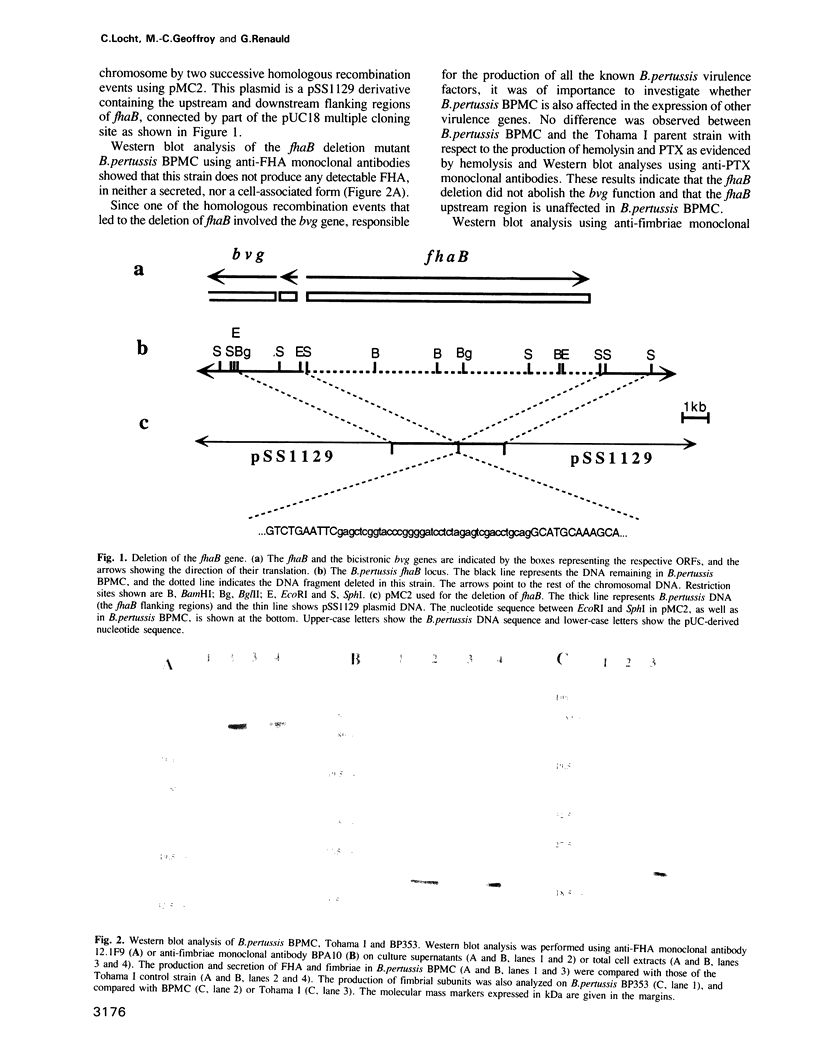
Abstract
The Bordetella pertussis filamentous hemagglutinin (FHA) is a major virulence factor responsible for attachment, one of the early events in bacterial pathogenesis. Deletion of its structural gene, fhaB, or a Tn5 insertion in fhaA, downstream of fhaB, resulted in a FHA- and fimbriae- phenotype, although fhaB and the fim genes are not linked. The fhaB downstream region therefore most likely encodes accessory proteins required for the biosynthesis of FHA and fimbriae, despite the lack of sequence similarities between these two proteins. The nucleotide sequence of this area contains the open reading frames fhaD and fhaA, whose products share sequence similarities with the papD and papC gene products, respectively. PapD is a periplasmic chaperone protein able to bind to the Escherichia coli P pilin subunits and to transport them towards the outer membrane protein PapC which is responsible for pilus membrane translocation. An additional open reading frame, fhaE, is located downstream of fhaA. Its amino acid sequence shares similarities with those of the fimbrial subunits. Deletion analyses suggest that fhaB and the downstream genes can be transcribed as a polycistronic operon, and primer extension analysis revealed the presence of a second promoter between fhaB and fhaD.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. L., Gerlach G. F., Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991 Jan;173(2):916–920. doi: 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine R., Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990 Jun;58(6):1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H., Munoz J. J. Fimbrial hemagglutinin in stationary and shake cultures of Bordetella pertussis. Infect Immun. 1979 Aug;25(2):764–767. doi: 10.1128/iai.25.2.764-767.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker D., Vader C. E., Roosendaal B., Mooi F. R., Oudega B., de Graaf F. K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol Microbiol. 1991 Apr;5(4):875–886. doi: 10.1111/j.1365-2958.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Hannah J. H., Leininger E. Adhesion of Bordetella pertussis to sulfatides and to the GalNAc beta 4Gal sequence found in glycosphingolipids. J Biol Chem. 1991 Oct 5;266(28):18827–18831. [PubMed] [Google Scholar]
- Brown D. R., Parker C. D. Cloning of the filamentous hemagglutinin of Bordetella pertussis and its expression in Escherichia coli. Infect Immun. 1987 Jan;55(1):154–161. doi: 10.1128/iai.55.1.154-161.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cuzzoni A., Pedroni P., Riboli B., Grandi G., de Ferra F. Nucleotide sequence of the fim3 gene from Bordetella pertussis and homology to fim2 and fimX gene products. Nucleic Acids Res. 1990 Mar 25;18(6):1640–1640. doi: 10.1093/nar/18.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisse-Gathoye A. M., Locht C., Jacob F., Raaschou-Nielsen M., Heron I., Ruelle J. L., de Wilde M., Cabezon T. Cloning, partial sequence, expression, and antigenic analysis of the filamentous hemagglutinin gene of Bordetella pertussis. Infect Immun. 1990 Sep;58(9):2895–2905. doi: 10.1128/iai.58.9.2895-2905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighini M., Relman D., Capiau C., Falkow S., Prugnola A., Scarlato V., Rappuoli R. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol. 1990 May;4(5):787–800. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect Immun. 1984 Oct;46(1):195–201. doi: 10.1128/iai.46.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galyov E. E., Karlishev A. V., Chernovskaya T. V., Dolgikh D. A., Smirnov OYu, Volkovoy K. I., Abramov V. M., Zav'yalov V. P. Expression of the envelope antigen F1 of Yersinia pestis is mediated by the product of caf1M gene having homology with the chaperone protein PapD of Escherichia coli. FEBS Lett. 1991 Jul 29;286(1-2):79–82. doi: 10.1016/0014-5793(91)80945-y. [DOI] [PubMed] [Google Scholar]
