac death | [25] |
| Nup155 | Homozygous null | Embryonic lethality | [25] |
| Nup214 | Homozygous null | Embryonic lethality | [24] |
| Nup358/RanBP2 | Homozygous null | Embryonic lethality | [23] |
| Nup358/RanBP2 | Heterozygous null | Reduced size and impaired glucose homeostasis | [23] |
| Nup358/RanBP2 | Hypomorph | Spontaneous and carcinogen-induced tumorigenesis | [22] |
| Rae1 | Homozygous null | Embryonic lethality | [21] |
| Nup98+Rae1 | Double Heterozygous Null | Increased carcinogen-induced tumorigenesis | [28] |
| Sec13 | Homozygous null | Embryonic lethality | [20] |
| Sec13 | Hypomorph | Reduced antigen presentation | [20] |
2. NPCs in neurological disorders and the aging brain
2.1. Triple A syndrome
The most well studied nucleoporin-related neurological disorder is called triple A syndrome. Achalasia-Addisonianism-Alacrima or Allgrove syndrome is a rare autosomal recessive disorder characterized by adrenocorticotropin resistant adrenal cortex, inability to relax esophageal sphincter, and inability to produce tears as well as other neurological symptoms [38]. The symptoms are highly variable in both severity and timing but because of the clear autosomal recessive inheritance pattern, genetic mapping of the mutation was possible. The disease was mapped to mutations in the AAAS gene, which codes for the protein Aladin [39]. At the time of the genetic mapping the subcellular location of Aladin was not known but it was subsequently found to be a component of the NPC [40]. There are many known Aladin mutations that have been linked to triple A syndrome but the ones with the greatest disease severity cause Aladin to not localize correctly to the NPC [41]. Surprisingly, Aladin knockout mice have only mild neurological defects and no other symptoms associated with triple A syndrome (Table 1) [36].
Although there is extensive information about the different mutations in the AAAS gene and there are descriptive models regarding how the nucleoporin mutations might lead to triple A syndrome, there is little evidence supporting a molecular mechanism. Aladin is directly anchored to the NPC by the transmembrane nucleoporin NDC1 [42]. Depletion of NDC1 not only affects Aladin NPC-anchoring but also impairs nuclear import [43]. Thus, an Aladin mutant that does not bind NDC1 will need to be used to discern if Aladin mislocalization results in a transport defect or if this is due to NDC1 depletion. But supporting a potential role for Aladin in nucleocytoplasmic transport, recent evidence suggests that triple A syndrome might be caused by the inability of the mutant or null allele of Aladin to transport proteins that are critical to protect cells from oxidative damage [44]. Nuclear import of a protein with known roles in oxidative stress response, Ferritin heavy chain (Fth1), is impaired in the absence of Aladin [45,46]. Subsequent research has shown that Aladin interacts directly with progesterone receptor membrane component 2 (PGRMC2), a microsomal protein known to regulate cytochrome P450 hydroxylases and oxidoreductases, which are critical for maintaining cellular oxidative state and for generating precursors for hormones like cortisol [47]. After Aladin knockdown, the cytochrome P450 enzymes are reduced and cells are more susceptible to oxidative stress [48]. These results support the hypothesis that Aladin mutations cause triple A syndrome by reducing the cells’ ability to respond to oxidative stress and possibly altering hormone production.
Supporting the oxidative stress model, Aladin-depleted human adrenal tumor cells had increased levels of oxidative damage and reduced cortisol production [49]. Cortisol is normally produced by the adrenal glands in response to ACTH but one of the characteristics of triple A syndrome is insufficient cortisol production in response to ACTH [38]. This is the first human evidence demonstrating how Aladin may be causing at least one of the symptoms of triple A syndrome, but much work remains to determine how the diverse symptoms of triple A syndrome manifest from Aladin mutations. The mechanistic studies to date have demonstrated that the loss of the Aladin protein leads to toxic cellular damage, but it sill remains to be seen if the known human mutations in the AAAS gene could cause this same damage. Additionally, how this cellular damage could cause the remaining physiological symptoms of triple A syndrome is still unclear.
2.2. Frontotemporal dementia, amyotrophic lateral sclerosis and Parkinson’s disease
Frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD) are all serious neurodegenerative disorders that cause progressive loss of cognitive ability (Figure 1). In recent years a link between these disparate disorders and NPC biology has emerged. A recent example of this connection is that a motor neuron specific knockout of Nup358 (also known as RanBP2), a cytoplasmic filament nucleoporin with known roles in oxidative stress response [50], causes a mouse ALS phenotype (Table 1). Conditional ablation of Nup358 in mouse motor neurons causes the onset of the ALS symptoms of hypoactivity, hind limb paralysis, respiratory distress, and eventually death [51]. The ubiquitous Nup358 heterozygous null mice suffer from increased severity of akinetic PD when they are exposed to the neurotoxin MPTP (Table 1) [52]. The connection between PD and ALS has not been determined, but Nup358 does appear to play a role in both of these neurodegenerative phenotypes.
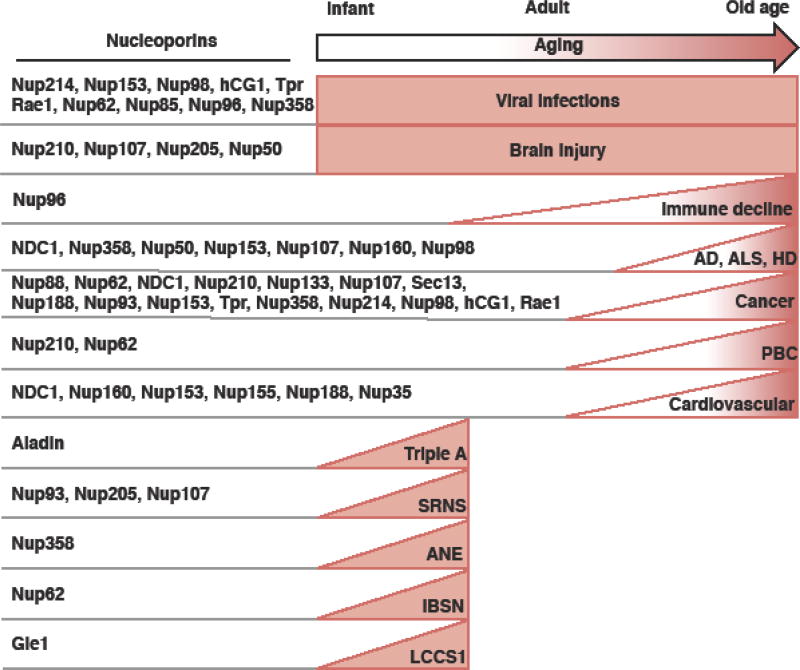
Figure 1.
Onset of nucleoporin-implicated health problems. Left: a list of the nucleoporins that are implicated in the health problems listed on the right. Right: a list of health problems with the age-based prevalence indicated by the box/triangle.
Triple A: Achalasia-Addisonianism-Alacrima or Allgrove syndrome; ALS: amyotrophic lateral sclerosis; PD: Parkinson’s disease; IBSN: Infantile bilateral striatal necrosis; LCCS1: lethal congenital contracture syndrome-1; ANE: acute necrotizing encephalopathy; PBC: Primary biliary cholangitis; SRNS: steroid-resistant nephrotic syndrome
The first discovered genetic determinant of FTD and ALS is the GGGGCC (G4C2) hexanucleotide repeat expansion (HRE) found in the C9orf72 gene. The HRE causes the transcription of RNA that form into nuclear foci [53,54]. Because the G4C2 HRE RNA undergoes repeat-associated non-AUG (RAN) translation, it forms insoluble high molecular weight polydipeptides [55,56]. Two groups simultaneously reported that, in Drosophila, the G4C2 HRE disrupts nucleocytoplasmic transport and causes the neurodegeneration typical of ALS [57,58]. Specifically, nuclear import is impaired and genetically increasing import or decreasing export rescues the neurodegeneration defect. The nucleocytoplasmic distribution of Ran in induced pluripotent stem cells (iPSCs) derived from patients with ALS is shifted towards the cytoplasm as expected in cells with reduced import. Excitingly, transport defects and neurodegeneration are rescued by pharmacotherapeutic intervention with nuclear export inhibitor KPT-276 (an analog of KPT-330, now known as Selinexor) opening up the possibility that these inhibitors might be useful for the treatment of ALS or other neurodegenerative diseases where nucleocytoplasmic transport is altered [57]. Nucleoporins were found to play a complex role in this Drosophila model of ALS. Knocking down most nucleoporins enhanced the phenotype while knocking down other nucleoporins suppressed the phenotype [58].
RAN translation of the sense and antisense G4C2 HRE RNAs produce 5 different polydipeptides: Poly glycine-alanine (GA), glycine-arginine (GR), glycine-proline (GP), proline-arginine (PR), and proline-alanine (PA) [59]. The formation of polyGA aggregates was prevented when a mutated form of the protein (a single proline amino acid was inserted every 5 repeats) was expressed instead of the pure polyGA variant. Toxic polyGA protein aggregates contain HR23A and HR23B (proteins critical for transferring ubiquitinated proteins to the proteasome) and nucleocytoplasmic transport proteins including the transmembrane nucleoporin Pom121 [60]. In a yeast genetic screen for polydipeptide repeat toxicity, the nucleoporin NDC1 and proteins critical to nucleocytoplasmic transport and regulators of the Ran-GTPase cycle were found [61]. The last few years of research into the pathological mechanisms of ALS increasingly suggest an important role for nucleocytoplasmic transport and the NPC. Thorough evaluations of the role of nucleocytoplasmic transport in ALS have been conducted recently [62,63].
2.3. Huntington’s Disease
Similar to the C9orf72 HRE commonly found in ALS, a CAG repeat expansion in the huntingtin gene leads to the formation of polyglutamine (polyQ) protein aggregates and causes Huntington’s disease (HD). HD is an autosomal dominant neurodegenerative disorder associated with neuronal cell death, motor impairment, personality changes, and dementia [64]. A mouse line with greater than 100 CAG repeats in the huntingtin gene was found to die at an advanced age after suffering from the development of a disease which possessed hallmark symptoms of HD. These mice formed neuronal intranuclear inclusions, which are also seen in patients with the disease [64,65]. Mutant forms of the huntingtin gene with greater than 20 CAG repeats cause perinuclear aggregates of the protein to form that are resistant to proteolytic degradation and cause cell death [66]. Protein aggregation in the cytoplasm but not the nucleus of cells leads to the mislocalization of proteins with low-complexity and disordered regions such as nuclear transport factors [67].
