Abstract
ETS proteins are a group of evolutionarily related, DNA-binding transcriptional factors. These proteins direct gene expression in diverse normal and disease states by binding to specific promoters and enhancers and facilitating assembly of other components of the transcriptional machinery. The highly conserved DNA-binding ETS domain defines the family and is responsible for specific recognition of a common sequence motif, 5′-GGA(A/T)-3′. Attaining specificity for biological regulation in such a family is thus a conundrum. We present the current knowledge of routes to functional diversity and DNA binding specificity, including divergent properties of the conserved ETS and PNT domains, the involvement of flanking structured and unstructured regions appended to these dynamic domains, posttranslational modifications, and protein partnerships with other DNA-binding proteins and coregulators. The review emphasizes recent advances from biochemical and biophysical approaches, as well as insights from genomic studies that detect ETS-factor occupancy in living cells.
Keywords: autoinhibition, ChIP-Seq, DNA binding, ETS domain, PNT domain, protein dynamics
INTRODUCTION
An overarching conundrum for eukaryotic transcription factors is how a set of related proteins displaying highly conserved DNA-binding domains execute diverse biological roles. This review addresses the current state of biochemical and genomic investigation of this specificity question in the ETS family. Initially, we focus on the common and distinct DNA binding properties of the ETS domain. Next, we discuss the PNT domain to illustrate the potential of semiconserved domains to provide a route to diverse function by different family members. Then, we consider new studies that provide the first views of what is happening in cells at the level of genome-wide ETS-factor DNA occupancy. We include genomic analysis of oncogenic ETS genes as these studies challenge our thinking about specificity and redundancy. Both biochemical and genomic approaches demonstrate the role of protein partnerships as a specificity mechanism. We emphasize systems for which biochemical and biophysical details are available because these provide a robust framework both for defining the specificity problem and informing the many experimental investigations aimed at understanding the role of ETS factors in biological regulation. To complement our structural and genomics focus, we refer the reader to previous reviews that cover biological properties of the ETS factors in more detail (1–6).
Evolution of the ETS Family
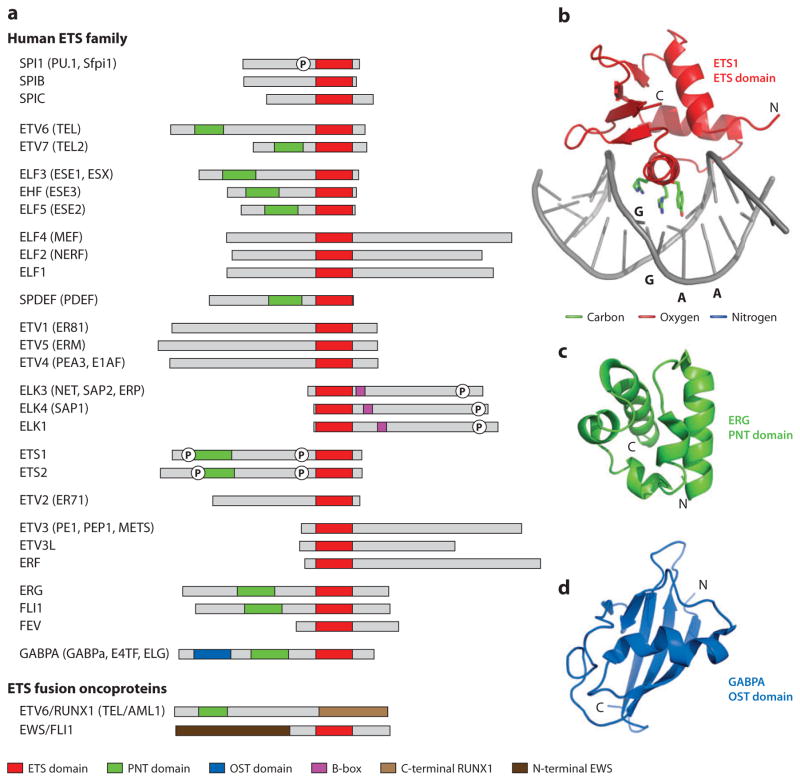
The ETS proteins are encoded by a gene family that is defined by the conserved ETS domain (Figure 1a,b) and present throughout the metazoan phyla (7). Family phylogeny suggests the duplication of an ancestral gene early in metazoan evolution. The continued growth of the family from invertebrates, represented by the 8 and 10 paralogs in Drosophila melanogaster and Caenorhabditis elegans, respectively (Supplemental Table 1: For all Supplemental Material, follow the link from the Annual Reviews home page at http://www.annualreviews.org), to vertebrates, represented by 28 human genes, was likely facilitated by large-scale duplications of vertebrate genomic regions (8). Hallmarks of these evolutionary steps are seen in the dendogram of the family (Figure 2) as well as in the relative positions of structural domains (Figure 1a).
Figure 1.
Structural and functional domains of the ETS family of transcription factors. (a) Nomenclature and domain organization of the 28 paralogous human ETS proteins (grouped according to the dendogram of Figure 2). We use HUGO nomenclature (191) for all ETS genes and proteins, with alternative names provided. Oncogenic ETS proteins, which are created by human chromosome rearrangements, are illustrated by two examples. Many ETS genes produce multiple protein products via alternative splicing/start sites, and in these cases, a single polypeptide was chosen arbitrarily. Boxes identify the DNA-binding ETS domain (red), PNT domain (green), OST domain (blue), and B-box (magenta) of the ETS factors as well as the C-terminal portion of RUNX1 (light brown) and the N-terminal portion of EWS (dark brown). The circled P symbolizes a phosphorylated region discussed in the text. Not identified are additional regions involved in transcriptional activation/repression, posttranslational modifications (including kinase docking and sumoylation), nuclear import/export, turnover, and so forth. (b) Ribbon diagram of the ETS1 ETS domain bound to a DNA duplex (1k79.pdb with the inhibitory helices deleted for clarity). The side chains of R391, R394, and Y395, which provide base-specific contacts to core GGAA, are also shown in stick format. Ribbon diagrams of (c) the ERG PNT domain (1sxe.pdb) and (d) the GABPA OST domain (1juo.pdb). See Supplemental Tables 2 and 3 for a referenced summary of all published ETS and PNT domain structures. Molecular diagrams were rendered with PyMOL (http://pymol.org) with coordinate files from the Protein Data Bank (http://pdb.org).
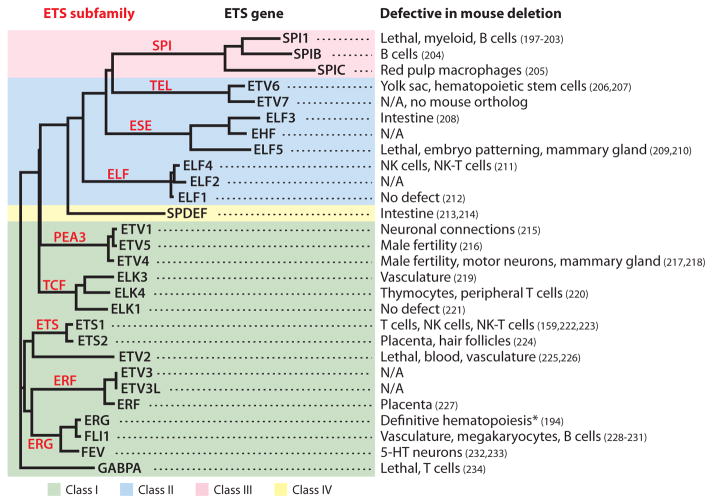
Figure 2.
Diversity in function of ETS family. (left) A dendogram shows the degree of relatedness of 28 human ETS domain sequences, built by ClustalW (192) using the neighbor-joining method (193). The length of each horizontal line indicates estimated evolutionary distance. Branches that separate an individual subfamily are labeled in red. Classes defined by differences in the in vitro derived binding site (62) are indicated by background colors. (right) A list of selected tissues or functions that are defective in each mouse gene deletion, as cataloged at http://www.knockoutmouse.org. See also citations 159 and 197–234, which are linked to defects in mouse deletion in figure. For the sake of brevity, some phenotypes are not listed. N/A indicates the gene deletion has not been reported. The asterisk indicates that this phenotype results from a point mutation of ERG that affects transactivation ability rather than a gene deletion (194). Mice with deletions or mutations of two members of a subfamily have phenotypes that indicate overlapping roles in endothelial cells (ETS1 and ETS2) (30), hematopoietic stem cells (ERG and FLI1) (28), thymocyte development (ELK1 and ELK4) (33), B-cell receptor signal transduction (SPI1 and SPIB) (195), and limb-bud development (ETV1 and ETV5) (196).
An understanding of the overall relatedness of ETS family members is critical for framing the question of functional specificity. For example, there are subfamilies with higher degrees of sequence similarity in both their ETS domains and PNT domains (9) (Figures 1a and 2). Closely related members of a subfamily may display redundant functions, whereas those in different subfamilies may have unique biochemical properties that could be utilized in distinct biological pathways.
Biochemical and Biological Diversity
Structural or functional domains and posttranslational modifications that are found outside of the highly conserved ETS domain illustrate the potential for unique properties of subfamilies or individual ETS proteins. The PNT domain (Figure 1a,c) has variable functions, discussed below, depending on the subfamily in which it resides. The OST domain appears unique to GABPA (Figure 1a,d) and serves in cofactor recruitment (10). The B-box, found in the three members of the ternary complex factor (TCF) subfamily, forms an induced structural element that interacts with serum response factor (SRF) on DNA (11). Although examined in only a few cases by biophysical methods (12–15), the remaining regions of the ETS factors, including those functioning as transactivation domains, likely display minimal stable secondary or tertiary structure. These intrinsically disordered regions can have numerous possible roles, including serving as flexible linkers, linear peptide motifs for binding to partner proteins, and sites of posttranslational modifications (16). Figure 1 is annotated to show only those examples of phosphorylation that are covered in this review owing to their interplay with structured domains. Other modifications found on ETS factors, including ubiquitinylation, sumoylation, glycosylation, and acetylation, have been reviewed elsewhere (17, 18). In conclusion, the overall diversification of the known biochemical properties of the family members suggests that different family members will play distinct biological roles.
Diverse biological roles of individual ETS family members are also suggested by the broad range of phenotypes displayed in mouse genetic disruptions (Figure 2). Genetic studies in C. elegans (19) and Drosophila (20) demonstrate this same trend, with a variety of phenotypes suggesting specialized functions ranging from organ cell fate and neuronal development to oogenesis and aging (Supplemental Table 1) (21–26). Although classic genetics approaches can be limited as a result of potential redundancy within the family, this diversity strongly indicates that different ETS family members carry out distinct biological roles. Indeed, recent reports that include analyses of transcriptional targets implicate specific ETS genes in development of mammalian hematopoietic stem cells (27–29), endothelial cells (30–32), and T cells (33). Although many putative transcriptional targets have been identified over the past 20 years for ETS family members through experimental analyses of transcriptional control elements by in vitro and cell-based assays (3), we focus only on genome-wide occupancy approaches and evaluate their contribution to the evaluation of the authentic targets of ETS factors.
Extensive Coexpression
In spite of the biological and biochemical evidence that different ETS proteins can execute diverse roles, an extensive overlap in the expression of various family members is found in many human tissues. Coexpression of the ETS genes has been systematically surveyed at the mRNA level in more than 20 tissues and transformed cell lines (34–35). Half of the family members are ubiquitously expressed in all cell types tested. In any particular cell type, 14 to 25 family members are coexpressed. This challenges the simplest notion that each family member is restricted to a tissue or cell type for a site of action. In some cases, however, high-level expression is confined to a single cell type (e.g., SPDEF in the prostate, SPI1 in myeloid cells). Additional levels of regulation beyond mRNA expression, such as translational control, alternative splicing, posttranslational modifications, cellular localization, and protein stability, will add to the complexity of this picture. Therefore, systematic proteomic studies that determine active protein levels will be necessary to describe the relative prevalence of ETS factors in diverse tissues.