- Gerlach G. F., Clegg S., Allen B. L. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989 Mar;171(3):1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Hirst T. R., Welch R. A. Mechanisms for secretion of extracellular proteins by gram-negative bacteria. Trends Biochem Sci. 1988 Jul;13(7):265–269. doi: 10.1016/0968-0004(88)90160-0. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Bränden C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature. 1989 Nov 16;342(6247):248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Normark S., Abraham S. N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- Imaizumi A., Suzuki Y., Ono S., Sato H., Sato Y. Effect of heptakis (2,6-O-dimethyl) beta-cyclodextrin on the production of pertussis toxin by Bordetella pertussis. Infect Immun. 1983 Sep;41(3):1138–1143. doi: 10.1128/iai.41.3.1138-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Evans P., Bergmeier L., Lee S. F., Progulske-Fox A., Harris A. C., Aitken A., Bleiweis A. S., Lehner T. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 1989 Nov 20;258(1):127–132. doi: 10.1016/0014-5793(89)81632-1. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Kuehn M. J., Normark S., Hultgren S. J. Immunoglobulin-like PapD chaperone caps and uncaps interactive surfaces of nascently translocated pilus subunits. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10586–10590. doi: 10.1073/pnas.88.23.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laoide B. M., Ullmann A. Virulence dependent and independent regulation of the Bordetella pertussis cya operon. EMBO J. 1990 Apr;9(4):999–1005. doi: 10.1002/j.1460-2075.1990.tb08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Tennent J. M., Hultgren S. J., Lund B., Normark S. PapD, a periplasmic transport protein in P-pilus biogenesis. J Bacteriol. 1989 Nov;171(11):6052–6058. doi: 10.1128/jb.171.11.6052-6058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintermans P., Pohl P., Deboeck F., Bertels A., Schlicker C., Vandekerckhove J., Van Damme J., Van Montagu M., De Greve H. Isolation and nucleotide sequence of the F17-A gene encoding the structural protein of the F17 fimbriae in bovine enterotoxigenic Escherichia coli. Infect Immun. 1988 Jun;56(6):1475–1484. doi: 10.1128/iai.56.6.1475-1484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livey I., Duggleby C. J., Robinson A. Cloning and nucleotide sequence analysis of the serotype 2 fimbrial subunit gene of Bordetella pertussis. Mol Microbiol. 1987 Sep;1(2):203–209. doi: 10.1111/j.1365-2958.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Pedroni P., Riboli B., de Ferra F., Grandi G., Toma S., Aricò B., Rappuoli R. Cloning of a novel pilin-like gene from Bordetella pertussis: homology to the fim2 gene. Mol Microbiol. 1988 Jul;2(4):539–543. doi: 10.1111/j.1365-2958.1988.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Pizza M., Bugnoli M., Manetti R., Covacci A., Rappuoli R. The subunit S1 is important for pertussis toxin secretion. J Biol Chem. 1990 Oct 15;265(29):17759–17763. [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Rioux C. R., Friedrich M. J., Kadner R. J. Genes on the 90-kilobase plasmid of Salmonella typhimurium confer low-affinity cobalamin transport: relationship to fimbria biosynthesis genes. J Bacteriol. 1990 Nov;172(11):6217–6222. doi: 10.1128/jb.172.11.6217-6222.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal B., de Graaf F. K. The nucleotide sequence of the fanD gene encoding the large outer membrane protein involved in the biosynthesis of K99 fimbriae. Nucleic Acids Res. 1989 Feb 11;17(3):1263–1263. doi: 10.1093/nar/17.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Falkow S. Identification of Bordetella pertussis regulatory sequences required for transcriptional activation of the fhaB gene and autoregulation of the bvgAS operon. J Bacteriol. 1991 Apr;173(7):2385–2392. doi: 10.1128/jb.173.7.2385-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato V., Prugnola A., Aricó B., Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Wada K., Wada Y., Doi H., Ishibashi F., Gojobori T., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):1981–1986. doi: 10.1093/nar/19.suppl.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Willems R., Paul A., van der Heide H. G., ter Avest A. R., Mooi F. R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990 Sep;9(9):2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]