Debate exists about the subcellular location of the initial polyQ aggregates [68]. Although mutant huntingtin protein is seen to accumulate initially in the nucleus of neurons in HD mouse models [69], cell culture live imaging studies suggest that mutant huntingtin protein exists diffusely in the cytoplasm and nucleoplasm of cells and that aggregates can form on either side of the nuclear membrane. When aggregates begin to form in the cytoplasm, they eventually become perinuclear deforming the nucleus and causing the normally postmitotic cells to re-enter the cell cycle and undergo cell death. Alternatively, if aggregates formed on the inside of the nucleus, the cells would not develop cytoplasmic aggregates and continue to survive [70]. Despite the disagreement about the initial subcellular location of the aggregates, clearance of mutant huntingtin protein via the autophagy or the ubiquitin proteasome pathways and maintaining efficient nucleocytoplasmic transport is thought to be critical to preventing the disease [68].
In a mouse model of HD, after polyQ aggregates formed, nucleocytoplasmic transport was greatly impaired, nucleoporins were severely mislocalized and they were labeled less with the posttranslational modification O-linked beta N-acetylglucosamine (O-GlcNAc) [71]. Many nucleoporins are constitutively modified with the O-GlcNAc carbohydrate on serine and threonine residues [72] and these modifications are critical to prevent NPC ubiquitination and subsequent proteasomal degradation. Loss of the O-GlcNAc modification from nucleoporins leads to the disruption of the permeability barrier of the NPCs [73]. Overexpression of nucleoporins and treatment with Thiamet-G, which inhibits the O-GlcNAc removing enzyme O-GlcNAcase [74], rescues not only the transport defect but also prevents the cell death associated with the formation of the polyQ aggregates [71].
Interestingly, some of the large perinuclear polyQ aggregates are decorated with NPCs even though the nuclear envelope continues to separate the aggregates from the nucleoplasm [70]. Unfortunately, it is not yet known how or why the NPCs come to encircle the perinuclear polyQ aggregates, but a proteomic analysis of all the proteins present in polyQ aggregates found many, if not all, nucleoporins [75]. Many questions remain about the role of NPCs in HD but there is sufficient evidence to indicate that alterations in nucleocytoplasmic transport are integral to the pathology of the disease.
2.4. Alzheimer’s disease
Alzheimer’s disease (AD) is a serious neurodegenerative disorder characterized by memory loss, cognitive dysfunction, and eventually early death. The prevalence of the disease is expected to quadruple in the next few decades and place an increasing burden on society [76]. The most common form of the disease has a genetic contribution of ~60–80% but no exact mechanism has been demonstrated. AD is difficult to diagnose early but can be characterized by the occurrence of extracellular amyloid beta protein aggregates, hyperphosphorylated tau (a microtubule associated protein), and intracellular neurofibrillary tangles (NFTs) composed of tau protein [76]. The role of NPCs in AD has not been established but in AD patient hippocampal neurons importin alpha1 is aberrantly localized to cytoplasmic inclusions [77], NPCs are found in aggregates, and nuclei are abnormally shaped [78].
The intracellular NFTs are analogous to the PolyQ aggregates seen in HD and the PolyGA aggregates seen in ALS because they all represent a failure in proteostasis, the cellular homeostasis of protein concentration, conformation, and location [79]. The evidence for the role the PolyQ and PolyGA aggregates play in nucleocytoplasmic transport is considerable and, while there is still little evidence that in AD NFTs might also alter transport, it seems likely that this disorder will also be found to have a critical NPC aspect.
2.5. Neurological development and brain injury
A study of the expression of nucleoporins in rats after a reduced blood flow brain injury called cerebral ischemia, found that nucleoporins Nup210, Nup107, Nup205, and Nup50 were all increased within days after the injury and the expression continued to rise for up to 4 weeks. RanGap1, an NPC binding protein with known roles in nucleocytoplasmic transport, was also found to have increased expression after injury. Expression of the nucleoporins surprisingly shifted from the classical perinuclear pattern to an intranuclear pattern [80]. The expression pattern of other nucleoporins was not examined and these experiments are still preliminary, but the possible role of nucleoporins in the resolution or pathology of brain injuries is exciting.
The first hint that nucleoporins might play a role directly in the development of the mammalian brain came from a study showing that mice a knockout of the nuclear basket nucleoporin Nup50 develop lethal neural tube defects in utero (Table 1) [32]. Additionally, scaffold nucleoporin Nup133 deficient mice are embryonically lethal and fail to develop terminally differentiated neurons (Table 1) [13]. Work looking at the role of NPCs in neurological development is still preliminary but there is corroborating evidence from cell culture assays. Knockdown of the transmembrane nucleoporin Nup210 prevents the differentiation of stem cells into neurons in vitro [12] and conversely knockdown of Nup153 in mouse embryonic stem cells induced differentiation into neurons with the associated loss of pluripotency [81].
2.6. Other neurological disorders and the aging brain
Infantile bilateral striatal necrosis (IBSN) is a neurological disorder characterized by the degradation of 2 to 3 specific areas of the brain: the caudate nucleus, the putamen, and sometimes the globus pallidus. A familial autosomal recessive form of the disease was discovered that was mapped to a Nup62 missense mutation. Surprisingly, the mutation did not cause the protein to be degraded or mislocalized [82]. How the mutated Nup62 protein leads to this disorder has yet to be determined.
Human lethal congenital contracture syndrome-1 (LCCS1), also known as fetal motor neuron disease, can be caused by a mutation in the predicted nucleoporin Gle1 [83]. A Zebrafish model showed that Gle1 is important for the survival of a subset of neuronal stem cells as well as arborization of axons [84]. In human cells the mutated form of the protein impairs Gle1 oligomerization, which is critical for the nucleoporin’s role in mRNA export [85].
Nup358 mutations have been shown to cause acute necrotizing encephalopathy (ANE), which is a sporadically occurring encephalopathy that presents in children after common viral infections (Figure 1) [86,87]. It is not known if the nucleoporin mutations are related to the infection or resolution of the viral response or what possible mechanism might cause the disease to occur in patients with the mutations.
Most but not all of these neurological disorders occur more frequently and more severely with age (Figure 1). Thus far we have not directly addressed the effect of aging on the NPCs in the brain and there are not many studies that directly look at this topic but the data that does exist warrants further study. Electron microscopy studies in rats show the density of NPCs in hippocampal neurons as the brain ages drops in the dentate gyrus but stays constant in the CA1 region [88,89]. A molecular study of aging rat brains shows that scaffold nucleoporins turn over at a finite but very slow rate while peripheral nucleoporins turn over much more quickly. Consistent with previous work using C. elegans, this study found that the number of NPCs did not change while the relative nucleoporin composition of NPCs were altered as rats aged [90–92]. This is interesting because NPCs undergo oxidative damage and become more leaky with age [90], suggesting that the NPC turnover rate is possibly too slow and damaged NPCs accumulate with age in long lived cells such as neurons.
3. NPCs in viral infections and immunity
3.1. NPC-based viral nuclear entry
As stated above, molecules larger than ~390 Angstrom are unable to pass into the nucleus, therefore large viruses such as herpes simplex virus (HSV), human immunodeficiency virus (HIV), and adenovirus need to use 1 of 3 established methods for entry to the nucleus: 1) capsid degradation in the cytosol followed by classical nuclear import, 2) docking at the NPC followed by capsid degradation and modified nuclear import, and 3) docking at the NPC followed by direct injection into the nucleus [93]. Even smaller viruses such as hepatitis B virus depend on nuclear entry, which is based on an active transport mechanism [94]. All of these methods involve the NPC either directly or indirectly. As we will see, viruses have evolved mechanisms to utilize the nucleocytoplasmic transport function of their hosts and their hosts have evolved mechanisms to combat these viral nuclear entry methods.
Nucleoporins that, under normal conditions, play a role in nuclear import are likely targets of viruses to establish nuclear entry. Nup214, a cytoplasmic ring nucleoporin with known roles in nucleocytoplasmic transport directly interacts with and acts as the functional import receptor of adenovirus 2 nucleocapsids [95]. The nuclear basket nucleoporin Nup153, which plays direct roles in importin alpha/beta mediated protein import [96], binds to the HIV capsid and knockdown of this nucleoporin reduces capsid entry [97]. In a proteomic analysis of HCV capsid interacting proteins, Nup98 was identified as critical for HCV entry into the nucleus and the subsequent propagation of the virus [98]. A nucleoporin found on both faces of the NPC, hCG1 [99], binds to the HIV protein Vpr and this interaction aids in the nuclear import of the viral protein [100]. These examples highlight how viruses utilize a variety of host nucleoporins, regardless of the NPC structural component, to gain entry into the nuclei of their hosts.
3.2. Antiviral immunity and evading the host immune defense
When a viral infection occurs mammalian cells produce an antiviral immune response to dampen virus production or signal for apoptosis. The most characterized example is that infected cells secrete Type 1 interferons (alpha and beta), which signal to nearby cells to activate an antiviral immune response [101]. For a virus to successfully propagate, it must evade or inhibit this antiviral immune response. Viruses are known to utilize many different methods to continue their proliferation and, considering the importance of nucleoporins in gene expression regulation [18], it is not surprising that viruses have evolved to utilize the NPC.
For example, the immune response to vesicular stomatitis virus (VSV) infection requires mRNA export, which occurs through the complex of NPC central channel nucleoporins Nup98 and Rae1. In order to block the host cell immune response, VSV produces a protein called matrix. The viral protein binds to Nup98/Rae1 and inhibits mRNA export which would effectively shut down the immune response, but immune cells such as lymphocytes produce the cytokine Type 2 interferon (IFN-gamma) which upregulates Nup98 protein to saturate the VSV block on export and allows an effective response to the intracellular pathogen [102,103]. Also targeting the role of Nup98 in antiviral signaling, polioviruses induce Nup98 degradation shortly after infection [104].