Expression profiles in transformed cell lines show strong similarity to those in matching normal tissues, although exceptions are worth noting. There is high expression of SPDEF and ESE subfamily members in mammary tumors (35), and a striking prevalence of ETV4 expression in cell lines versus normal matching adult tissues (34). The high expression levels of ERG and PEA3 subfamily members in a subset of prostate tumors led to the discovery of common chromosomal rearrangements associated with this human cancer (36). Thus, relative differences in expression of ETS genes may have a significant role in both normal biology and cancer.
ETS DOMAIN: BIOCHEMICAL OVERVIEW OF DNA BINDNG AND AUTOINHIBITION
Structure and DNA-Binding Interface
The overall structure and DNA-binding mechanism of the ETS domain is based on high-resolution molecular models of ten family members derived from both X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy (Figure 1b, Supplemental Table 2) (37–51). The conserved ETS domain consists of three α-helices on a small four-stranded, antiparallel β-sheet scaffold. This variant of the prototypic helix-turn-helix DNA-binding motif, termed the winged helix architecture, is also found in eukaryotic FOX proteins, heat shock factors, and linker histones as well as the prokaryotic CAP protein (52). The ETS domain binds DNA over a region spanning 12 to 15 base pairs (bp), but it displays sequence preference for only ~9 bp (numbered 1–9 in this review) with a central, invariant 5′-GGA(A/T)-3′ core. The interface is characterized both by direct readout, which involves base-pair contact by ETS-domain sidechains, and indirect readout, which utilizes the sequence-dependent positioning of the phosphodiester backbone. The most important direct interactions in the major groove involve hydrogen bonding between the two invariant arginines in the recognition helix H3 and the two guanines of the GGA(A/T) core. Additional direct and water-mediated hydrogen bonding, hydrophobic, and electrostatic interactions involved in the interface are provided primarily by residues in the “turn” between helices H2 and H3, the “wing” between strands S3 and S4, and the N terminus of helix H1. Strikingly, there are no direct contacts to any base pair outside of the central GGA(A/T), thus implicating sequence-dependent backbone configurations for specificity in the flanking regions (53). Indeed, thermodynamic studies revealed an ~400-fold variation of the affinity of SPI1 depending on sequences flanking the GGA core (54). The structural basis for this indirect readout is currently not well defined, although DNA duplexes bound by ETS domains are typically distorted from an ideal B-DNA geometry.
DNA-Sequence Preferences
The selectivity of multiple ETS proteins has been determined by in vitro methods that demonstrate a binding preference for a particular DNA sequence. Ten proteins were assayed in independent experiments in a variety of laboratories that selected preferred sequences from pools of DNA duplexes, using either the SELEX (systematic evolution of ligands by exponential enrichment) or length-encoded multiplex approach (53, 55–61). More recently, 26 mouse and 27 human ETS proteins were subjected to either microwell binding assays or protein-binding microarrays to determine sequence preferences (62). The results of such analyses performed by different laboratories and across the entire ETS family are striking in their similarity. The most common high-affinity, consensus sequence identified, ACCGGAAGT, consists of an invariant GGA core surrounded by positions with lower preference (see also Genomic Approaches to the Specificity Question, section below).
Sequence preferences observed in systematic analysis of the whole family suggest four classes of ETS proteins that track with subfamilies (Figure 2) (62). Class I, containing more than half of the ETS family (PEA3, TCF, ETS, ERF, and ERG subfamilies), displays a consensus sequence identical to the most common in vitro–derived sequence (ACCGGAAGT). Class II contains eight members (TEL, ESE, and ELF subfamilies) and has a consensus that differs primarily in the first nucleotide (CCCGGAAGT). Members of this second group tend also to be more sensitive to the adenine-to-thymine substitution at position 7, as has been previously reported for ELF1 (63–65). However, variation in the affinity for the alternative GGAT core can also occur between Class I members, such as ELK1 and ELK4 (58). Class III is composed of only the SPI subfamily, whose members prefer binding sites with an adenine-rich sequence 5′ to the GGA (57). Class IV includes only SPDEF, which has a unique preference for a GGAT core sequence instead of GGAA (66). Quantitative DNA-binding studies indicate that the affinity of different ETS proteins for the same consensus sequence many vary by only ~10-fold, whereas the affinity of the same protein for variant consensus sites differs by ~5- to 80-fold (53, 56, 65).
A rationale for some sequence preferences comes from detailed biochemical investigations. For example, two TCF subfamily members, ELK1 and ELK4, have identical DNA-recognition helices, but detailed crystallographic and mutagenic studies revealed that residues at the DNA-binding interface, including a key tyrosine in helix H3, differ conformationally owing to distinct tertiary contacts with adjacent, nonconserved residues (41, 42). Collectively, these effects restrict the ability of ELK1, but not ELK4, to bind variants of the ETS consensus sequence including GGAT.
In conclusion, the differences in DNA-sequence preference of most ETS proteins identified from in vitro studies are subtle. Furthermore, these sequences, which were selected owing to their relative affinity in vitro, could occur rarely in transcriptional-control elements as a result of this lack of selectivity. Specificity in cells could derive predominantly from interactions with cofactors, including partner transcription factors, as discussed below. Lower affinity sites that vary from the high-affinity selected sequences could provide more specificity by poising ETS factors to be dependent on these cofactors.
Structural Elements Appended to the ETS Domain
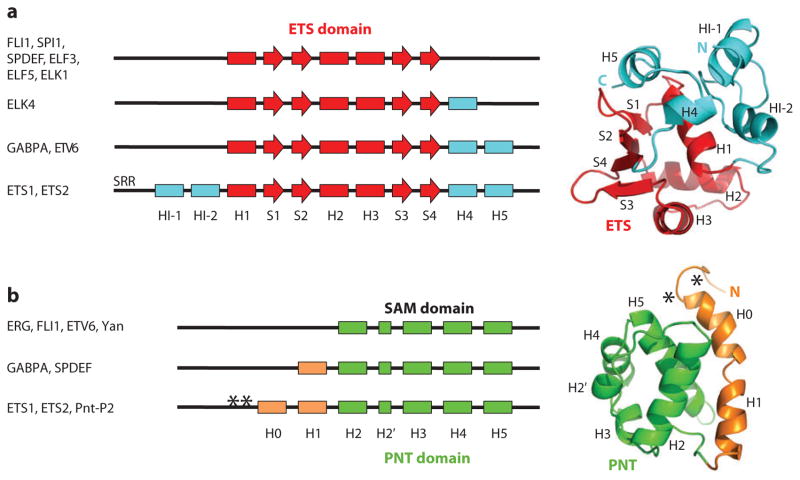
Structural elements appended to the ETS domain regulate its function and demonstrate diversification in the family (Figures 3a and 4). In several ETS proteins, a short α- or 310-helix, or in the case of ELF3 an ordered coil sequence, lies immediately C terminal to the final β-strand of the ETS domain. This may be an even more common feature than currently recognized because the analogous C-terminal regions have not been characterized structurally for all family members. These flanking residues contribute to the tertiary structure and stability of several ETS domains (51, 67). GABPA, ETS1, ETS2, and ETV6 have two C-terminal helices, termed H4 and H5. In the case of GABPA, these serve as a platform for binding GABPB1 (GABPβ), which indirectly enhances DNA affinity, slowing the half-time for dissociation by at least 100-fold (Figure 4a) (40). The two appended C-terminal helices in ETV6 participate in DNA-binding autoinhibition, likely via a steric mechanism (50). In ETS1 and, by sequence similarity, ETS2 and their Drosophila ortholog Pnt-P2, two more helices, HI-1 and HI-2, reside N terminal to the ETS domain and pack with helices H4 and H5 (Figure 3a, right) (45, 48, 68). The resulting helical bundle is central to the allosteric autoinhibition of DNA binding by these family members (discussed below).
Figure 3.
Appended structures to the conserved ETS and PNT domains expand regulatory potential. (a) The core ETS domain (red) contains three α-helices (rectangles) on a four-stranded, antiparallel β-sheet (arrows). Appended N- and C-terminal helices (cyan), as well as an ordered C-terminal coil region in ELF3 (not shown) and dynamic sequences such as the serine-rich region (SRR) of ETS1, provide additional functional diversity. Shown (right) is a ribbon diagram of residues 301–441 of ETS1 (1r36.pdb), with the appended helices (cyan) packed against helix H1 of the core ETS domain. Helices HI-1, HI-2, H4, and H5 inhibit DNA binding of ETS1. Helix HI-1, which is distal from the recognition helix H3, unfolds upon DNA binding as part of the autoinhibition mechanism (Figures 4c,d and 5a). (b) The core PNT domain (green) consists of four α-helices and a small α- or 310-helix (H2′) with a fold similar to that of the SAM domain. Although not shown for clarity, residues preceding H2 form a conserved helical-like turn. Again, appended N-terminal (H0, H1) helices (orange) provide additional routes to functional diversity. Shown (right) is the ribbon diagram of residues 29–138 of ETS1 (2jv3.pdb), including the PNT domain, which constitute a docking site for both MAP kinase and the TAZ1 domain of an ETS1 cofactor, CBP (Figure 5b). Two conserved phosphoacceptors (asterisk) preceding the dynamic helix H0 of ETS1 are indicated. See Supplemental Tables 2 and 3 for a referenced summary of all published ETS and PNT domain structures. The codes shown are from the Protein Data Bank (http://pdb.org).
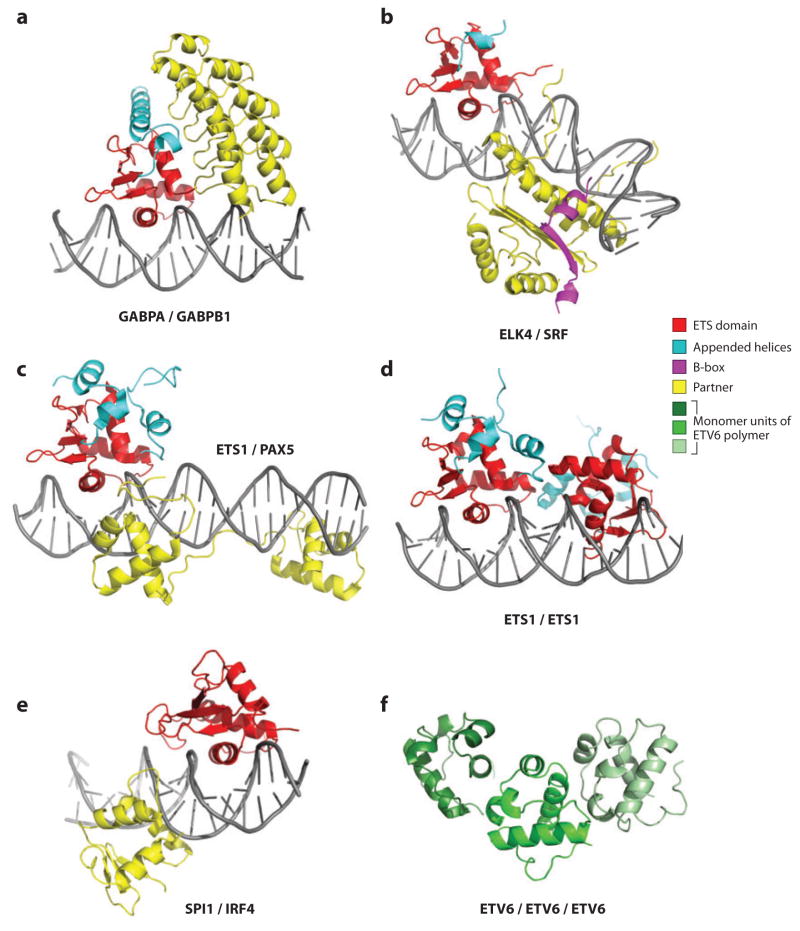
Figure 4.