Nup98 is not the only nucleoporin targeted to alter nucleocytoplasmic transport. Human rhinovirus encodes the 2A protease family, which proteolytically cleaves Nup62, a central channel nucleoporin with known roles in nucleocytoplasmic transport [105–108]. Different strains of the rhinovirus produce variants of the 2A proteases, which cleave Nup62 in distinct ways. Nup62 cleavage changes nucleocytoplasmic transport receptors depending on how the nucleoporin is cleaved, which partially explains some of the differences in disease phenotypes and host responses to the different rhinovirus strains [109]. The nuclear basket nucleoporin Nup153 is also degraded during picornavirus infections such as poliovirus and rhinovirus [105,106].
Viruses are also able to modulate the selectivity of the host NPC to directly favor viral proliferation. Herpes simplex virus (HSV-1) protein ICP27 directly binds Nup62 and inhibits nuclear import. At the same time ICP27 also plays a role in nuclear export of viral mRNA and protein. This allows ICP27 to simultaneously block host nuclear import receptors, which prevents an antiviral response and facilitates viral production [110]. A more in-depth review of the viral modulation of nucleocytoplasmic transport has been conducted recently [111].
It is interesting to consider if treatment with drugs that block nucleocytoplasmic transport could be used to treat a viral infection or if they could help elucidate the mechanisms used by viruses to hijack cellular transport machinery. There is some precedent for this idea. Chromosome region maintenance 1 (CRM1) nuclear export inhibitor Leptomycin B (LMB) blocks export of p53 which would be targeted for degradation in the cytoplasm by an HPV protein after HPV infection [112]. LMB also blocks mRNA export of an HIV protein critical to viral replication preventing HIV replication in monocytes [113]. Notably, Selinexor, an analog of the nuclear export inhibitor LMB, is in clinical trials for the treatment of many cancers. Because Selinexor has already proven safe enough for clinical trials, if nuclear export inhibitors are shown to be an effective treatment for viral infections, many of the barriers to approval are already reduced.
3.3. The role of nucleoporins in the immunological response
As we have seen the role of nucleoporins in virology and antiviral responses has been well studied, but the role of these proteins in immunological responses to not just viruses but also other infections is still preliminary. In a monocyte cell line the scaffold nucleoporin Nup85 (FROUNT) was found to bind CCR2 and CCR5: chemokine receptors responsible for signaling for cell motility. The binding is essential to CCR2/CCR5 signaling for pseudopodia formation and chemotaxis [114,115]. Surprisingly, in these studies Nup85 was found mainly not at the NPC but at the leading edge of the cells.
Demonstrating the first in vivo evidence of a nucleoporin modulating the immune response heterozygous null mice for the scaffold nucleoporin Nup96 were shown to have an impaired response to viral infections (Table 1). The mice have a specific reduction in Nup96 with normal levels of other nucleoporins and normal numbers of NPCs. Lower Nup96 protein levels specifically reduce export of mRNA for major histocompatibility complexes (MHC) I and II. Because MHCs are critical for antigen presentation to immune effector cells, the immune response to virus in these mice was dampened [30]. Like Nup96 heterozygous mice, mice with hypomorphic Sec13, a scaffold nucleoporin found in a subcomplex with Nup96 [116], have reduced MHC I and II expression and lower levels of cytokine production in stimulated T cells (Table 1) [20]. Both of these mouse models demonstrate the importance of nucleoporins in antigen presenting cells.
A direct role of nucleoporins in effector cells of the immune system has yet to be shown in vivo but, in a mouse T cell line, binding of the immune adaptor SLP-76 to NPC cytoplasmic filament-associated protein RanGap1 is required for nuclear entry of SLP-76 and other proteins critical for an effective immune response [70].
4. NPCs in the development and progression of cancers
The roles of nucleoporins in a broad spectrum of cancers have been reviewed extensively [117–120]. We will briefly examine the literature in the context of the overall effect on healthy aging and highlight some newer findings. The role of nucleoporins in cancer can be put into 2 broad categories: fusion proteins and altered gene expression.
4.1. Oncogenic nucleoporin fusion proteins
Over the years, several nuclear pore complex components have been found as part of chromosomal translocations that generate aberrant fusion proteins in cancers. Tpr, Nup98, Nup358, and Nup214 are termed ‘promiscuous’ because they fuse to different partners to produce a variety of oncogenic fusion proteins [117].
Tpr (translocated promoter region) was the first nucleoporin described as a fusion protein [121]. Although unknown at that time, Tpr is a nuclear basket nucleoporin, which forms filaments that extend inside the nucleus. In Drosophila, the Tpr homolog Megator and another nuclear basket nucleoporin, Nup153, bind to ~25% of the genome [122]. In different cancers Tpr is found fused to 4 different tyrosine kinase receptors Met, NTrk1, FGFR1, and ALK. All of these fusions have been shown to create or are predicted to create constitutive activators of growth pathways including the Ras/MAPK and PI3K pathways [118].
The cytoplasmic filament nucleoporin Nup214 is another nucleoporin found as part of oncogenic fusion proteins. In T cell acute lymphocytic leukemia (T-ALL) and acute myeloid leukemia (AML) Nup214 is commonly fused to Dek and Set, which are modifiers of chromatin structure [123]. Nup214 also forms an active tyrosine kinase fusion protein with Abl and functions as an adaptor protein with SQSTM1 [124,125]. Although they are less well characterized than the Nup98 fusions described later, some mechanisms have been demonstrated for Nup214 fusion proteins. Recently, the Set and Dek fusions to Nup214 have been shown to alter gene expression regulation by inhibiting nuclear export of mRNA and transcription factors by tethering the export factors Crm1 and Nxf1 to nucleoplasmic aggregates [126]. In various cancers another cytoplasmic filament nucleoporin, Nup358, is fused to Alk, Abl, and Fgfr. The mechanism of oncogenesis in these fusions is still largely unexplored [117].
The central channel nucleoporin Nup98 has been shown to play a key role in cancer as a fusion protein with many binding partners that promote transformation in leukemias [118]. The Nup98/HoxA9 fusion was simultaneously first reported by two groups [127,128] and has subsequently been shown to be sufficient to cause leukemic transformation in human cells and in vivo in mice [129]. HoxA9 is one of a family of transcription factors, called homeodomain proteins, which are known to control development and differentiation, and are commonly mutated in cancers. Nup98 has also been found fused to other homeodomain proteins as well as non-homeodomain proteins in leukemias having ~32 known fusion partners [117].
Much research has been done studying the different Nup98 fusion proteins and many mechanisms of oncogenesis have been proposed. The homeodomain fusions exogenously upregulate HoxA cluster genes, inhibit differentiation, and increase self-renewal [130]. Recently, Nup98 fusion proteins were found to alter nuclear envelope morphology, which further expands the possible mechanisms [131]. Additionally, Nup98 fusions to TopII beta and SETBP1 were shown to displace wild-type Nup98 and subsequently reduce Crm1-dependent nucleocytoplasmic export [132]. More recently, a variety of Nup98 fusion proteins were shown to interact with mixed lineage leukemia 1 (Mll1) and these interactions are necessary for binding to Hox gene promoter regions [133]. The therapeutic implications are that Mll1 will be a good drug target in Nup98 fusion-induced leukemia.
The Nup98-HoxA9 fusion protein is found to reside in the nucleoplasm of cells [134] and many of the fusion partners have known roles in the nucleus such as histone acetyltransferases and deacetylases. Recent evidence shows that expression of Nup98-HoxA9 fusion protein in mouse embryonic stem cells induces Hox cluster genes by binding to Crm1, which is bound at specific sites. The Crm1 inhibitor LMB disassembles the fusion protein clusters and reduces the expression of the Hox genes [135]. These data are particularly interesting as this could be a secondary mechanism of action for nuclear export inhibitors, which would increase the efficacy of the drug in Nup98-HoxA9 fusion positive cancers.
4.2. Changes in nucleoporin expression and nucleocytoplasmic transport
Expression of the cytoplasmic ring nucleoporin Nup88 is elevated in cancer cell lines and many tumors [136]. Nup88 is known to interact with mitotic spindles and its overexpression causes an increase in aneuploidy [137]. The overexpressed nucleoporin is found in aggregates in the cytoplasm and higher expression correlates with cancer progression and poor prognosis [138,139]. A recent study has mapped the interactome of Nup88 and demonstrated that the nucleoporin blocks Misp spindle localization that is critical for normal spindle formation and chromosome separation [140].
An analysis of proteome alterations during prostate cancer progression revealed that Nup62 is upregulated in metastatic and clinically localized prostate cancer when compared to benign prostate [141]. Nup62 was shown to directly regulate calcium signaling through calmodulin-dependent kinase kinase 2 (CaMKK2) in castrate resistant prostate cancer cells. Nup62 and CaMKK2 expression are increased in advanced prostate cancer and knockdown of Nup62, and not other nucleoporins (Nup98 or Nup88), reduced growth more strongly in an advanced prostate cancer cell line when compared to a non-malignant prostate cell line [142].
NDC1 is highly overexpressed in NSCLC and higher expression levels correlate with increased progression and poorer survival. Knockdown of NDC1 in NSCLC cell lines reduced cell proliferation and caused cell cycle alterations. In vivo knockdown of NDC1 in xenograft cancer models was able to reduce tumor growth [143].
Although the mechanisms of pathogenesis are not well characterized, most other nucleoporins and transport proteins have also been found dysregulated in various cancers including Nup210, Nup133, Nup107, Sec13, Nup188, Nup93, Nup153, Tpr, Nup358, Nup214, Nup98, hCG1, RanGap1, and Rae1 [117,144].
Interestingly, in a whole genome RNAi screen against colon cancers with expression profiles similar to Braf(V600E) mutants, Nup358 was the gene found to have the greatest specific lethality when knocked down. The Braf-like mutant colon cancer cells depended on Nup358 for efficient microtubule dynamics at kinetochores and the cells were more sensitive to the microtubule polymerizing poison vinorelbine. Treatment with vinorelbine was sufficient to block Braf-like mutant colon cancer xenograft tumor growth [145]. The critical role for Nup358 in mitotic spindle assembly was previously described in HeLa cells [146] but this study highlights how nucleoporins are capable of playing roles in cancer pathogenesis while not directly associated with the NPC and without being fused to other proteins. The example of Nup62 seen above and this case of Nup358 demonstrate how targeting nucleoporins, even ubiquitously expressed ones with broad cellular roles, can have differential lethality in cancer cells.
Thus far we have discussed the direct role of NPC components in cancer, but new research suggests that nucleocytoplasmic transport through the NPC may play a role in some forms of cancer. Not surprisingly, potent inhibitors of nuclear export like Leptomycin B prevent growth of cells including cancer cells. Recent work has shown that the selective inhibitor of nuclear export, Selinexor, is capable of specifically inhibiting the growth of human leukemia cells with little toxicity to the hematopoietic cells in a mouse model [147]. This form of treatment has proved effective and safe enough to enter into multiple late stage clinical trials [148]. Additionally, it was recently published that Selinexor is specifically lethal to Kras mutant non-small cell lung cancers when compared to Kras wild type cancer cells. In these cells nuclear export was necessary to prevent the cell growth factor Nfkb inactivation by Ikba accumulation in the nucleus [149].
4.3. Nucleoporin-based mouse cancer models
As we have seen, many nucleoporins play a significant role in different cancers. With a variety of nucleoporin haploinsufficient, knockout, and overexpression mice available, it is interesting to examine if these mice are prone to cancer. In most cases, nucleoporin knockout mice are embryonic lethal and tumorigenesis has not been reported in many of the non-lethal knockout mice but overexpression and heterozygous null mice have been studied (Table 1).
In mice Nup88 overexpression was shown to cause spontaneous intestinal tumors and although global nucleocytoplasmic transport was unaffected, aneuploidy and chromosome instability were induced. The mechanism demonstrated was that high levels of Nup88 sequester Nup98-Rae1 away from APC/CCDH1 where they normally function to ensure correct centrosome segregation [31]. This is further proof that the role of Nup88 in cancer is mediated by its transport-independent mitotic functions.
Nup98 and Rae1 double heterozygous mice and Rae1 heterozygous mice have increased aneuploidy. The mice have a normal rate of spontaneous tumorigenesis but have a higher incidence of tumors when treated with the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) [28].
Mice with Nup358 haploinsufficiency or hypomorphism develop severe aneuploidy from a failure to properly segregate chromosomes during mitosis. The aneuploidy manifests in an increased incidence of spontaneous tumors and DMBA-induced tumors [22]. Interestingly, in humans Nup358 levels are reduced in non-small cell lung carcinoma when compared to healthy controls [150], although a prospective study would better explain if low Nup358 levels increase the risk of this type of cancer.
5. NPCs in other disorders
5.1. Primary biliary cholangitis (PBC) and other autoimmune disorders
PBC is an autoimmune T lymphocyte mediated chronic non-suppurative destructive cholangitis (CNSDC) disease. Nup210 and Nup62 autoantibodies were found to be associated with more severe primary biliary cirrhosis and poorer prognosis [151]. Nup210 and Nup62 autoantibodies, along with automitochondrial antibodies, are so infrequent outside of the disease context that they are considered to be a biomarker for PBC [152]. Additionally, Tpr and some nuclear envelope autoantibodies have been found in PBC as well as other autoimmune disorders such as arthritis, systemic lupus erythematous (SLE) and autoimmune liver disease (ALD) [153,154].
Interestingly, Nup210 is not expressed in normal liver biliary epithelial cells, lowly expressed in hepatitis diseased liver cells, and highly expressed in PBC liver cells [155] suggesting that the abnormal expression of Nup210 in these cells may be a consequence of or play a role in the development of the diseases. Nup358 was found to play a critical role in maintaining bile acid homeostasis in biliary epithelial cells and protein levels were increased shortly after exposure to a primary biliary acid [156].
The role of nuclear envelope proteins including the nucleoporins Nup210, Nup62, and Tpr in autoimmune disorders has yet to be determined. It has been suggested that the autoantibodies seen in PBC are a consequence of molecular mimicry of bacterial antigens, which are similar to the mammalian antigens. The best evidence for this is that in a mouse model of CNSDC, mice inoculated with Streptococcus intermedius develop Nup210 autoantibodies [157] but, it would be informative to determine if the autoantibodies are a result of the molecular mimicry or the host antigens. Another group created a mouse model of autoimmunity in the bile ducts by inoculating mice with Novosphingobium aromaticivorans, and although they did not test for Nup210 autoantibodies they did demonstrate that transferring the NK T lymphocytes from mice where the disease had already been observed, was sufficient to establish the disease in recipient mice [158]. This suggests that a bacterial infection may be required to trigger the disease but PBC is mediated by the host immune system after disease onset.
5.2. Steroid-resistant nephrotic syndrome (SRNS)
Dysfunction of the glomerular podocytes in the kidney causes SRNS, which often leads to chronic kidney disease. Recently homozygous and compound heterozygous mutations in Nup93, Nup205, and Xpo5 were identified as monogenic causes of SRNS. Nup205 or Nup93 mutations disrupted NPC assembly and their knockdown reduced the presence of the other nucleoporins at the NPC [159]. Nup107 homozygous mutations have been found that cause early childhood onset SRNS [160]. All of the nucleoporins that have been shown to be involved in SNRS are known to be members of the critical NPC scaffold. Much research remains to be done but it is possible that these mutations disrupt the formation or transport function of the NPC.
5.3. Cardiovascular
There is building evidence that nucleoporins play a role in the cardiovascular system. An analysis of copy number variants in humans with heterotaxy, a rare but serious birth defect that affects heart development, found a duplication event in the Nup188 gene. Morpholino knockdown of the gene in Xenopus caused a defect in left-right patterning [161].
Patients who have had heart failure (ischemic and dilated hearts) have increased levels of specific nucleoporins. Specifically the transmembrane nucleoporin NDC1, the scaffold nucleoporin Nup160, and the nuclear basket nucleoporin Nup153 are increased in hearts of patients with ischemic and dilated cardiomyopathy, while Nup93 was increased only in dilated heart patients. NDC1 was also shown to be mislocalized in cardiomyocytes from both ischemic and dilated hearts [162]. Supporting these data, a point mutation in the scaffold nucleoporin Nup155 was sufficient to delocalize the nucleoporin from the NPC, which led to early sudden death due to atrial fibrillation. Additionally, Nup155 hypomorphic mice have atrial fibrillation and die of early sudden cardiac death (Table 1) [25].
The scaffold nucleoporin Nup35 has been shown to regulate intracellular cardiomyocyte pH by controlling the mRNA export of the Na+–H+ exchanger- 1 (NHE1) transcript [163]. NHE1 protein is responsible for maintaining intracellular pH [164]. In response to an acid challenge cardiomyocytes deficient in Nup35 failed to maintain intracellular pH homeostasis and overexpression of Nup35 blocked hypoxia-induced intracellular acidification. Both Nup35 and NHE1 are downregulated in ischemic cardiomyocytes [163].
After cardiomyocyte hypertrophic stimulus nuclear import is downregulated to increase nuclear export by changing the conformation of the cytoplasmic face of the NPC [165]. During cardiomyocyte differentiation there is a conformational change in the NPC that results in increased NPC density, larger pore size, greater likelihood of cargo occupancy, and Mef2c translocation from the cytoplasm to the nucleus [166,167]. Cardiomyocytes derived from a knockout mouse for the calcium sequestering protein, calreticulin (which is necessary for proper cardiac development) had altered NPC composition. NPCs from the knockout mouse were found to have smaller pores and Nup62, Nup214, and Nup153 were reduced even though Nup98 levels were unaffected and the density of NPCs remained the same [168]. Supporting evidence in plants for the change in conformation state of the NPC shows that the selectivity of pores can be altered in response to cellular stresses [169]. Although these studies are preliminary they suggest a new paradigm for examining the role of NPCs in development and diseases: not just composition of NPCs but conformational states can alter the fate and function of cells.
5.4. Beta Thalassemia
The insights gained from the study of Nup98 fusion proteins have led to an unexpected possible treatment for the autosomal recessive hematological malignancy Beta Thalassemia, which causes anemia from reduced hemoglobin synthesis. A Nup98-HoxA10HD fusion protein was engineered and when transfected in hematopoietic stem cells, was able to increase the proliferative ability and maintain the undifferentiated state of the stem cells in vitro [170]. The Nup98 fusion protein was able to increase the number of human hematopoietic stem cells 4 months after initial transfer into immunodeficient mouse hosts [171]. This strategy has been shown to be effective in the treatment of a murine model of Beta Thalassemia [172].
5.5. Aging
Aging plays a role in almost all of the diseases discussed above but the normal aging process is also involved in NPC biology. It was identified that in postmitotic tissues NPC assembly does not occur or takes place at a very low rate. In postmitotic C. elegans cells, expression of the scaffold nucleoporins, which are essential for NPC assembly, is unnecessary after development as NPC scaffolds do not significantly turn over for the remainder of their lives (~40–50 days). This low, or lack of turnover, of NPCs in postmitotic tissues has also been described in mammals [173]. Pulse chase experiments in cells and rats showed that scaffold NPC components turn over very slowly or not at all in postmitotic cells while non-scaffold nucleoporins are short lived [90,91]. Subsequent experiments have shown that scaffold nucleoporins do turn over but very slowly [92]. Due to their extremely long life, in aging neurons NPCs accumulate damage to the point where they can no longer perform their functions [90]. This results in the loss of nuclear compartmentalization and the abnormal distribution of nuclear and cytoplasmic proteins in old neurons, which can have devastating effects for cellular homeostasis. Similarly, recent evidence indicates that as rats age their myocytes also have compromised nuclear compartmentalization and lower levels of the structural nucleoporin Nup93 which is critical for nuclear permeability [174,175].
Nucleocytoplasmic transport is also perturbed in aging by alterations in the levels of non-nucleoporin transport proteins. In a study comparing transcriptional profiles of iPSC-derived neurons and directly reprogrammed induced neurons (iNs) from old and young humans, the nuclear import receptor RanBP17 was identified as a cause of the age-associated loss in nuclear compartmentalization. The protein is reduced in aged neurons and is necessary for maintaining nuclear compartmentalization [176].
The accumulation of protein aggregates, which have pathogenic effects in diseases like AD, have also been shown to be an inherent part of the normal aging process in C. elegans [177]. As discussed above, nucleoporins play a direct role in the prevention of protein aggregates and some of their negative consequences. Demonstrating that protein aggregation is inherently part of the aging in mammals would significantly expand the possible roles of nucleoporins in the aging and aging-related diseases.