Structurally characterized ETS protein partnerships. Shown are molecular models of the ETS partnerships involving the ETS domains of (a) GABPA with GABPB1 (1awc.pdb), (b) ELK4 with serum response factor (SRF) (Ihbx.pdb), (c) ETS1 with PAX5 (1 mdm.pdb), (d) the ETS1 homodimer (2nny.pdb), and (e) SPI1 with IRF4 (46). In each case, the ribbon diagrams are colored as ETS domain (red), appended helices (cyan), B-box (magenta), and partner (yellow). (f) Three monomer units of an ETV6 PNT domain polymer associated head-to-tail (greens; 1ji7.pdb). See Supplemental Tables 2 and 3 for a referenced summary of all published ETS and PNT domain structures. The codes listed are from the Protein Data Bank (http://pdb.org).
Autoinhibition of ETS1 DNA Binding: A Dynamic Mechanism
Several ETS proteins exhibit autoinhibition of DNA binding. Diagnostic of this phenomenon is a reduction in the overall affinity for DNA of the full-length protein relative to smaller fragments that retain the ETS domain. Autoinhibition offers many routes for the regulation of proteins, including alternative splicing or proteolysis that would remove inhibitory elements, and posttranslational modifications or protein partnerships that can either reinforce or antagonize inhibition (69).
The best-characterized example of autoinhibition among the ETS proteins is that of ETS1, in which all the above regulatory mechanisms are at play. Autoinhibition of ETS1 DNA binding was discovered initially by deletion analyses (56, 70, 71). However, two naturally occurring variants confirm the biological relevance of inhibitory sequences. The oncogenic version of ETS1 found in the p135 oncoprotein of the E26 retrovirus has the C-terminal inhibitory sequences deleted. Its higher DNA-binding activity may contribute to oncogenesis (72). Likewise, an alternatively spliced ETS1 isoform, which lacks the N-terminal inhibitory sequences encoded by exon VII, binds DNA with higher affinity and slightly broader specificity (73). As discussed below, ETS1 autoinhibition is reinforced by phosphorylation (74, 75) and counteracted by protein partnerships, such as those with RUNX1 (also termed AML1/CBFα2) and PAX5.
Detailed analyses implicate the three structural components of ETS1 in the mechanism of autoinhibition, namely the ETS domain, the appended inhibitory module (Figure 3a), and an adjacent intrinsically disordered serine-rich region (SRR) (Figure 5a). The inhibitory module comprises a helical bundle (HI-1, HI-2, H4, and H5) and interfaces with H1 of the ETS domain. Collectively, these two components, the inhibitory module and ETS domain, are described as the regulatable unit of ETS1. The basic framework of ETS1 autoinhibition was established by early studies demonstrating that HI-1 unfolds upon DNA binding, thus suggesting a thermodynamic penalty to reduce net DNA affinity (76). Subsequent structural analyses showed that the inhibitory module is distal to the DNA-binding interface of ETS1, indicative of an allosteric mechanism linking binding and helix unfolding (45, 48).
Figure 5.
Dynamic properties of the ETS and PNT domains regulate ETS1 function. (a) ETS1 autoinhibition involves a conformational equilibrium between a rigid inactive state and a flexible active state. Upon DNA binding, helix HI-1 unfolds. Transient phosphorylation-dependent interactions of the unstructured serine-rich region (SRR) with the regulatable unit, formed by the core ETS domain (red) and the appended inhibitory module (cyan), stabilize the inactive state (80). This stabilization increases progressively with increasing levels of CaM kinase II multisite phosphorylation of the SRR, leading to rheostatic control of ETS1 DNA binding (79). (b) The PNT domain of ETS1 interacts with the TAZ1 domain of CBP. Phosphorylation of ETS1 causes a conformational change and increases the affinity of the interaction (97). The ETS1 PNT domain consists of a core helical bundle (green) with appended helices H0 and H1 (orange). MAP kinase phosphorylation of Thr38 and Ser41 shifts a conformational equilibrium of the dynamic helix H0 toward a more open state. This conformational switch, along with increased complementary electrostatic interactions, favors binding of the TAZ1 domain (yellow). Note that the structure of the PNT-TAZ1 complex has not been determined, and though not shown in panel a or b, both the unmodified and phosphorylated forms of ETS1 can bind CBP and DNA, respectively.
More recent NMR-based studies have revealed that dynamic properties across a wide timescale range are central to ETS1 autoinhibition (Figure 5a). Helix HI-1 is only marginally stable and thus poised to unfold. The recognition helix H3 also shows significant local fluctuations (48) supporting the hypothesis that DNA-binding domains, in general, require flexibility for high-affinity DNA recognition (77, 78). Furthermore, a network of hydrophobic residues spanning from helix HI-1 to H3 undergoes millisecond to microsecond timescale motions (79). Most importantly, the ETS domain appended with all four inhibitory helices binds DNA with half the affinity of the isolated ETS domain, whereas the presence of an adjacent SRR reduces binding 10- to 20-fold. Most strikingly, the SRR is intrinsically disordered and interacts only transiently with the ETS domain and inhibitory module (80).
Collectively, these data lead to the current model of ETS1 autoinhibition in which the regulatable unit exists in a conformational equilibrium between a flexible state competent for DNA binding and a more rigid inactive state (Figure 5a). Transient interactions with the SRR allosterically favor the latter state. Increasing levels of multisite phosphorylation of the SRR by CaM kinase II leads to a progressive reduction in DNA affinity by promoting these transient interactions (79). Consistent with in vivo studies that show a role for phosphorylation (81), this rheostatic mechanism provides a graded control of ETS1DNA affinity (82). Although the mechanism linking HI-1 unfolding with DNA binding is not readily apparent in static molecular structures (45), molecular dynamics simulations point to correlated motions involving HI-1, H4, and H1 that change upon hydrogen bonding of H1 to the phosphodiester backbone of DNA (83). This theoretical evidence for a conduit from the DNA interface through the regulatable unit is consistent with the dynamics of this region observed in the absence of DNA by NMR.
Autoinhibition in Other ETS Proteins
Other ETS factors also exhibit DNA-binding autoinhibition. A C-terminal inhibitory sequence, bearing helix H5, strongly attenuates ETV6 DNA binding by steric blockage of its ETS domain DNA-binding interface (Figure 3a) (50). Members of the PEA3 subfamily, including ETV4 (12, 84) and ETV5 (85), contain autoinhibitory sequences appended to both sides of their ETS domains. However, based on proline-scanning mutagenesis, these sequences do not appear to be helical. A cooperative partnership with USF-1 enhances the affinity of ETV4 for DNA, at least in part by interacting with these inhibitory sequences (84).
In cases of the ESE and TCF subfamilies, DNA binding is repressed by sequences distant from the ETS domain. The C-terminal ETS domain of ELF3 is inhibited by its central transactivation domain (86), whereas related ELF5 is inhibited by sequences at its N terminus (87). The TCF subfamily members, ELK1, ELK3, and ELK4, exhibit even more complex inhibitory behaviors involving an interplay of the B-box, the C-terminal transactivation domain, and a NID (NET inhibitory domain), as well as interactions with the helixloop-helix Id proteins (88–90). Furthermore, phosphorylation of the transactivation domain results in enhanced DNA binding (91).
An interesting question is whether the autoinhibition mechanisms in these other ETS proteins have features in common with ETS1. A critical feature of autoinhibition in ETS1 is the dynamic character of the ETS domain. The dynamic character of DNA-binding domains, in general, is proposed to be necessary for nonspecific scanning of DNA in search of specific, high-affinity sites (77, 78), with motions quenched upon specific complex formation. Thus, all ETS domains could have these dynamic properties. Different appended inhibitory elements of diverse ETS factors could impinge on these dynamic properties similarly to how the inhibitory helices of ETS1 regulate its motions. Importantly, autoinhibition sets up the potential for regulation of DNA binding through protein partnerships, as described below.
PNT DOMAIN: DIVERSE FUNCTION AND REGULATION
PNT-Domain Structure and Appended Elements
Approximately one-third of ETS family members encode a second structured domain, termed the PNT domain (Figure 1a). This ~80-residue conserved domain plays unique roles in different ETS proteins. High-resolution molecular models of the PNT domain are derived from both X-ray crystallography and NMR spectroscopy performed on one Drosophila and multiple vertebrate proteins (Supplemental Table 3) (92–97). The core of this domain is formed by four α-helices (H2–H5) along with a short 310- or α-helix (H2′) (Figures 1c and 3b), which is highly similar to that of the more widespread SAM domain. SAM domains exhibit remarkably diverse association states and function in a variety of protein-protein and -RNA interactions (98). Similar to the ETS domain, additional helical elements (H0 and H1) appended to the conserved-core of the PNT domain provide unique regulatory paths for distinct family members (Figure 3b).
Ras Signal Transduction and MAP-Kinase Docking
The transcriptional activation by vertebrate ETS1 and ETS2 and the Drosophila ortholog Pnt-P2 of Ras-responsive genes is increased by mitogen-activated protein (MAP) kinase phosphorylation of threonine and serine residues immediately preceding their highly similar PNT domains (68, 99). Detailed in vitro kinetic studies revealed that the PNT domains facilitate signaling by enhancing substrate binding (ETS1 or ETS2 to the MAP kinase ERK2 and Drosophila Pnt-P2 to the orthologous Rolled kinase) rather than altering the kinetic properties of the enzymes (100–102). Thus, the PNT domains are docking modules, interacting with complementary docking sites on the kinases (103, 104). This presumably increases the effective local concentration of the adjacent phosphoacceptors at the catalytic sites of the enzymes, thereby enhancing both the specificity and rate of their modification (105).
Although a detailed structural model of a PNT-domain/MAP-kinase complex has not been solved, insights into the interfaces have been obtained from mutagenesis studies, chemical footprinting, and competition experiments with peptides that bind known docking sites on kinases. Key to PNT-domain binding are residues from helices H2, H4, and H5 surrounding an exposed phenylalanine side chain (100, 102). Thus, unlike the well-characterized linear peptide motifs that bind docking sites on kinases (106, 107), the PNT domains interact via a three-dimensional surface. Conversely, this region of the ETS1 PNT domain has been modeled to associate with the ERK2 substrate binding groove centered near loop 12 and the αG helix (108). Additional contacts involving the unstructured N-terminal tail of ETS1 with the common D-recruitment site on the kinase also have been proposed (109, 110). To both accommodate this docking mode and position the phosphoacceptors in the active site of the kinase, a structural change in the PNT domain, such as the unfolding of helix H0, would be required. Indeed, as discussed below, this helix is dynamic and forms part of a phospho-switch mechanism for CBP recruitment.