The role of nucleoporins in the aging of higher eukaryotes has yet to be directly determined because many of the nucleoporin knockout mice are unable to complete embryogenesis (Table 1) but aging and age-related diseases could be studied with the use of genetic mouse tools. Overexpression, hypomorphic, inducible knockout and inducible shRNA expression mouse lines would allow the induction, reduction or depletion of specific components of the NPC, at different times of development/disease progression, and in specific cells of the mouse. This would greatly enhance our ability to study the functions of nucleoporins in specific tissues and how those functions play a role in aging and aging-related diseases.
6. Conclusions
Many new advances in the study of nucleocytoplasmic transport, the NPC, and nucleoporins suggest that the ability of organisms to regulate these processes and structures must be tightly controlled to prevent diseases from arising or progressing. Many of the diseases discussed here are directly associated with aging such as AD and HD but most of the remaining diseases such as cancer and cardiovascular disease become worse indirectly with age. Regardless, the aging of humans increases the occurrence and severity of diseases that nucleoporins have been shown to play a direct role in (Figure 1). These diseases, and therefore nucleoporins, play at least an indirect role on human lifespan.
The original identification of a gene duplication of the Pom121 nucleoporin creating a protein called Pom121C opened up new possibilities for nucleoporins. Knocking down either Pom121 protein caused NPC clustering in HeLa cells [178]. More recently it was shown that the Pom121C gene codes for a soluble form of the protein that utilizes the Pom121 NPC interacting domains to bring a subset of nucleoporins into Nup98 nucleoplasmic clusters. Although the role of this particular gene duplication event is not known, it was found that it arose multiple times in evolutionary history suggesting that there is a convergent advantage [179]. As seen above, this process parallels the one used by cancer cells to tether or bring oncogenic transcription factors into the nucleus through nucleoporin fusion proteins. Utilizing nucleoporins to direct subsets of proteins (including other nucleoporins) to specific sites in the genome could expand the mechanisms by which these proteins are capable of altering cell function and fate as well as diseases and aging.
Acknowledgments
Due to lack of space the authors apologize for our inability to cite relevant publications. We thank Dr. Joana Borlido, Dr. Marcela Raices, and Leanora Hernandez for their helpful discussions and comments on the manuscript. This publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR065083. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M.A.D. is a Pew Scholar in the Biomedical Sciences.
Abbreviations
- NPC
Nuclear Pore Complex
- triple A syndrome
Achalasia-Addisonianism-Alacrima or Allgrove syndrome
- FTD
Frontotemporal dementia
- ALS
amyotrophic lateral sclerosis
- PD
Parkinson’s disease
- HRE
hexanucleotide repeat expansion
- RAN
repeat-associated non-AUG
- GA
translation, Poly glycine-alanine
- GR
glycine-arginine
- GP
glycine-proline
- PR
proline-arginine
- PA
proline-alanine
- polyQ
polyglutamine
- O-GlcNAc
O-linked beta N-acetylglucosamine
- NFTs
neurofibrillary tangles
- IBSN
Infantile bilateral striatal necrosis
- LCCS1
lethal congenital contracture syndrome-1
- ANE
acute necrotizing encephalopathy
- VSV
vesicular stomatitis virus
- HSV
herpes simplex virus
- CRM1
Chromosome region maintenance 1
- LMB
Leptomycin B
- MLL
mixed lineage leukemia
- CaMKK2
calmodulin-dependent kinase kinase 2
- DMBA
7,12-dimethylbenz[a]anthracene
- PBC
Primary biliary cholangitis
- CNSDC
chronic non-suppurative destructive cholangitis
- SLE
systemic lupus erythematous
- ALD
autoimmune liver disease
- SRNS
Steroid-resistant nephrotic syndrome
- NHE1
Na+–H+ exchanger- 1
- iNs
induced neurons
- iPSCs
induced pluripotent stem cells
- T-ALL
T cell acute lymphocytic leukemia
- AML
acute myeloid leukemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callan HG, Randall JT, Tomlin SG. An electron microscope study of the nuclear membrane. Nature. 1949;163:280. doi: 10.1038/163280a0. [DOI] [PubMed] [Google Scholar]
- 2.Callan HG, Tomlin SG. Experimental studies on amphibian oocyte nuclei. I. Investigation of the structure of the nuclear membrane by means of the electron microscope. Proc. R. Soc. Lond., B, Biol. Sci. 1950;137:367–378. doi: 10.1098/rspb.1950.0047. [DOI] [PubMed] [Google Scholar]
- 3.Field MC, Koreny L, Rout MP. Enriching the pore: splendid complexity from humble origins. Traffic. 2014;15:141–156. doi: 10.1111/tra.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 5.Kosinski J, Mosalaganti S, von Appen A, Teimer R, DiGuilio AL, Wan W, et al. Molecular architecture of the inner ring scaffold of the human nuclear pore complex. Science. 2016;352:363–365. doi: 10.1126/science.aaf0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franke WW. On the universality of nuclear pore complex structure. Z Zellforsch Mikrosk Anat. 1970;105:405–429. doi: 10.1007/BF00335464. [DOI] [PubMed] [Google Scholar]
- 7.Gall JG. Octagonal nuclear pores. J. Cell Biol. 1967;32:391–399. doi: 10.1083/jcb.32.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeGrasse JA, DuBois KN, Devos D, Siegel TN, Sali A, Field MC, et al. Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Mol. Cell Proteomics. 2009;8:2119–2130. doi: 10.1074/mcp.M900038-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raices M, D'Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat. Rev. Mol. Cell Biol. 2012;13:687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- 10.Olsson M, Ekblom M, Fecker L, Kurkinen M, Ekblom P. cDNA cloning and embryonic expression of mouse nuclear pore membrane glycoprotein 210 mRNA. Kidney Int. 1999;56:827–838. doi: 10.1046/j.1523-1755.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 11.Olsson M, Schéele S, Ekblom P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp. Cell Res. 2004;292:359–370. doi: 10.1016/j.yexcr.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.D'Angelo MA, Gomez-Cavazos JS, Mei A, Lackner DH, Hetzer MW. A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell. 2012;22:446–458. doi: 10.1016/j.devcel.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupu F, Alves A, Anderson K, Doye V, Lacy E. Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev. Cell. 2008;14:831–842. doi: 10.1016/j.devcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asally M, Yasuda Y, Oka M, Otsuka S, Yoshimura SH, Takeyasu K, et al. Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis. Febs J. 2011;278:610–621. doi: 10.1111/j.1742-4658.2010.07982.x. [DOI] [PubMed] [Google Scholar]
- 15.Cerveny KL, Cavodeassi F, Turner KJ, de Jong-Curtain TA, Heath JK, Wilson SW. The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development. 2010;137:2107–2115. doi: 10.1242/dev.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan T, Kehlenbach RH, Schirmer EC, Kehlenbach A, Fan F, Clurman BE, et al. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol. Cell. Biol. 2000;20:5619–5630. doi: 10.1128/mcb.20.15.5619-5630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2:a000562–a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raices M, D'Angelo MA. Nuclear pore complexes and regulation of gene expression. Current Opinion in Cell Biology. 2017;46:26–32. doi: 10.1016/j.ceb.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge L. Some highlights of research on aging with invertebrates, 2010. Aging Cell. 2011;10:5–9. doi: 10.1111/j.1474-9726.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira TG, Zhang L, Shaulov L, Harel A, Kuss SK, Williams J, et al. Sec13 Regulates Expression of Specific Immune Factors Involved in Inflammation In Vivo. Sci Rep. 2015;5:17655. doi: 10.1038/srep17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu JR, Jeganathan KB, Baker DJ, Wu X, Kang-Decker N, van Deursen JM. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J. Cell Biol. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133:103–115. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aslanukov A, Bhowmick R, Guruju M, Oswald J, Raz D, Bush RA, et al. RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficits in glucose metabolism. PLoS Genet. 2006;2:e177. doi: 10.1371/journal.pgen.0020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Deursen J, Boer J, Kasper L, Grosveld G. G2 arrest and impaired nucleocytoplasmic transport in mouse embryos lacking the proto-oncogene CAN/Nup214. The EMBO Journal. 1996;15:5574–5583. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y-W, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106:287–295. doi: 10.1182/blood-2004-12-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki M, Kuwata T, Yamazaki Y, Jenkins NA, Copeland NG, Osato M, et al. Identification of cooperative genes for NUP98-HOXA9 in myeloid leukemogenesis using a mouse model. Blood. 2005;105:784–793. doi: 10.1182/blood-2004-04-1508. [DOI] [PubMed] [Google Scholar]
- 28.Jeganathan KB, Baker DJ, van Deursen JM. Securin associates with APCCdh1 in prometaphase but its destruction is delayed by Rae1 and Nup98 until the metaphase/anaphase transition. Cell Cycle. 2006;5:366–370. doi: 10.4161/cc.5.4.2483. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Kasper LH, Mantcheva RT, Mantchev GT, Springett MJ, van Deursen JM. Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc Natl Acad Sci USA. 2001;98:3191–3196. doi: 10.1073/pnas.051631598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faria AMC, Levay A, Wang Y, Kamphorst AO, Rosa MLP, Nussenzveig DR, et al. The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity. 2006;24:295–304. doi: 10.1016/j.immuni.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Naylor RM, Jeganathan KB, Cao X, van Deursen JM. Nuclear pore protein NUP88 activates anaphase-promoting complex to promote aneuploidy. J. Clin. Invest. 2016;126:543–559. doi: 10.1172/JCI82277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol. Cell. Biol. 2000;20:5631–5642. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parish IA, Stamp LA, Lorenzo AMD, Fowler SM, Sontani Y, Miosge LA, et al. A Novel Mutation in Nucleoporin 35 Causes Murine Degenerative Colonic Smooth Muscle Myopathy. Am. J. Pathol. 2016;186:2254–2261. doi: 10.1016/j.ajpath.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama K, Noguchi J, Hirose M, Kajita S, Katayama K, Khalaj M, et al. A mutation in the nuclear pore complex gene Tmem48 causes gametogenesis defects in skeletal fusions with sterility (sks) mice. J. Biol. Chem. 2013;288:31830–31841. doi: 10.1074/jbc.M113.492306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okita K, Kiyonari H, Nobuhisa I, Kimura N, Aizawa S, Taga T. Targeted disruption of the mouse ELYS gene results in embryonic death at peri-implantation development. Genes Cells. 2004;9:1083–1091. doi: 10.1111/j.1365-2443.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 36.Huebner A, Mann P, Rohde E, Kaindl AM, Witt M, Verkade P, et al. Mice lacking the nuclear pore complex protein ALADIN show female infertility but fail to develop a phenotype resembling human triple A syndrome. Mol. Cell. Biol. 2006;26:1879–1887. doi: 10.1128/MCB.26.5.1879-1887.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao N, Davuluri G, Gong W, Seiler C, Lorent K, Furth EE, et al. The nuclear pore complex protein Elys is required for genome stability in mouse intestinal epithelial progenitor cells. Gastroenterology. 2011;140:1547–55. e10. doi: 10.1053/j.gastro.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cronshaw JM, Matunis MJ. The nuclear pore complex: disease associations and functional correlations. Trends Endocrinol. Metab. 2004;15:34–39. doi: 10.1016/j.tem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Tullio-Pelet A, Salomon R, Hadj-Rabia S, Mugnier C, de Laet MH, Chaouachi B, et al. Mutant WD-repeat protein in triple-A syndrome. Nat. Genet. 2000;26:332–335. doi: 10.1038/81642. [DOI] [PubMed] [Google Scholar]
- 40.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cronshaw JM, Matunis MJ. The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci USA. 2003;100:5823–5827. doi: 10.1073/pnas.1031047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kind B, Koehler K, Lorenz M, Huebner A. The nuclear pore complex protein ALADIN is anchored via NDC1 but not via POM121 and GP210 in the nuclear envelope. Biochem. Biophys. Res. Commun. 2009;390:205–210. doi: 10.1016/j.bbrc.2009.09.080. [DOI] [PubMed] [Google Scholar]
- 43.Yamazumi Y, Kamiya A, Nishida A, Nishihara A, Iemura S-I, Natsume T, et al. The transmembrane nucleoporin NDC1 is required for targeting of ALADIN to nuclear pore complexes. Biochem. Biophys. Res. Commun. 2009;389:100–104. doi: 10.1016/j.bbrc.2009.08.096. [DOI] [PubMed] [Google Scholar]
- 44.Hirano M, Furiya Y, Asai H, Yasui A, Ueno S. ALADINI482S causes selective failure of nuclear protein import and hypersensitivity to oxidative stress in triple A syndrome. Proc Natl Acad Sci USA. 2006;103:2298–2303. doi: 10.1073/pnas.0505598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storr HL, Kind B, Parfitt DA, Chapple JP, Lorenz M, Koehler K, et al. Deficiency of ferritin heavy-chain nuclear import in triple a syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol. Endocrinol. 2009;23:2086–2094. doi: 10.1210/me.2009-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, et al. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J. Neurosci. Res. 2003;71:46–63. doi: 10.1002/jnr.10463. [DOI] [PubMed] [Google Scholar]
- 47.Jühlen R, Landgraf D, Huebner A, Koehler K. Identification of a novel putative interaction partner of the nucleoporin ALADIN. Biol Open. 2016;5:1697–1705. doi: 10.1242/bio.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jühlen R, Idkowiak J, Taylor AE, Kind B, Arlt W, Huebner A, et al. Role of ALADIN in human adrenocortical cells for oxidative stress response and steroidogenesis. PLoS ONE. 2015;10:e0124582. doi: 10.1371/journal.pone.0124582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prasad R, Metherell LA, Clark AJ, Storr HL. Deficiency of ALADIN impairs redox homeostasis in human adrenal cells and inhibits steroidogenesis. Endocrinology. 2013;154:3209–3218. doi: 10.1210/en.2013-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho K-I, Yi H, Yeh A, Tserentsoodol N, Cuadrado L, Searle K, et al. Haploinsufficiency of RanBP2 is neuroprotective against light-elicited and age-dependent degeneration of photoreceptor neurons. Cell Death Differ. 2009;16:287–297. doi: 10.1038/cdd.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho K-I, Yoon D, Qiu S, Danziger Z, Grill WM, Wetsel WC, et al. Loss of Ranbp2 in motor neurons causes the disruption of nucleocytoplasmic and chemokine signaling and proteostasis of hnRNPH3 and Mmp28, and the development of amyotrophic lateral sclerosis (ALS)-like syndromes. Dis. Model. Mech. 2017 doi: 10.1242/dmm.027730. dmm.027730–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho K-I, Searle K, Webb M, Yi H, Ferreira PA. Ranbp2 haploinsufficiency mediates distinct cellular and biochemical phenotypes in brain and retinal dopaminergic and glia cells elicited by the Parkinsonian neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Cell. Mol. Life Sci. 2012;69:3511–3527. doi: 10.1007/s00018-012-1071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin W-L, DeJesus-Hernandez M, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gendron TF, Bieniek KF, Zhang Y-J, Jansen-West K, Ash PEA, Caulfield T, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee K-H, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–133. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. U.S.a. 2013;110:E4968–77. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y-J, Gendron TF, Grima JC, Sasaguri H, Jansen-West K, Xu Y-F, et al. C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nat. Neurosci. 2016;19:668–677. doi: 10.1038/nn.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovičić A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat. Neurosci. 2015;18:1226–1229. doi: 10.1038/nn.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jovičić A, Paul JW, Gitler AD. Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia. J. Neurochem. 2016;138(Suppl 1):134–144. doi: 10.1111/jnc.13642. [DOI] [PubMed] [Google Scholar]
- 63.Boeynaems S, Bogaert E, Van Damme P, Van Den Bosch L. Inside out: the role of nucleocytoplasmic transport in ALS and FTLD. Acta Neuropathol. 2016;132:159–173. doi: 10.1007/s00401-016-1586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 65.Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 66.Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, et al. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol. Biol. Cell. 2001;12:1393–1407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woerner AC, Frottin F, Hornburg D, Feng LR, Meissner F, Patra M, et al. Cytoplasmic protein aggregates interfere with nucleocytoplasmic transport of protein and RNA. Science. 2016;351:173–176. doi: 10.1126/science.aad2033. [DOI] [PubMed] [Google Scholar]
- 68.Zhao T, Hong Y, Li X-J, Li S-H. Subcellular Clearance and Accumulation of Huntington Disease Protein: A Mini-Review. Front Mol Neurosci. 2016;9:27. doi: 10.3389/fnmol.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gasset-Rosa F, Chillon-Marinas C, Goginashvili A, Atwal RS, Artates JW, Tabet R, et al. Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport. Neuron. 2017;94:48–57. e4. doi: 10.1016/j.neuron.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu K-Y, Shyu Y-C, Barbaro BA, Lin Y-T, Chern Y, Thompson LM, et al. Disruption of the nuclear membrane by perinuclear inclusions of mutant huntingtin causes cell-cycle re-entry and striatal cell death in mouse and cell models of Huntington's disease. Hum. Mol. Genet. 2015;24:1602–1616. doi: 10.1093/hmg/ddu574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, et al. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017;94:93–107. e6. doi: 10.1016/j.neuron.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li B, Kohler JJ. Glycosylation of the nuclear pore. Traffic. 2014;15:347–361. doi: 10.1111/tra.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu Y, Liu T-W, Madden Z, Yuzwa SA, Murray K, Cecioni S, et al. Post-translational O-GlcNAcylation is essential for nuclear pore integrity and maintenance of the pore selectivity filter. J Mol Cell Biol. 2016;8:2–16. doi: 10.1093/jmcb/mjv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuzwa SA, Shan X, Jones BA, Zhao G, Woodward ML, Li X, et al. Pharmacological inhibition of OGlcNAcase (OGA) prevents cognitive decline and amyloid plaque formation in bigenic tau/APP mutant mice. Mol Neurodegener. 2014;9:42. doi: 10.1186/1750-1326-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suhr ST, Senut MC, Whitelegge JP, Faull KF, Cuizon DB, Gage FH. Identities of sequestered proteins in aggregates from cells with induced polyglutamine expression. J. Cell Biol. 2001;153:283–294. doi: 10.1083/jcb.153.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H, Ueda M, Miyamoto Y, Yoneda Y, Perry G. Aberrant localization of importin 1 in hippocampal neurons in Alzheimer disease. Brain Research. 2006 doi: 10.1016/j.brainres.2006.09.084. [DOI] [PubMed] [Google Scholar]
- 78.Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS. Nuclear pore complex proteins in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:45–54. doi: 10.1097/01.jnen.0000195939.40410.08. [DOI] [PubMed] [Google Scholar]
- 79.Yerbury JJ, Ooi L, Dillin A, Saunders DN, Hatters DM, Beart PM, et al. Walking the tightrope: proteostasis and neurodegenerative disease. J. Neurochem. 2016;137:489–505. doi: 10.1111/jnc.13575. [DOI] [PubMed] [Google Scholar]
- 80.Li C, Goryaynov A, Yang W. The selective permeability barrier in the nuclear pore complex. Nucleus. 2016;7:430–446. doi: 10.1080/19491034.2016.1238997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacinto FV, Benner C, Hetzer MW. The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 2015;29:1224–1238. doi: 10.1101/gad.260919.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann. Neurol. 2006;60:214–222. doi: 10.1002/ana.20902. [DOI] [PubMed] [Google Scholar]