In contrast, members of the TCF subfamily (ELK1, ELK3, and ELK4) lack a PNT domain and dock on the D- and F-recruitment sites of MAP kinases via linear peptide sequences (88, 89). These sequences, termed the D- and FxFP-motifs, respectively, flank the phosphoacceptor-containing transactivation domains of the TCF factors. In addition to increasing phosphorylation efficiency and directing acceptor specificity, these motifs differentially target TCF subfamily members to the MAP-kinase isoforms ERK2, JNK, or p38. The resulting phosphorylation regulates the TCF proteins at the levels of DNA binding, coactivator or -repressor recruitment, and cellular localization (2, 88, 91, 111).
Role of the PNT Domain in Phosphorylation-Enhanced Interactions of ETS1 and CBP
The mechanism by which Ras/MAP-kinase signaling enhances ETS1-regulated gene expression via phosphorylation-enhanced recruitment of the general transcriptional coactivator CBP has been characterized recently using cell-based assays and biophysical measurements (65, 97, 112). ERK2 phosphorylates Thr38, as well as a nonconsensus acceptor Ser41, within the unstructured N-terminal region of ETS1, immediately adjacent to its PNT domain. NMR spectroscopic studies revealed that, in addition to the four α-helices that match the SAM domain (H2–H5), the PNT domain of ETS1 is appended with two additional helices (H0–H1) (Figure 3b). Importantly, helix H0 is only marginally stable and undergoes significant local structural fluctuations (97).
Dual phosphorylation perturbs a “closed-open” conformational equilibrium of the PNT domain, displacing the dynamic helix H0 from the core bundle (Figure 5b) (97). These modifications increase the affinity of ETS1 for the TAZ1 (or CH1) domain of CBP by ~30-fold (97). NMR-monitored titration experiments mapped TAZ1 binding to both the phosphoacceptors and helices H0, H2, and H5 of the ETS1 PNT domain. Reciprocal experiments mapped the interaction surface of the TAZ1 domain bound by ETS1. This same CBP region is also implicated in several peptide models of transactivation domains bound to the TAZ1 domain (113). The charge complementarity of these surfaces indicates that electrostatic forces act in concert with the conformational equilibrium to mediate phosphorylation effects. As discussed above, the same interface of the PNT domain also serves as a docking site for the ERK2 kinase. Thus, the PNT domain of ETS1 is key to relaying signal transduction, both “accepting” and “transmitting” the effects of phosphorylation.
As is the case for many transcription factors, other ETS proteins function in association with CBP or its closely related coactivator, p300. GABPA contains an OST domain, which displays a ubiquitin-like fold and binds the TAZ1 and TAZ2 domains of CBP (Figure 1a,d) (10). ELK1 binds p300, and Ras/MAP-kinase-dependent phosphorylation within a transactivation domain enhances the interaction (89, 114–116). In addition, direct interactions between CBP and p300 have been reported for SPI1 (117–118) and ETV1 (119, 120). Note, however, ELK1, ETV1, and SPI1 have no PNT domain (Figure 1a), and the structural bases for CBP or p300 binding remain to be established. The Mediator has also been identified as a coactivator for the TCF subfamily (121–124). The interplay of multiple coactivators for transcription factors, including the ETS proteins, remains an area of current investigation.
PNT-Domain Self-Association
The PNT domain of ETV6 and its Drosophila ortholog Yan form helical homopolymers in a head-to-tail configuration as deduced from crystallographic studies (Figure 4f) (93, 94, 102). The polymer results from the association of two complementary surfaces of neighboring PNT domains, termed mid-loop (ML) and end-helix (EH). Mutant proteins bearing a substituted hydrophobic to charged residue on either the ML or EH surface are monomeric. However, a mixture of two mutants, one with an ML-mutated surface and one with an EH-mutated surface, yields dimers by stable association of their remaining wild-type interfaces. This dimer has been used as a model polymer and subjected to functional analysis of the role of self-association by ETV6 and Yan (26, 50, 96, 102). As discussed below, the dimeric form of ETV6 facilitates DNA binding, but other roles for a longer polymer are possible, such as recruitment of cofactors. With the exception of Yan, ETV6, and likely ETV7 (125), all other PNT domains characterized to date have one or both of these surfaces blocked by amino acid substitutions or appended helices and, thus, are monomeric (95, 126).
The EGF-receptor signaling pathway in Drosophila eye development provides a well-studied example of the interplay of PNT domains from ETS family members that relies on both kinase docking and self-association (reviewed in Reference 127). Yan functions as a repressor and self-associates via its PNT domain (26). Pnt-P2 acts as an activator of common developmental genes. These two ETS factors are targets of the Drosophila MAP kinase Rolled. Upon signaling, Rolled phosphorylates Yan, leading to its export from the nucleus and subsequent degradation in the cytoplasm. Mae, which bears a PNT domain but no ETS domain (128, 129), facilitates Yan phosphorylation by serving as a docking site for Rolled. In addition, the affinity of Mae for Yan is 1000-fold higher than Yan for itself; thus, the presence of Mae favors Yan depolymerization (96). In parallel, phosphorylation of Pnt-P2 adjacent to its PNT domain stimulates activation of its target genes presumably owing to enhanced recruitment of CBP. The Mae PNT domain binds to the PNT domain of Pnt-P2, blocking its Rolled docking site and, thereby, preventing its phosphorylation-dependent activation (102). Thus, PNT-domain homoand hetero-typic interactions provide a robust response to signaling.
The PNT domain is present in certain subfamilies (ETS, TEL, ERG) plus GABPA and SPDEF, but absent in others (SPI, ERF, TCF, ELF) (Figures 1a and 2). Multiple evolutionary events likely led to the loss of this domain as the family duplicated and diversified. The PNT domains in the ERG and ELF subfamilies, as well as in SPDEF, have no reported activities. We anticipate that potential novel functions are yet to be discovered.
GENOMIC APPROACHES TO THE SPECIFICITY QUESTION: REDUNDANT AND SPECIFIC BINDING
Recent genome-wide analyses of ETS-factor occupancy provide a new approach to the specificity question by identifying genomic regions, both promoters and enhancers, bound by individual ETS proteins in living cells. Genomics technology combines chromatin immunoprecipitation (ChIP) with either microarrays (ChIP-chip) or massively parallel sequencing (ChIP-Seq) for identification of the factor-bound DNA regions (130, 131). Nine ETS proteins have been assayed for genome-wide occupancy by either promoter microarrays (ETS1, GABPA, ELF1, ELK1, EWS-FLI1, and SPI1) (132–135) or high-throughput sequencing (GABPA, ETS1, ERG, FLI1, EWS-ERG, EWS-FLI1, SPI1, SPDEF, ETV1, and ELF1) (29, 62, 65, 136–140). (See Supplemental Table 4 for public database access.) Although there are caveats with the current state of this technology (see Caveats of Genome-Wide Occupancy Techniques, sidebar), recent progress with these approaches has generated interesting occupancy patterns for ETS proteins. Furthermore, the availability of multiple data sets from several family members is providing insights into specificity and genomic views of cooperative partnerships.
CAVEATS OF GENOME-WIDE OCCUPANCY TECHNIQUES.
Microarrays and massively parallel sequencing can identify the sequences of DNA derived from ChIP experiments. Mapping these sequences to the genome results in peaks of reads above background that indicate the genomic region bound by the immunoprecipitated transcription factor. Lists of bound regions identified by peak-calling algorithms are not comprehensive, but they generally reflect cut-off values that compromise between false-negative and false-positive results. Resolution ranges from tens to hundreds of base pairs depending on the quality of the peak (136, 190). Bioinformatics approaches can predict binding sequences by searching for overrepresented patterns in large numbers of bound regions or by directly searching for a particular motif. Neither method proves the use of a particular motif. It is also important to consider that bound regions may differ by cell type or growth condition. Another caveat of the current technology is the limited information regarding the function of a bound transcription factor, including information about which gene(s) may be targets of regulation. Currently, bound regions are usually assigned to the closest genes to make functional correlations. Compilation of correlative genomics and bioinformatics data is essential at this stage with anticipation that technology can drive high-throughput tests of functionality in the future.
Redundant Binding of ETS Proteins in Living Cells
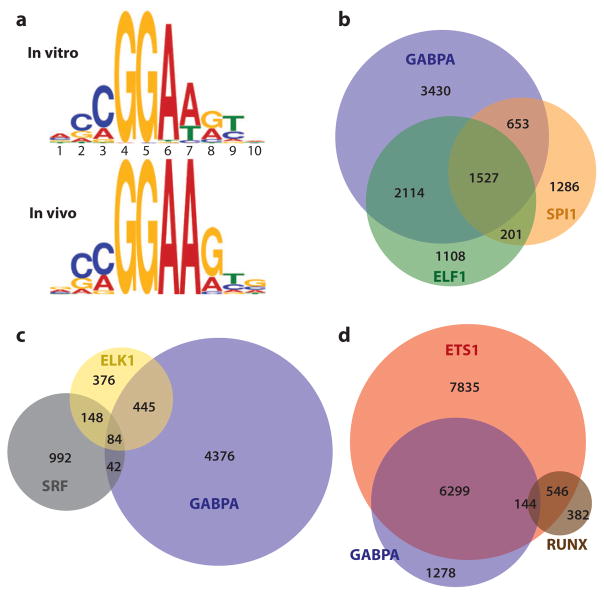
Genome-wide occupancy data for several ETS proteins have been compared and found to have a high degree of similarity (Figure 6). The reported overlap for ETS1 and GABPA shows most GABPA-bound regions are also occupied by ETS1 in Jurkat cells (65, 136). Many of these same regions are occupied by ELK1 in HeLa cells (134, 141). In Figure 6b, we compare data for ELF1 (62) and GABPA from Jurkat T cells (136) and SPI1 from mouse macrophages (138). From even these limited data sets, which use different techniques, cell lines, and species, overlapping occupancy is observable with statistically significant frequency. We speculate that the redundancy may encompass the entire family because the diverse ETS proteins, which have already been implicated, including GABPA, ETS1, ELK1, ELF1, and SPI1, represent five subfamilies.
Figure 6.
Genomic analyses via ChIP-chip and ChIP-Seq detect specific and redundant ETS binding. (a) Redundantly occupied regions in cells have a DNA sequence similar to preferred ETS-binding sequences derived in vitro. Sequence frequencies are compiled (top) from all 27 in vitro–derived ETS-binding sites (62) or (bottom) from the most overrepresented sequence in regions co-occupied in cells by GABPA, ETS1, and ELF1 (62, 65, 136). Frequencies are shown in logo form, which depicts base pair (A:T, T:A, G:C, C:G) frequency as relative heights of A, T, G, and C, respectively. (b) Redundant occupancy is observed for even the most divergent family members and between species. The relative distribution of specific and overlapping bound regions are shown for GABPA (136) and ELF1 (62) in human Jurkat T cells and SPI1 in mouse macrophages (138) by numbers of bound regions within a Venn diagram display. Analysis of SPI1 mouse data was limited to promoter regions, which could be compared with orthologous human regions using the LiftOver tool at http://genome.UCSC.edu (235). Data were reprocessed for this comparison, as previously reported (65). (c,d) Serum response factor (SRF) and RUNX preferentially co-occupy regions bound specifically by ELK1 and ETS1, respectively. Diagrams (c,d) illustrate data from Boros et al. (134) and Hollenhorst et al. (65), respectively. See Supplemental Table 4 for database sources of all genomics data.
Overlapping binding within the resolution of the ChIP technology could be the result of clusters of binding sites specific for each family member. Alternatively, interchangeable occupancy of multiple ETS factors at one site over time could be sampled in the pool of multiple cells (Figure 7). Favoring the latter hypothesis, high-resolution comparisons of binding data for ETS1 and GABPA reveal sharp colocalization of binding events (65). Furthermore, bioinformatics analyses of regions with broad redundancy identified a striking enrichment of the sequence CCGGAAGT (132, 134). This sequence motif matches exactly the in vitro-derived DNA-binding consensus of all but four ETS proteins (Classes I and II) (Figure 6a). Thus, the sequence motifs in redundantly occupied regions are likely able to bind most, if not all, ETS proteins.