- 83.Nousiainen HO, Kestilä M, Pakkasjärvi N, Honkala H, Kuure S, Tallila J, et al. Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. Nat. Genet. 2008;40:155–157. doi: 10.1038/ng.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jao L-E, Appel B, Wente SR. A zebrafish model of lethal congenital contracture syndrome 1 reveals Gle1 function in spinal neural precursor survival and motor axon arborization. Development. 2012;139:1316–1326. doi: 10.1242/dev.074344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Folkmann AW, Collier SE, Zhan X, Aditi, Ohi MD, Wente SR. Gle1 functions during mRNA export in an oligomeric complex that is altered in human disease. Cell. 2013;155:582–593. doi: 10.1016/j.cell.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sell K, Storch K, Hahn G, Lee-Kirsch MA, Ramantani G, Jackson S, et al. Variable clinical course in acute necrotizing encephalopathy and identification of a novel RANBP2 mutation. Brain Dev. 2016;38:777–780. doi: 10.1016/j.braindev.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Neilson DE, Adams MD, Orr CMD, Schelling DK, Eiben RM, Kerr DS, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am. J. Hum. Genet. 2009;84:44–51. doi: 10.1016/j.ajhg.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Topple A, Smith G, Fifkova E, Cullen-Dockstader K. Nuclear pore complex frequency in CA1 pyramidal cells of the aging rat. Mechanisms of Ageing and Development. 1990;51:33–39. doi: 10.1016/0047-6374(90)90159-d. [DOI] [PubMed] [Google Scholar]
- 89.Fifková E, Tonks M, Cullen-Dockstader K. Changes in the nuclear pore complexes of the dentate granule cells in aged rats. Exp. Neurol. 1987;95:755–762. doi: 10.1016/0014-4886(87)90314-1. [DOI] [PubMed] [Google Scholar]
- 90.D'Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savas JN, Toyama BH, Xu T, Yates JR, Hetzer MW. Extremely Long-Lived Nuclear Pore Proteins in the Rat Brain. Science. 2012;335:942–942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, III, et al. Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greber UF, Fassati A. Nuclear import of viral DNA genomes. Traffic. 2003;4:136–143. doi: 10.1034/j.1600-0854.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 94.Rabe B, Vlachou A, Panté N, Helenius A, Kann M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc. Natl. Acad. Sci. U.S.a. 2003;100:9849–9854. doi: 10.1073/pnas.1730940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol. 2001;3:1092–1100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- 96.Walther TC, Fornerod M, Pickersgill H, Goldberg M, Allen TD, Mattaj IW. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. The EMBO Journal. 2001;20:5703–5714. doi: 10.1093/emboj/20.20.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen N-Y, Zhou L, Gane PJ, Opp S, Ball NJ, Nicastro G, et al. HIV-1 capsid is involved in post-nuclear entry steps. Retrovirology. 2016;13:28. doi: 10.1186/s12977-016-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lussignol M, Kopp M, Molloy K, Vizcay-Barrena G, Fleck RA, Dorner M, et al. Proteomics of HCV virions reveals an essential role for the nucleoporin Nup98 in virus morphogenesis. Proc. Natl. Acad. Sci. U.S.a. 2016;113:2484–2489. doi: 10.1073/pnas.1518934113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strahm Y, Fahrenkrog B, Zenklusen D, Rychner E, Kantor J, Rosbach M, et al. The RNA export factor Gle1p is located on the cytoplasmic fibrils of the NPC and physically interacts with the FG-nucleoporin Rip1p, the DEAD-box protein Rat8p/Dbp5p and a new protein Ymr 255p. The EMBO Journal. 1999;18:5761–5777. doi: 10.1093/emboj/18.20.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le Rouzic E, Mousnier A, Rustum C, Stutz F, Hallberg E, Dargemont C, et al. Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1. J. Biol. Chem. 2002;277:45091–45098. doi: 10.1074/jbc.M207439200. [DOI] [PubMed] [Google Scholar]
- 101.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Enninga J, Levy DE, Blobel G, Fontoura BMA. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- 103.Faria PA, Chakraborty P, Levay A, Barber GN, Ezelle HJ, Enninga J, et al. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol. Cell. 2005;17:93–102. doi: 10.1016/j.molcel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 104.Park N, Katikaneni P, Skern T, Gustin KE. Differential targeting of nuclear pore complex proteins in poliovirus-infected cells. J. Virol. 2008;82:1647–1655. doi: 10.1128/JVI.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gustin KE, Sarnow P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. The EMBO Journal. 2001;20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gustin KE, Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Percipalle P, Clarkson WD, Kent HM, Rhodes D, Stewart M. Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import. J. Mol. Biol. 1997;266:722–732. doi: 10.1006/jmbi.1996.0801. [DOI] [PubMed] [Google Scholar]
- 108.Park N, Skern T, Gustin KE. Specific cleavage of the nuclear pore complex protein Nup62 by a viral protease. J. Biol. Chem. 2010;285:28796–28805. doi: 10.1074/jbc.M110.143404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watters K, Inankur B, Gardiner JC, Warrick J, Sherer NM, Yin J, et al. Differential Disruption of Nucleocytoplasmic Trafficking Pathways by Rhinovirus 2A Proteases. J. Virol. 2017;91:e02472–16. doi: 10.1128/JVI.02472-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malik P, Tabarraei A, Kehlenbach RH, Korfali N, Iwasawa R, Graham SV, et al. Herpes simplex virus ICP27 protein directly interacts with the nuclear pore complex through Nup62, inhibiting host nucleocytoplasmic transport pathways. J. Biol. Chem. 2012;287:12277–12292. doi: 10.1074/jbc.M111.331777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuss SK, Mata MA, Zhang L, Fontoura BMA. Nuclear imprisonment: viral strategies to arrest host mRNA nuclear export. Viruses. 2013;5:1824–1849. doi: 10.3390/v5071824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolff B, Sanglier JJ, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleocytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 114.Terashima Y, Onai N, Murai M, Enomoto M, Poonpiriya V, Hamada T, et al. Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat. Immunol. 2005;6:827–835. doi: 10.1038/ni1222. [DOI] [PubMed] [Google Scholar]
- 115.Toda E, Terashima Y, Sato T, Hirose K, Kanegasaki S, Matsushima K. FROUNT is a common regulator of CCR2 and CCR5 signaling to control directional migration. J. Immunol. 2009;183:6387–6394. doi: 10.4049/jimmunol.0803469. [DOI] [PubMed] [Google Scholar]
- 116.Fontoura BM, Blobel G, Matunis MJ. A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J. Cell Biol. 1999;144:1097–1112. doi: 10.1083/jcb.144.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nofrini V, Di Giacomo D, Mecucci C. Nucleoporin genes in human diseases. Eur. J. Hum. Genet. 2016;24:1388–1395. doi: 10.1038/ejhg.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simon DN, Rout MP. Cancer and the nuclear pore complex. Adv. Exp. Med. Biol. 2014;773:285–307. doi: 10.1007/978-1-4899-8032-8_13. [DOI] [PubMed] [Google Scholar]
- 119.Takeda A, Yaseen NR. Nucleoporins and nucleocytoplasmic transport in hematologic malignancies. Semin. Cancer Biol. 2014;27:3–10. doi: 10.1016/j.semcancer.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 120.Xu S, Powers MA. Nuclear pore proteins and cancer. Semin. Cell Dev. Biol. 2009;20:620–630. doi: 10.1016/j.semcdb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 122.Vaquerizas JM, Suyama R, Kind J, Miura K, Luscombe NM, Akhtar A. Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 2010;6:e1000846. doi: 10.1371/journal.pgen.1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou M-H, Yang Q-M. NUP214 fusion genes in acute leukemia (Review) Oncol Lett. 2014;8:959–962. doi: 10.3892/ol.2014.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Port SA, Mendes A, Valkova C, Spillner C, Fahrenkrog B, Kaether C, et al. The Oncogenic Fusion Proteins SET-Nup214 and Sequestosome-1 (SQSTM1)-Nup214 Form Dynamic Nuclear Bodies and Differentially Affect Nuclear Protein and Poly(A)+ RNA Export. J. Biol. Chem. 2016;291:23068–23083. doi: 10.1074/jbc.M116.735340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Keersmaecker K, Rocnik JL, Bernad R, Lee BH, Leeman D, Gielen O, et al. Kinase activation and transformation by NUP214-ABL1 is dependent on the context of the nuclear pore. Mol. Cell. 2008;31:134–142. doi: 10.1016/j.molcel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Saito S, Cigdem S, Okuwaki M, Nagata K. Leukemia-Associated Nup214 Fusion Proteins Disturb the XPO1-Mediated Nuclear-Cytoplasmic Transport Pathway and Thereby the NF-κB Signaling Pathway. Mol. Cell. Biol. 2016;36:1820–1835. doi: 10.1128/MCB.00158-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamura T, Largaespada DA, Lee MP, Johnson LA, Ohyashiki K, Toyama K, et al. Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat. Genet. 1996;12:154–158. doi: 10.1038/ng0296-154. [DOI] [PubMed] [Google Scholar]
- 128.Borrow J, Shearman AM, Stanton VP, Becher R, Collins T, Williams AJ, et al. The t(7;11)(p15;p15) translocation in acute myeloid leukaemia fuses the genes for nucleoporin NUP98 and class I homeoprotein HOXA9. Nat. Genet. 1996;12:159–167. doi: 10.1038/ng0296-159. [DOI] [PubMed] [Google Scholar]
- 129.Kroon E, Thorsteinsdottir U, Mayotte N, Nakamura T, Sauvageau G. NUP98-HOXA9 expression in hemopoietic stem cells induces chronic and acute myeloid leukemias in mice. The EMBO Journal. 2001;20:350–361. doi: 10.1093/emboj/20.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gough SM, Slape CI, Aplan PD. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood. 2011;118:6247–6257. doi: 10.1182/blood-2011-07-328880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fahrenkrog B, Martinelli V, Nilles N, Fruhmann G, Chatel G, Juge S, et al. Expression of Leukemia-Associated Nup98 Fusion Proteins Generates an Aberrant Nuclear Envelope Phenotype. PLoS ONE. 2016;11:e0152321. doi: 10.1371/journal.pone.0152321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saito S, Yokokawa T, Iizuka G, Cigdem S, Okuwaki M, Nagata K. Function of Nup98 subtypes and their fusion proteins, Nup98-TopIIβ and Nup98-SETBP1 in nuclear-cytoplasmic transport. Biochem. Biophys. Res. Commun. 2017 doi: 10.1016/j.bbrc.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 133.Xu H, Valerio DG, Eisold ME, Sinha A, Koche RP, Hu W, et al. NUP98 Fusion Proteins Interact with the NSL and MLL1 Complexes to Drive Leukemogenesis. Cancer Cell. 2016;30:863–878. doi: 10.1016/j.ccell.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, van Deursen JM. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol. Cell. Biol. 1999;19:764–776. doi: 10.1128/mcb.19.1.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oka M, Mura S, Yamada K, Sangel P, Hirata S, Maehara K, et al. Chromatin-prebound Crm1 recruits Nup98-HoxA9 fusion to induce aberrant expression of Hox cluster genes. Elife. 2016;5:e09540. doi: 10.7554/eLife.09540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martínez N, Alonso A, Moragues MD, Pontón J, Schneider J. The nuclear pore complex protein Nup88 is overexpressed in tumor cells. Cancer Res. 1999;59:5408–5411. [PubMed] [Google Scholar]