Figure 7.
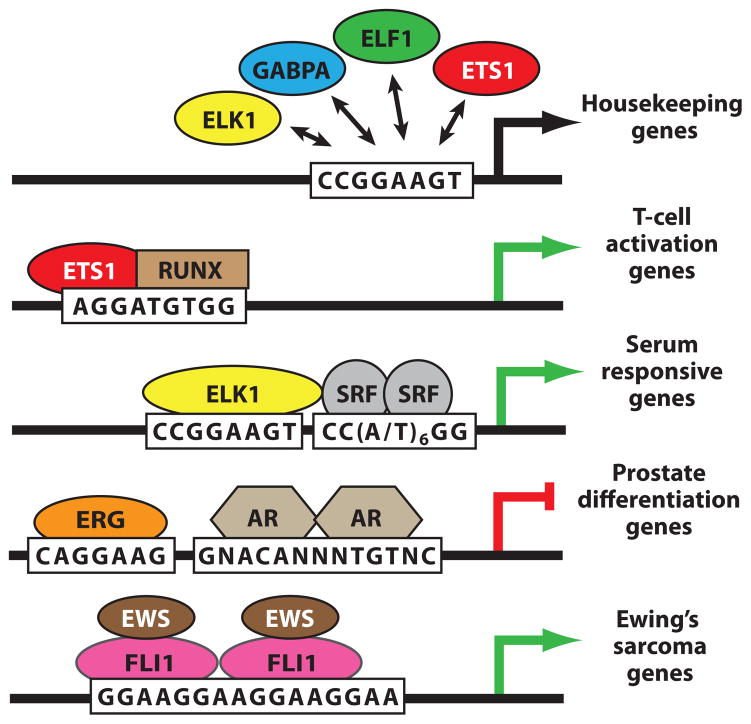
Partnerships provide a route to specificity in normal and disease states. Models depict ETS protein interactions with other DNA-binding proteins at the promoters or enhancers of distinct target classes. ETS proteins exhibit redundant occupancy of a consensus ETS-binding site in housekeeping promoters (132, 134). In contrast, ETS proteins bind specifically near genes that agree with predicted biological functions in either normal or cancer cells (65, 133–134, 139). Specific interactions are associated with activation (green arrows) or repression (red bar) of target genes. Specific targets are highlighted by co-occupancy of multiple transcription factors [RUNX, serum response factor (SRF), androgen receptor (AR)] implicating partnerships as drivers of specificity. EWS-FLI1 tandem occupancy is inferred from functional and biochemical analyses of binding to GGAA microsatellite repeats (133, 137, 187). See Supplemental Table 4 for database sources of all genomics data.
DNA regions occupied redundantly by many ETS proteins are most frequently found a short distance (~20 to 40 bp) upstream of transcription start sites and are correlated with characteristics of active promoters, as evidenced by DNase I sensitivity and presence of histone H3K4 tri-methylation (65, 142, 143). There is also an indication from gene ontology studies that “housekeeping-type” genes are more likely to be characterized by this redundant ETS-factor binding. Searching for overrepresented motifs in the human genome identified an ETS-binding sequence located ~25 bp upstream of the transcription start site of CpG island-containing housekeeping gene promoters (144–146). It has been proposed that housekeeping genes, which are actively expressed in most cell types, could be regulated by different ETS factors in different cell types. The frequency of this sequence (in ~25% of human promoters) (65), the association with commonly expressed housekeeping genes, and its occurrence near transcription start sites suggest a common role for ETS factors in promoter proximal regions, such as transcription initiation, promoter clearance, or maintenance of active chromatin. Functional studies will be necessary to resolve whether individual ETS proteins have distinct activities at these sites or whether a redundant function could be executed by alternative family members.
Redundancy within subfamilies has not been extensively investigated by genome-wide techniques. However, such redundancy may be expected and has been investigated in a detailed analysis of the EGR1 promoter. This promoter is regulated by the TCF subfamily, in part by recruitment of Mediator, as mentioned above. Direct ChIP experiments for ELK1, ELK3, and ELK4 demonstrated alternate occupancy that appeared to correspond to relative abundance of the factors in different cell types (121). In this case, findings suggest the TCF proteins differed in Mediator binding, thus providing distinct EGR1 expression and responsiveness to signaling in different cell types.
Specific Binding of ETS Proteins in Living Cells
Specific binding by an ETS factor is defined as the binding of only one or possibly only highly related subfamily members. Because comparisons of ETS transcription factor genomic occupancy data reveal a significant amount of redundant binding, it can be difficult to differentiate specific binding sites from false-negative or false-positive results. Furthermore, until all ETS family members expressed in a single cell are assayed by ChIP, specificity cannot be definitively concluded. Despite these limitations, current genome-wide studies have identified specific genomic occupancy that correlates with predicted ETS protein roles. For example, in hematopoietic stem cells, the closely related ETS proteins ERG and FLI1 bind to a largely redundant set of targets, whereas targets of the more divergent ETS protein SPI1 have much less overlap (29). However, the mechanism of specificity is yet to be determined.
In some cases the subtle differences in DNA-sequence preference observed in vitro are supported by genomic studies. Regions bound specifically by ETS1 are found in Jurkat cells and have a higher proportion of sites with a thymine at position 7 compared with regions co-occupied by ETS1 and GABPA (65). This observation may be due to the relative tolerance of this change by ETS1 compared with other ETS proteins (147). Genomic occupancy data for SPI1 in myeloid cells and SPDEF in prostate cells enabled bioinformatics analyses for these divergent family members (62). Position-weight matrices derived from unbiased searches of top-scoring regions show similarities to the subtly different sequences derived from the high-throughput proteomics approaches that are discussed above. However, analysis of ELK1, SPI1, GABPA, ERG, FLI1, and ETV1 by other groups report no distinct ETS motifs (29, 134, 136, 138–140). More parsing of bound regions into different classes, such as redundant proximal promoter versus specific distal enhancers, may be necessary to ascertain signatures of specificity determinants.
One sequence change that is often seen in specific binding sites is the presence of an adenine instead of a cytosine at position 3 (ACAGGAAGT). This change may influence specificity in some cases. However, it may also be a result of selection against a CpG, which would be vulnerable to DNA methylation and, thus, inhibition of ETS-protein binding (148, 149). This selection would be necessary at enhancers, where ERG (62) and ETS1 specificity (65) is observed, but not at redundant promoter-binding sites where the ETS-binding sequence can be protected from DNA methylation as a result of proximity to, or inclusion in, hypomethylated CpG islands.
In conclusion, genomic-binding studies have revealed both redundant and specific occupancy by ETS factors. The power of genomics to detect redundancy contrasts with the limits of genetic analyses in which such redundancy may easily be masked. However, the relevance of all genomic occupancy awaits more detailed functional tests, especially ones that can distinguish roles of distinct ETS factors. In addition, many ETS sites predicted on the basis of biochemical understanding, and bioinformatics tools are not occupied in vivo, and conversely, many actual sites of genomic occupancy are not predicted. These discrepancies are consistent with studies in other transcription factor families (130, 150) and likely reflect in vivo complexities such as competition with chromatin packaging and other DNA-binding proteins. Thus, identification of other chromatin-associated factors and specificity partnerships, as described below, will provide a necessary backdrop to understand ETS-protein binding detected in living cells by genomics approaches.
COOPERATIVE PARTNERSHIPS PROVIDE ETS FAMILY SPECIFICITY: GENOMIC AND BIOCHEMICAL PERSPECTIVES
A well-established theme in the regulation of eukaryotic gene expression is combinatorial assembly of multiple factors within the same control elements (e.g., a promoter or enhancer). This phenomenon can provide added specificity and thus synergistic control if there is positive or negative cooperative DNA binding between juxtaposed factors or joint recruitment of additional components of the transcriptional machinery. ETS proteins display a variety of such cooperative partnerships, as previously reviewed (1, 2, 151, 152). To provide a mechanistic framework for understanding this route to specificity, we focus on the high-resolution structural models of ETS partnerships that delineate underlying protein-protein interactions (Figure 4; Supplemental Table 2) and on cases supported by unbiased identification of composite elements through genome-wide analysis (Figure 7).
Ternary Complex Factors/Serum Response Factor
Serum response elements that mediate activation of immediate-early genes such as c-FOS include both ETS- and SRF-binding sites. The cooperative binding interactions between SRF and the TCF subfamily of ETS transcription factors, including ELK1, ELK3, and ELK4, have been well characterized (11; reviewed in Reference 89). Crystallographic studies demonstrate that DNA binding is enhanced by a direct protein contact between ELK4 and the SRF MADS domain (44). The partnership also depends on the TCF B-box, a 21-residue region that adopts a helix-sheet-helix motif when bound to the MADS domain of SRF. This conserved box is limited to the TCF subfamily and, thus, illustrates a route to both redundancy within a subfamily and specificity within the entire ETS family (Figures 1a and 4b).
Chromatin occupancy studies that relied on ChIP-chip reveal co-occupancy of ELK1 and SRF at more than 200 promoters (Figure 6c) (134, 153). These co-occupied regions are more likely than other sites to be bound specifically by ELK1 and not by another ETS protein. This specific binding mode occurs at only 22% of ELK1-bound regions, whereas the redundant binding mode, characterized by a strong consensus ETS-binding sequence and overlapping occupancy with other ETS transcription factors, is observed more frequently (50%). Thus, similar to ETS1, the TCF subfamily can undergo both specific and redundant binding (Figure 7).
SPI1/IRF4
The ETS factor SPI1 partners with the interferon regulatory family transcription factor IRF4 at immunoglobulin light-chain gene enhancers. The low affinity of the winged helix-turn-helix DNA-binding domain of IRF4 for the λB enhancer is increased 20–40-fold by the presence of SPI1 at this site (154, 155). The crystallographically determined structure of the two DNA-binding domains on a composite enhancer sequence reveals that this cooperativity arises from both direct protein-protein contacts across the central minor groove and induced DNA conformational changes (Figure 4e) (46). More dramatically, however, the phosphorylated PEST sequence of SPI1 interacts with an autoinhibitory region of IRF4 to enhance DNA binding by almost three orders of magnitude (154). This enhanced interaction awaits structural characterization.
ETS1/RUNX1 and Autoinhibition
ETS1 and RUNX1 form a partnership that is well characterized by biochemical and genomic approaches (156–158). The level of cooperativity of ETS1 and RUNX1 on ETS sites similar to that in the Moloney murine leukemia virus enhancer approximates the 10-fold repression of ETS1 DNA binding by autoinhibition. Also, ETS1 variants with a deleted or disrupted inhibitory module no longer show such enhancement. These observations suggest that cooperativity results from a relief of autoinhibition. Intriguingly, the effect also requires autoinhibitory sequences in RUNX1 (157). At the time of writing this review, molecular coordinates for several ternary complexes of ETS1 and RUNX1 are on hold in the Protein Data Bank. Thus, we can anticipate informative structural data on this partnership in the near future.
Both redundant and specific occupancy were characterized for ETS1 in Jurkat T cells with more than 14,000 bound regions detected (65). Specific ETS1 occupancy occurs primarily in regions relatively distal to transcription start sites that have enhancer characteristics, such as DNase I sensitivity and histone H3K4 mono-methylation (142, 143). More than 1000 of these specifically bound regions detected by ChIP-Seq display the sequence AGGA(A/T)GTGG. This motif has been characterized in the T-cell receptor enhancers as an ETS1/RUNX1 cooperative binding element with the RUNX1 site comprising the (A/T)GTGG (Figure 7). Indeed, co-occupancy by RUNX factors at many of the ETS1-specific regions was verified by ChIP-Seq (Figure 6d). More than 30-fold cooperative binding is detected with composite sequences bearing this close spacing (156); thus, there may be additional mechanisms at play beyond simply counteracting the 10-fold effect of autoinhibition. Ontology analysis of genes with the composite motif included components of the T-cell activation machinery, including ZAP70, LCK, CXCR3, CXCR4, and CD38. Thus, the ETS/RUNX sequence motif is predictive of the function of nearby genes (65). These sites also recruit the transcriptional coactivator CBP, which adds further evidence for their functional relevance. These genomic studies are consistent with genetics experiments in which mice with a deletion of ETS1 have defects in T-cell activation (159).
Despite the evidence that ETS1 regulates T-cell genes via ETS/RUNX-binding sites, it is not clear that these sites are completely specific for ETS1. Currently, only GABPA and ELF1 ChIP-Seq data are available in Jurkat T cells to compare with ETS1 (62, 136). However, other ETS proteins such as ELF2, ELF4, and FLI1 have been reported to interact with RUNX proteins (160, 161). Furthermore, ChIP-Seq in hematopoietic stem cells identifies extensive co-occupancy of RUNX1 with FLI1 and ERG (29). Thus, it is possible that ETS/RUNX binding sites have only partial specificity for subsets of ETS proteins and that combinations of ETS and RUNX factors may vary with cell type. The forthcoming structural analysis of ETS and RUNX combinations will be important for deciphering rules of their partnering.
ETS1/PAX5
ETS1 and PAX5 bind cooperatively to a composite sequence located in the promoter of mb-1, a gene expressed in early B cell differentiation (Figure 4c) (162). This ETS-binding site displays a variant GGAG core. Crystallographic studies of this cooperative partnership demonstrate a protein contact that leads to an altered conformation of a tyrosine conserved in most ETS domains (Tyr395 in ETS1). Gln22, found in the β-hairpin of the PAX5 paired domain, hydrogen bonds to Tyr395 in the recognition helix H3 of ETS1 to stabilize this altered conformation. This enables new DNA contacts for ETS1, while avoiding unfavorable interactions with the variant GC bp (43). Interestingly, this same conserved tyrosine is involved in ELK4’s ability to bind the variant GGAT core (41).
This partnership also provides insights into the linkage of protein interactions and ETS1 autoinhibition. The PAX5 paired domain enhances the binding of both the autoinhibited and uninhibited variants of ETS1 to the same affinity (163). Thus, the PAX5 partnership appears to counteract the effects of autoinhibition, possibly through the ETS domain rather than direct interactions with inhibitory elements. Gln22 of the paired domain also contacts Gln336 near the N-terminus of helix H1 of the ETS domain (43), providing a conduit into the dynamic core of the ETS domain. There is specificity of this PAX5 partnership for ETS1 rather than other family members, thus illustrating the use of protein interactions to select individual ETS proteins (162). However, the biochemical basis for this selectivity is not clear, as the key residues Tyr395 and Gln336, which contact PAX5, are present in other family members. Furthermore, there are no genomic data in the B-cell system to ascertain the frequency and specificity of this partnership in cells or the common occurrence of a PAX5-ETS composite element.
ETS/FOX
The major historical route to discovery of partnerships in the ETS family, including those mentioned above, has been mapping of tissue-specific enhancer regions that leads to the identification of a functional ETS-binding site and neighboring synergistic elements. A number of additional ETS partnerships have been implicated at this level, but they are not the focus of this review. However, one is mentioned to illustrate emerging topics and critical directions for investigation of partnerships. The ETS and FOX families converge, as illustrated by the role of ETV2 and FOXC2 in the endothelial-cell gene-expression program (31, 32). The partnership is characterized by a composite motif, detected in genome-wide bioinformatics analyses, which can be co-occupied by ETV2 and FOXC2 in DNA-binding assays. Endothelial-cell-specific genes, including FLK1, TAL1, NOTCH4, and VE-CADHERIN, display the motif. Importantly, a genome-wide search with this motif identified endothelial-specific genes. A combination of cell-based transcription assays, mouse transgenics, and zebrafish reverse genetics demonstrates the function of this partnership. The basis of transcriptional synergy is not yet established, and thus it will be important to perform biochemical and structural studies on an ETS-FOX ternary complex.
ETV6 Self-Association and Autoinhibition
In contrast to most ETS proteins, ETV6 is a transcriptional repressor. ETV6 monomers bind DNA extremely weakly owing to autoinhibition of the ETS domain, as discussed above (50). In contrast, an engineered ETV6 dimer shows higher affinity, but only on multiple ETS-binding motifs. It is proposed that self-association via the PNT domain (see Figure 4f) mediates cooperative DNA binding, which compensates for the low affinity of the monomeric ETS domain. Furthermore, this cooperativity is very tolerant of the orientation and spacing (within five helical turns) of the multiple ETS-binding sites, suggesting a substantial degree of flexibility of the middle region of ETV6 (~200 residues), which links the ETS and PNT domains (Figure 1a). Collectively, these results suggest that autoinhibition and polymerization could define the specificity of ETV6 by targeting promoters bearing multiple binding sites. Such sites need not have a well-ordered array of GGA core sequences. Furthermore, polymerization of ETV6 is speculated to create an extended complex, which, along with recruitment of corepressors, may facilitate transcriptional repression (93).
Reports on Drosophila Yan, the apparent ortholog of ETV6, strongly suggest that self-association is a key to the function of this class of ETS factors (26). Biological relevance is also drawn from the chromosome rearrangements of the ETV6 locus that encode oncogenic fusion proteins associated with B cell leukemias. The fusions include the PNT domain of ETV6 and the DNA-binding domain of either RUNX1 (Figure 1a) (164) or PAX5 (165, 166). We speculate that the rules regarding DNA binding learned from the native ETV6 polymer, as modeled by the dimer, may also apply to these oncogenic fusion proteins. Genomic and additional biochemical data will be helpful to understand fully how the biophysical properties of ETV6 impact target selection of both native and oncogenic versions of ETV6.
Other Dimeric Configurations
In addition to ETV6, several ETS proteins have been implicated in dimeric interactions. ETS1 binds cooperatively as a homodimer to tandem ETS motifs, such as that found in the MMP3 promoter. The cooperativity of dimerization is the same 10-fold effect as autoinhibition, suggesting that the dimer configuration may counteract autoinhibition (167). The crystallography-based model of the ternary complex reveals that residues in the loop between inhibitory helix HI-2 and helix H1 of one monomer interact with those in the turn between helices H2 and H3 of the ETS domain in the other monomer (Figure 4d) (49). An alternatively spliced isoform of ETS1, lacking inhibitory elements N terminal to the ETS domain, fails to dimerize, thus implicating the contact made by the inhibitory elements in the cooperativity mechanism (168). However, as with ETS1/PAX5 and ETS1/RUNX1 partnerships, a complete mechanism for linking partnerships and autoinhibition is still lacking.
Other ETS proteins possibly use multiple ETS-binding motifs. On the basis of the crystallographic structure of a single ELF3 ETS domain bound to the B site of a tandem type II TGF-B receptor promoter, enhanced binding of a second ELF3 to the adjacent A site was proposed to result from protein-induced DNA conformational changes (51). This configuration has not been investigated by either biochemical or genomics approaches. In addition, GABPA has two alternative non-DNA-binding partners GABPB1 and GABPB2, which enhance its DNA-binding affinity and provide a homodimeric interface to form GABPA-(GABPB)2-GABPA heterotetramers on DNA (40). The spacing requirement for the tetrameric GABPA/B complex has not been determined, and genomic data for GABPA have not yet elucidated whether such complexes distinguish specific sites in promoters. Note that most of the GABPA sites are also used by monomeric ETS1 or other ETS proteins (65), thus implicating no GABPA specificity determinants.
The ability of ETS factors to use multiple ETS motifs either by ETV6 polymers or other dimeric configurations begs the question of whether clusters of ETS consensus sites occur in the genome. We found no evidence for use of tandem ETS sites in Jurkat cells with the dimeric spacing of the MMP3 promoter (P.C. Hollenhorst, unpublished data). However, a high density of many transcription factor binding motifs, including ETS sites, is noted in promoter and enhancer regions (134, 136, 169). Testing of ETS-factor polymeric binding in cells awaits discovery of additional authentic ETS targets.
In conclusion, combinatorial control of transcription employs multiple DNA-binding transcription factors in close proximity. Although the DNA recognition sequences for ETS proteins and their partners may be closely juxtaposed or even overlapping, the resulting protein-protein interfaces are often small and the effects on their DNA binding rather subtle. Nevertheless, the thermodynamic result of the direct protein-protein interactions and/or the indirect changes in protein-DNA interactions is net-positive cooperativity relative to the independent binding of each transcription factor. The added specificity of larger composite DNA-binding sites, which is gained through the cooperative binding of two factors, may constitute the greatest biological impact of such partnerships. Extrapolating from the detailed mechanism of ETS1 autoinhibition, the dynamic character of the ETS domain could be impacted within the context of a cooperative partnership. It remains to be determined how many of these ETS factors and their respective partnerships tap into the potential to regulate an ETS domain by autoinhibition and/or by affecting protein dynamics.
GENOMIC INSIGHTS INTO ONCOGENIC ETS FACTORS
ERG and Androgen Receptor in Prostate Cancer
Rearrangements of ETS loci are a hallmark of prostate cancer with ~50% of tumors showing alterations at an ETS gene locus (36). In the most common scenario, a promoter region from an androgen-responsive, prostate-specific gene, TMPRSS2, is attached to the ERG locus to drive aberrantly regulated ERG expression in prostate cells. Chromosomal rearrangements that result in the overexpression of members of the PEA3 subfamily (ETV1, ETV4, or ETV5) are found less frequently (36, 170, 171). These genetic changes are implicated in tumorigenesis (170, 172–176). ChIP-Seq of ERG in both cell lines and tumors that overexpress this transcription factor identifies a striking level of cooccupancy (44%) with the androgen receptor (AR) (62, 139). The involvement of the AR in normal prostate development, coupled with the prevalence of ETS-binding sequences at AR-bound regions (177), implies a cooperative interaction that could play a role in ETS-mediated prostate oncogenesis (Figure 7). Expression studies indicate that a potential mechanism of oncogenic ETS proteins, such as ERG and ETV5, at these sites is antagonism of normal AR function (139, 178, 179). Supporting this idea, tumor-suppressor ETS proteins, such as SPDEF, synergize with the AR (66). However, the genetic finding that ETS and AR genes cooperate in oncogenesis (175) indicates that this mechanism is more complex than our current understanding and may differ on a target-by-target basis (180).
The biochemical mechanism for interactions between ETS factors and the AR is also not known. There is some evidence that the AR can physically interact with ERG (139), ETV5 (179), SPDEF (66), ETV1 (181), and ETS1 (177) through their ETS domains, even in the absence of DNA. However, whether this interaction has any ETS family specificity or how it impacts ETS function remains unknown. DNA-binding assays have not tested cooperative binding, and bioinformatics analyses have not identified an overrepresented spacing of ETS- and AR-binding sites functionally analogous to that observed with the ETS1/RUNX partnership. Therefore, the interaction between ETS proteins and the AR may lead to specificity of function, but not necessarily cooperative DNA binding. Thus, ETS proteins may influence cancer via AR target genes, but the mechanism of this effect remains to be discovered.