- 137.Hashizume C, Nakano H, Yoshida K, Wong RW. Characterization of the role of the tumor marker Nup88 in mitosis. Mol. Cancer. 2010;9:119. doi: 10.1186/1476-4598-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gould VE, Orucevic A, Zentgraf H, Gattuso P, Martinez N, Alonso A. Nup88 (karyoporin) in human malignant neoplasms and dysplasias: correlations of immunostaining of tissue sections, cytologic smears, and immunoblot analysis. Hum. Pathol. 2002;33:536–544. doi: 10.1053/hupa.2002.124785. [DOI] [PubMed] [Google Scholar]
- 139.Agudo D, Gómez-Esquer F, Martínez-Arribas F, Núñez-Villar MJ, Pollán M, Schneider J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. Int. J. Cancer. 2004;109:717–720. doi: 10.1002/ijc.20034. [DOI] [PubMed] [Google Scholar]
- 140.Li J, Zhao J, Li Y. Multiple biological processes may be associated with tumorigenesis under NUP88-overexpressed condition. Genes Chromosomes Cancer. 2017;56:117–127. doi: 10.1002/gcc.22417. [DOI] [PubMed] [Google Scholar]
- 141.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 142.Karacosta LG, Kuroski LA, Hofmann WA, Azabdaftari G, Mastri M, Gocher AM, et al. Nucleoporin 62 and Ca(2+)/calmodulin dependent kinase kinase 2 regulate androgen receptor activity in castrate resistant prostate cancer cells. Prostate. 2016;76:294–306. doi: 10.1002/pros.23121. [DOI] [PubMed] [Google Scholar]
- 143.Qiao W, Han Y, Jin W, Tian M, Chen P, Min J, et al. Overexpression and biological function of TMEM48 in non-small cell lung carcinoma. Tumour Biol. 2016;37:2575–2586. doi: 10.1007/s13277-015-4014-x. [DOI] [PubMed] [Google Scholar]
- 144.Tsuji S, Midorikawa Y, Seki M, Takayama T, Sugiyama Y, Aburatani H. Network-based analysis for identification of candidate genes for colorectal cancer progression. Biochem. Biophys. Res. Commun. 2016;476:534–540. doi: 10.1016/j.bbrc.2016.05.158. [DOI] [PubMed] [Google Scholar]
- 145.Vecchione L, Gambino V, Raaijmakers J, Schlicker A, Fumagalli A, Russo M, et al. A Vulnerability of a Subset of Colon Cancers with Potential Clinical Utility. Cell. 2016;165:317–330. doi: 10.1016/j.cell.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 146.Hashizume C, Kobayashi A, Wong RW. Down-modulation of nucleoporin RanBP2/Nup358 impaired chromosomal alignment and induced mitotic catastrophe. Cell Death Dis. 2013;4:e854. doi: 10.1038/cddis.2013.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br. J. Haematol. 2013;161:117–127. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, Gerecitano JF, Shields AF, Unger TJ, et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2016;34:4142–4150. doi: 10.1200/JCO.2015.65.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim J, McMillan E, Kim HS, Venkateswaran N, Makkar G, Rodriguez-Canales J, et al. XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature. 2016;538:114–117. doi: 10.1038/nature19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J. Hepatol. 2001;34:366–372. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 152.Nesher G, Margalit R, Ashkenazi YJ. Anti-nuclear envelope antibodies: Clinical associations. Semin. Arthritis Rheum. 2001;30:313–320. doi: 10.1053/sarh.2001.20266. [DOI] [PubMed] [Google Scholar]
- 153.Konstantinov K, von Mikecz A, Buchwald D, Jones J, Gerace L, Tan EM. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. J. Clin. Invest. 1996;98:1888–1896. doi: 10.1172/JCI118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ou Y, Enarson P, Rattner JB, Barr SG, Fritzler MJ. The nuclear pore complex protein Tpr is a common autoantigen in sera that demonstrate nuclear envelope staining by indirect immunofluorescence. Clin. Exp. Immunol. 2004;136:379–387. doi: 10.1111/j.1365-2249.2004.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakamura M, Takii Y, Ito M, Komori A, Yokoyama T, Shimizu-Yoshida Y, et al. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis. J. Autoimmun. 2006;26:138–145. doi: 10.1016/j.jaut.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Kim D-H, Kwon S, Byun S, Xiao Z, Park S, Wu S-Y, et al. Critical role of RanBP2-mediated SUMOylation of Small Heterodimer Partner in maintaining bile acid homeostasis. Nat Commun. 2016;7:12179. doi: 10.1038/ncomms12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haruta I, Kikuchi K, Hashimoto E, Nakamura M, Miyakawa H, Hirota K, et al. Long-term bacterial exposure can trigger nonsuppurative destructive cholangitis associated with multifocal epithelial inflammation. Lab. Invest. 2010;90:577–588. doi: 10.1038/labinvest.2010.40. [DOI] [PubMed] [Google Scholar]
- 158.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Braun DA, Sadowski CE, Kohl S, Lovric S, Astrinidis SA, Pabst WL, et al. Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat. Genet. 2016;48:457–465. doi: 10.1038/ng.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miyake N, Tsukaguchi H, Koshimizu E, Shono A, Matsunaga S, Shiina M, et al. Biallelic Mutations in Nuclear Pore Complex Subunit NUP107 Cause Early-Childhood-Onset Steroid-Resistant Nephrotic Syndrome. Am. J. Hum. Genet. 2015;97:555–566. doi: 10.1016/j.ajhg.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fakhro KA, Choi M, Ware SM, Belmont JW, Towbin JA, Lifton RP, et al. Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc. Natl. Acad. Sci. U.S.a. 2011;108:2915–2920. doi: 10.1073/pnas.1019645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tarazón E, Rivera M, Roselló-Lletí E, Molina-Navarro MM, Sánchez-Lázaro IJ, España F, et al. Heart failure induces significant changes in nuclear pore complex of human cardiomyocytes. PLoS ONE. 2012;7:e48957. doi: 10.1371/journal.pone.0048957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu L, Pan L, Li J, Huang B, Feng J, Li C, et al. Nucleoporin 35 regulates cardiomyocyte pH homeostasis by controlling Na+-H+ exchanger-1 expression. J Mol Cell Biol. 2015;7:476–485. doi: 10.1093/jmcb/mjv054. [DOI] [PubMed] [Google Scholar]
- 164.Kaila K, Vaughan-Jones RD. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J. Physiol. (Lond.) 1987;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perez-Terzic C, Gacy AM, Bortolon R, Dzeja PP, Puceat M, Jaconi M, et al. Directed inhibition of nuclear import in cellular hypertrophy. J. Biol. Chem. 2001;276:20566–20571. doi: 10.1074/jbc.M101950200. [DOI] [PubMed] [Google Scholar]
- 166.Perez-Terzic C, Behfar A, Méry A, van Deursen JMA, Terzic A, Puceat M. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ. Res. 2003;92:444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- 167.Perez-Terzic C, Faustino RS, Boorsma BJ, Arrell DK, Niederländer NJ, Behfar A, et al. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S68–76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 168.Faustino RS, Behfar A, Groenendyk J, Wyles SP, Niederlander N, Reyes S, et al. Calreticulin secures calcium-dependent nuclear pore competency required for cardiogenesis. J. Mol. Cell. Cardiol. 2016;92:63–74. doi: 10.1016/j.yjmcc.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 169.Gu Y, Zebell SG, Liang Z, Wang S, Kang B-H, Dong X. Nuclear Pore Permeabilization Is a Convergent Signaling Event in Effector-Triggered Immunity. Cell. 2016;166:1526–1538. e11. doi: 10.1016/j.cell.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ohta H, Sekulovic S, Bakovic S, Eaves CJ, Pineault N, Gasparetto M, et al. Near-maximal expansions of hematopoietic stem cells in culture using NUP98-HOX fusions. Experimental Hematology. 2007;35:817–830. doi: 10.1016/j.exphem.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abraham A, Kim Y-S, Zhao H, Humphries K, Persons DA. Increased Engraftment of Human Short Term Repopulating Hematopoietic Cells in NOD/SCID/IL2rγnull Mice by Lentiviral Expression of NUP98-HOXA10HD. PLoS ONE. 2016;11:e0147059. doi: 10.1371/journal.pone.0147059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao HF, Abraham A, Kim Y-S, Wang Y-D, Pestina T, Zhan J, et al. Lentiviral Transfer of γ-Globin with Fusion Gene NUP98-HOXA10HD Expands Hematopoietic Stem Cells and Ameliorates Murine β-Thalassemia. Mol. Ther. 2017;25:593–605. doi: 10.1016/j.ymthe.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harel A, Orjalo AV, Vincent T, Lachish-Zalait A, Vasu S, Shah S, et al. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol. Cell. 2003;11:853–864. doi: 10.1016/s1097-2765(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 174.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, et al. Cardiomyogenesis in the adult human heart. Circ. Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 175.Galy V, Mattaj IW, Askjaer P. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol. Biol. Cell. 2003;14:5104–5115. doi: 10.1091/mbc.E03-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mertens J, Paquola ACM, Ku M, Hatch E, Böhnke L, Ladjevardi S, et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Funakoshi T, Maeshima K, Yahata K, Sugano S, Imamoto F, Imamoto N. Two distinct human POM121 genes: requirement for the formation of nuclear pore complexes. FEBS Lett. 2007;581:4910–4916. doi: 10.1016/j.febslet.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 179.Franks TM, Benner C, Narvaiza I, Marchetto MCN, Young JM, Malik HS, et al. Evolution of a transcriptional regulator from a transmembrane nucleoporin. Genes Dev. 2016;30:1155–1171. doi: 10.1101/gad.280941.116. [DOI] [PMC free article] [PubMed] [Google Scholar]