EWS-FLI1 in Ewing’s Sarcoma
Ewing’s sarcoma, a common pediatric cancer, is invariably associated with ETS gene translocations (182) that result in a fusion oncogene consisting of the 5′ end of EWS and the 3′ end of FLI1, or less frequently ERG, ETV1, ETV4, or FEV (Figure 1a) (183, 184). The strong transactivation activity in the N terminus of EWS contributes to the function of the resulting oncogenic fusion protein (185). However, insights from genomic studies suggest that the fusion protein also directs regulation of new transcriptional target genes. ChIP-chip and ChIP-Seq have identified the genomic regions bound by the most common ETS fusion proteins in Ewing’s sarcoma, EWS-FLI1 and EWS-ERG (62, 133, 137). Two overrepresented DNA sequences are found in these regions: a consensus ETS-binding site that is similar to promoters discussed above, which have redundant occupancy of ETS factors, and microsatellites that bear tandem repeats of the sequence GGAA. Of these two classes of target sequences, only genes near the microsatellite repeats correlate with expression changes associated with transformation, including NROB1 (133) and GSTM4 (186). Thus, GGAA repeat targets, but not redundant promoter targets, mediate the oncogenic role of these fusions.
GGAA microsatellites, which can have more than 10 copies of the tetrameric repeat, constitute arrays of ETS-binding sites that could mediate cooperative binding between neighboring factors. In fact, four or more repeats are necessary for EWS-FLI1 transcriptional activation in reporter assays, and two EWS-FLI1 monomers bind to this four-motif array in biochemical assays (Figure 7) (133, 137, 187). However, whether cooperativity is at play is unclear. A recent study revealed that the EWS portion of the fusion oncoprotein contributes only to the transcriptional activation, not the DNA-binding activity. This implicates the ETS-domain component of the fusion protein in microsatellite binding (187). Indeed, multiple ETS proteins, including examples not involved in Ewing’s sarcoma, can bind to GGAA microsatellites. Furthermore, as discussed above, ETS proteins ETV6, ETV7, GABPA, and ELF3 bind as homo-oligomers to neighboring ETS-binding sites. Whether the GGAA microsatellites that are involved in Ewing’s sarcoma are the natural targets of any of these ETS proteins remains to be tested.
In conclusion, the oncogenic potential of ETS factors is likely derived from their transcriptional properties. In addition to the chromosome rearrangements described above, there is mounting evidence for an oncogenic role for dysregulation of ETS genes in prostate (188, 188a), melanoma (189), and gastrointestinal stromal tumors (140). Alterations in any ETS protein can have multiple downstream effects, amplifying the primary genetic defect. Thus, a potentially effective intervention in ETS-driven cancers would be a disruption in the function of the oncogenic ETS protein. The range of macromolecular interactions, which include DNA-protein and various homo- or hetero-typic protein-protein complexes, all serve as potential interfaces for targeted therapeutics.
In considering the route to oncogenesis as a window into the specificity question of the ETS family, it is noteworthy that multiple family members can be involved in sarcoma and prostate cancer with an interesting overlap between the two diseases. There are no substantial data to indicate that the various family members cause different disease progression or outcome. Future questions include whether these family members are selected as a result of susceptibility of the genetic locus to rearrangements or distinct properties of these, but not other, ETS family members. Finally, continued genomic and biochemical analysis of the oncogenic ETS factors can help elucidate oncogenesis pathways as well as shed light on specificity routes within the family.
Supplementary Material
SUMMARY POINTS.
Conservation of the ETS domain leads to similar DNA interactions among all ETS factors as detected by in vitro biochemical assays. However, deviation from these highest-affinity sites is encountered in biological settings.
The PNT domain provides diversity to ETS proteins, both by its variable presence and differing functions, ranging from kinase and coactivator binding to self-association.
The DNA-binding affinity of many, if not most, ETS proteins is tempered by autoinhibition. Specific DNA motifs used in cells can diverge from the optimal consensus, thus lowering affinity further. This combination of circumstances poises an ETS factor to participate in partnerships with other factors that have additional preferences for DNA sequence and architecture, thus enhancing specificity for genomic locations. High-resolution molecular models demonstrate the relatively small protein-interaction surfaces that can mediate such cooperativity.
Protein dynamics underlie phosphorylation-dependent regulatory processes, including autoinhibition of the ETS domain and enhanced binding of CBP by the PNT domain. Flexible regions, which are likely sites of modification, function in concert with more structured regions, which also exhibit dynamic conformational equilibria.
ETS binding sites displaying a strong match to the highest-affinity consensus motifs are common in CpG island-containing housekeeping promoters. These sequences serve as interchangeable binding sites for many ETS proteins. Every cell type expresses multiple ETS factors that could potentially participate in this redundant occupancy.
Genome-wide occupancy studies identify many (hundreds to thousands) binding sites for a single ETS protein in a particular cell type. Although the functional relevance of many of these sites awaits further analysis, bioinformatics approaches have identified ETS consensus motifs and composite elements that are beginning to define genomic determinants for specificity and cooperative partnerships.
Oncogenic ETS proteins alter numerous transcriptional targets within cells that express many other ETS factors. Selection of oncogenic target genes is likely achieved by altered use of the specificity mechanisms for normal cells described in this review.
FUTURE ISSUES.
Dynamics are increasingly implicated as a powerful regulatory mechanism in protein function. Studying how intra- and intermolecular interactions establish the amplitude and timescale of these dynamics—and, thus potentially, the DNA-binding properties of transcription factors—is a new avenue for understanding the regulation of gene expression. Biophysical studies of increasing sophistication will help define the physicochemical bases for ETS autoinhibition and dissect the role of dynamics in protein and target DNA recognition by the PNT and ETS domains, respectively.
A high level of redundant genomic occupancy in the ETS family was detected in promoter proximal regions. Further work will determine whether ETS proteins play a redundant mechanistic role in transcription regulation at these targets and whether these sites have functions distinct from more distal enhancer elements.
As genome-wide binding data become available for more ETS proteins, as well as other transcription factors, comparisons will allow classification of redundant, specific, and partially specific binding sites. This categorization will improve the robustness of the new data sets to investigate mechanisms that enable specificity within the ETS family, including composite elements for cooperative binding partnerships and other routes to synergistic transcriptional regulation. Redundancy within subfamilies versus the entire family needs to be clarified.
The ETS genes that are involved in chromosomal abnormalities in solid tumors are limited largely to those in ERG and PEA3 subfamilies. It is not known to what degree the oncogenic functions of these genes overlap. Determining the specific roles of these two subfamilies, compared with the rest of the ETS family, will be important to understanding the mechanisms of ETS-mediated oncogenesis.
The development of therapeutic strategies, including small-molecule modulators of protein interactions, to regulate ETS proteins could have high clinical impact. Currently available and new structural and biochemical information about ETS proteins will be valuable in achieving this challenging research goal.
Acknowledgments
We thank Tim Parnell and Krista Meyer for critical comments on the manuscript, David Nix and Katherine Chandler for help with data analysis, and Carlos Escalante and Aneel Aggarwal for providing the coordinates of the SPI1/IRF4/DNA complex. This work was supported by the National Institutes of Health through awards GM38663 (to B.J.G.) and CA42014 (to the Huntsman Cancer Institute for support of shared resources) as well as the Canadian Cancer Society Research Institute (017308 to L.P.M.). Huntsman Cancer Institute/Huntsman Cancer Foundation funding (to B.J.G.); Indiana University School of Medicine support (to P.C.H.); and instrument support from the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, the British Columbia Knowledge Development Fund, the Michael Smith Foundation for Health Research, and the UBC Blusson Fund (to L.P.M.) are gratefully acknowledged.
Glossary
- ETS domain
conserved structural domain in all ETS proteins that is necessary and sufficient for DNA binding
- PNT domain
structural domain conserved in approximately one-third of ETS proteins, which functions in protein interactions and resembles the SAM domain
- SRF
serum response factor
- Intrinsically disordered protein sequences
regions of proteins that are biologically active, yet lack a well-defined three-dimensional structure under physiological conditions
- MAP kinase
mitogen-activated protein kinase
- Promoter
a genomic region in relatively close proximity to the transcription start site that regulates transcription of the nearby gene
- Enhancer
a genomic region that regulates transcription at variable distances from the transcription start site, often greater than 1 kb
- ChIP
chromatin immunoprecipitation
- ChIP-Seq
a genomics technique that combines chromatin immunoprecipitation with massively parallel sequencing to identify DNA sequences bound by proteins in living cells
- Housekeeping genes
a loosely defined set of genes that are expressed in all cell types and function in normal cell growth and maintenance
- CpG islands
genomic regions with many occurrences of the DNA sequence 5′-CG-3′, which is modified by DNA methyltransferase to bear a methyl-cytosine
- AR
androgen receptor
- Microsatellite
a genomic region with 10 to 100 tandem repeats of a 2–6-bp sequence
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding or financial holding that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Graves BJ, Petersen JM. Specificity within the ETS family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 2.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–37. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 3.Oikawa T, Yamada T. Molecular biology of the ETS family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 4.Hsu T, Trojanowska M, Watson DK. ETS proteins in biological control and cancer. J Cell Biochem. 2004;91:896–903. doi: 10.1002/jcb.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez-Hartmann A, Duval DL, Bradford AP. ETS transcription factors in endocrine systems. Trends Endocrinol Metab. 2007;18:150–58. doi: 10.1016/j.tem.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degnan BM, Degnan SM, Naganuma T, Morse DE. The ETS multigene family is conserved throughout the Metazoa. Nucleic Acids Res. 1993;21:3479–84. doi: 10.1093/nar/21.15.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Zhang Q. Genome-wide identification and evolutionary analysis of the animal specific ETS transcription factor family. Evol Bioinform Online. 2009;5:119–31. doi: 10.4137/ebo.s2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laudet V, Hanni C, Stehelin D, Duterque-Coquillaud M. Molecular phylogeny of the ETS gene family. Oncogene. 1999;18:1351–59. doi: 10.1038/sj.onc.1202444. [DOI] [PubMed] [Google Scholar]
- 10.Kang HS, Nelson ML, Mackereth CD, Scharpf M, Graves BJ, McIntosh LP. Identification and structural characterization of a CBP/p300-binding domain from the ETS family transcription factor GABP alpha. J Mol Biol. 2008;377:636–46. doi: 10.1016/j.jmb.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassler M, Richmond TJ. The B-box dominates SAP-1-SRF interactions in the structure of the ternary complex. EMBO J. 2001;20:3018–28. doi: 10.1093/emboj/20.12.3018. Demonstrates the structural role of the B-box in an ELK4/SRF DNA ternary complex formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bojovic BB, Hassell JA. The PEA3 ETS transcription factor comprises multiple domains that regulate transactivation and DNA binding. J Biol Chem. 2001;276:4509–21. doi: 10.1074/jbc.M005509200. [DOI] [PubMed] [Google Scholar]
- 13.Augustijn KD, Duval DL, Wechselberger R, Kaptein R, Gutierrez-Hartmann A, van der Vliet PC. Structural characterization of the PIT-1/ETS-1 interaction: PIT-1 phosphorylation regulates PIT-1/ETS-1 binding. Proc Natl Acad Sci USA. 2002;99:12657–62. doi: 10.1073/pnas.192693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macauley MS, Errington WJ, Scharpf M, Mackereth CD, Blaszczak AG, et al. Beads-on-a-string, characterization of ETS-1 sumoylated within its flexible N-terminal sequence. J Biol Chem. 2006;281:4164–72. doi: 10.1074/jbc.M510488200. [DOI] [PubMed] [Google Scholar]
- 15.Lens Z, Dewitte F, Monte D, Baert JL, Bompard C, et al. Solution structure of the N-terminal transactivation domain of ERM modified by SUMO-1. Biochem Biophys Res Commun. 2010;399:104–10. doi: 10.1016/j.bbrc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 16.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 17.Tootle TL, Rebay I. Post-translational modifications influence transcription factor activity: a view from the ETS superfamily. Bioessays. 2005;27:285–98. doi: 10.1002/bies.20198. [DOI] [PubMed] [Google Scholar]
- 18.Charlot C, Dubois-Pot H, Serchov T, Tourrette Y, Wasylyk B. A review of post-translational modifications and subcellular localization of ETS transcription factors: possible connection with cancer and involvement in the hypoxic response. Methods Mol Biol. 2010;647:3–30. doi: 10.1007/978-1-60761-738-9_1. [DOI] [PubMed] [Google Scholar]
- 19.Hart AH, Reventar R, Bernstein A. Genetic analysis of ETS genes in C. elegans. Oncogene. 2000;19:6400–8. doi: 10.1038/sj.onc.1204040. [DOI] [PubMed] [Google Scholar]
- 20.Hsu T, Schulz RA. Sequence and functional properties of Ets genes in the model organism Drosophila. Oncogene. 2000;19:6409–16. doi: 10.1038/sj.onc.1204033. [DOI] [PubMed] [Google Scholar]
- 21.Schmid C, Schwarz V, Hutter H. AST-1, a novel ETS-box transcription factor, controls axon guidance and pharynx development in C. elegans. Dev Biol. 2006;293:403–13. doi: 10.1016/j.ydbio.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 22.Miley GR, Fantz D, Glossip D, Lu X, Saito RM, et al. Identification of residues of the Caenorhabditis elegans LIN-1 ETS domain that are necessary for DNA binding and regulation of vulval cell fates. Genetics. 2004;167:1697–709. doi: 10.1534/genetics.104.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thyagarajan B, Blaszczak AG, Chandler KJ, Watts JL, Johnson WE, Graves BJ. ETS-4 is a transcriptional regulator of life span in Caenorhabditis elegans. PLoS Genet. 2010;6:e1001125. doi: 10.1371/journal.pgen.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–89. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher JC, Burtis KC, Hogness DS, Thummel CS. The Drosophila E74 gene is required for metamorphosis and plays a role in the polytene chromosome puffing response to ecdysone. Development. 1995;121:1455–65. doi: 10.1242/dev.121.5.1455. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Graham TGW, Vivekanand P, Cote L, Cetera M, Rebay I. Sterile alpha motif domainmediated self-association plays an essential role in modulating the activity of the Drosophila ETS family transcriptional repressor Yan. Mol Cell Biol. 2010;30:1158–70. doi: 10.1128/MCB.01225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciau-Uitz A, Pinheiro P, Gupta R, Enver T, Patient R. Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev Cell. 2010;18:569–78. doi: 10.1016/j.devcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Kruse EA, Loughran SJ, Baldwin TM, Josefsson EC, Ellis S, et al. Dual requirement for the Ets transcription factors Fli-1 and Erg in hematopoietic stem cells and the megakaryocyte lineage. Proc Natl Acad Sci USA. 2009;106:13814–19. doi: 10.1073/pnas.0906556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–44. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, et al. Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114:1123–30. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, et al. Combinatorial regulation of endothelial gene expression by Ets and Forkhead transcription factors. Cell. 2008;135:1053–64. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–95. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costello P, Nicolas R, Willoughby J, Wasylyk B, Nordheim A, Treisman R. Ternary complex factors SAP-1 and Elk-1, but not Net, are functionally equivalent in thymocyte development. J Immunol. 2010;185:1082–92. doi: 10.4049/jimmunol.1000472. [DOI] [PubMed] [Google Scholar]
- 34.Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the Ets family of transcription factors. Nucleic Acids Res. 2004;32:5693–702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galang CK, Muller WJ, Foos G, Oshima RG, Hauser CA. Changes in the expression of many Ets family transcription factors and of potential target genes in normal mammary tissue and tumors. J Biol Chem. 2004;279:11281–92. doi: 10.1074/jbc.M311887200. [DOI] [PubMed] [Google Scholar]
- 36.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. Recurrent fusion of TMPRSS2 and Ets transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 37.Liang H, Mao XH, Olejniczak ET, Nettesheim DG, Yu LP, et al. Solution structure of the Ets domain of Fli-1 when bound to DNA. Nat Struct Biol. 1994;1:871–6. doi: 10.1038/nsb1294-871. [DOI] [PubMed] [Google Scholar]
- 38.Kodandapani R, Pio F, Ni CZ, Piccialli G, Klemsz M, et al. A new pattern for helix-turn-helix recognition revealed by the PU.1 ETS-domain-DNA complex. Nature. 1996;380:456–60. doi: 10.1038/380456a0. [DOI] [PubMed] [Google Scholar]
- 39.Werner MH, Clore GM, Fisher CL, Fisher RJ, Trinh L, et al. Correction of the NMR structure of the ETS1/DNA complex. J Biomol NMR. 1997;10:317–28. doi: 10.1023/a:1018399711996. [DOI] [PubMed] [Google Scholar]
- 40.Batchelor AH, Piper DE, de la Brousse FC, McKnight SL, Wolberger C. The structure of GABP alpha/beta: An Ets domain ankyrin repeat heterodimer bound to DNA. Science. 1998;279:1037–41. doi: 10.1126/science.279.5353.1037. [DOI] [PubMed] [Google Scholar]
- 41.Mo Y, Vaessen B, Johnston K, Marmorstein R. Structures of SAP-1 bound to DNA targets from the E74 and c-fos promoters: Insights into DNA sequence discrimination by Ets proteins. Mol Cell. 1998;2:201–12. doi: 10.1016/s1097-2765(00)80130-6. [DOI] [PubMed] [Google Scholar]
- 42.Mo Y, Vaessen B, Johnston K, Marmorstein R. Structure of the Elk-1-DNA complex reveals how DNA-distal residues affect Ets domain recognition of DNA. Nat Struct Biol. 2000;7:292–7. doi: 10.1038/74055. [DOI] [PubMed] [Google Scholar]
- 43.Garvie CW, Hagman J, Wolberger C. Structural studies of Ets-1/Pax5 complex formation on DNA. Mol Cell Biol. 2001;8:1267–76. doi: 10.1016/s1097-2765(01)00410-5. [DOI] [PubMed] [Google Scholar]
- 44.Mo Y, Ho W, Johnston K, Marmorstein R. Crystal structure of a ternary SAP-1/SRF/c-fos SIRE DNA complex. J Mol Biol. 2001;314:495–506. doi: 10.1006/jmbi.2001.5138. [DOI] [PubMed] [Google Scholar]
- 45.Garvie CW, Pufall MA, Graves BJ, Wolberger C. Structural analysis of the autoinhibition of Ets-1 and its role in protein partnerships. J Biol Chem. 2002;277:45529–36. doi: 10.1074/jbc.M206327200. [DOI] [PubMed] [Google Scholar]
- 46.Escalante CR, Brass AL, Pongubala JM, Shatova E, Shen L, et al. Crystal structure of PU.1/IRF-4/DNA ternary complex. Mol Cell. 2002;10:1097–105. doi: 10.1016/s1097-2765(02)00703-7. [DOI] [PubMed] [Google Scholar]
- 47.Wang YZ, Feng L, Said M, Balderman S, Fayazi Z, et al. Analysis of the 2.0 angstrom crystal structure of the protein-DNA complex of the human PDEF Ets domain bound to the prostate specific antigen regulatory site. Biochemistry. 2005;44:7095–106. doi: 10.1021/bi047352t. [DOI] [PubMed] [Google Scholar]
- 48.Lee GM, Donaldson LW, Pufall MA, Kang HS, Pot I, et al. The structural and dynamic basis of Ets-1 DNA binding autoinhibition. J Biol Chem. 2005;280:7088–99. doi: 10.1074/jbc.M410722200. [DOI] [PubMed] [Google Scholar]
- 49.Lamber EP, Vanhille L, Textor LC, Kachalova GS, Sieweke MH, Wilmanns M. Regulation of the transcription factor Ets-1 by DNA-mediated homo-dimerization. EMBO J. 2008;27:2006–17. doi: 10.1038/emboj.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green SM, Coyne HJ, McIntosh LP, Graves BJ. DNA binding by the Ets protein TEL (ETV6) is regulated by autoinhibition and self-association. J Biol Chem. 2010;285:18496–504. doi: 10.1074/jbc.M109.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarkar VB, Babayeva ND, Wilder PJ, Rizzino A, Tahirov TH. Crystal structure of mouse Elf3 C-terminal DNA-binding domain in complex with Type II TGF-beta receptor promoter DNA. J Mol Biol. 2010;397:278–89. doi: 10.1016/j.jmb.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajiwala KS, Burley SK. Winged helix proteins. Curr Opin Struct Biol. 2000;10:110–6. doi: 10.1016/s0959-440x(99)00057-3. [DOI] [PubMed] [Google Scholar]
- 53.Szymczyna BR, Arrowsmith CH. DNA binding specificity studies of four Ets proteins support an indirect read-out mechanism of protein-DNA recognition. J Biol Chem. 2000;275:28363–70. doi: 10.1074/jbc.M004294200. [DOI] [PubMed] [Google Scholar]
- 54.Poon GMK, Macgregor RB. Base coupling in sequence-specific site recognition by the Ets domain of murine PU.1. J Mol Biol. 2003;328:805–19. doi: 10.1016/s0022-2836(03)00362-0. [DOI] [PubMed] [Google Scholar]
- 55.Brown TA, McKnight SL. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992;6:2502–12. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- 56.Nye JA, Petersen JM, Gunther CV, Jonsen MD, Graves BJ. Interaction of murine Ets-1 with GGA-binding sites establishes the Ets domain as a new DNA-binding motif. Genes Dev. 1992;6:975–90. doi: 10.1101/gad.6.6.975. [DOI] [PubMed] [Google Scholar]
- 57.Ray-Gallet D, Mao C, Tavitian A, Moreau-Gachelin F. DNA binding specificities of Spi-1/PU.1 and Spi-B transcription factors and identification of a Spi-1/Spi-B binding site in the c-fes/c-fps promoter. Oncogene. 1995;11:303–13. [PubMed] [Google Scholar]
- 58.Shore P, Sharrocks AD. The Ets-domain transcription factors Elk-1 and SAP-1 exhibit differential DNA binding specificities. Nucleic Acids Res. 1995;23:4698–706. doi: 10.1093/nar/23.22.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.John S, Marais R, Child R, Light Y, Leonard WJ. Importance of low affinity Elf-1 sites in the regulation of lymphoid-specific inducible gene expression. J Exp Med. 1996;183:743–50. doi: 10.1084/jem.183.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi YS, Sinha S. Determination of the consensus DNA-binding sequence and a transcriptional activation domain for ESE-2. Biochem J. 2006;398:497–507. doi: 10.1042/BJ20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao X, Miesfeldt S, Yang H, Leiden JM, Thompson CB. The FLI-1 and chimeric EWS-FLI-1 oncoproteins display similar DNA binding specificities. J Biol Chem. 1994;269:18216–22. [PubMed] [Google Scholar]
- 62.Wei GH, Badis G, Berger MF, Kivioja T, Palin K, et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 2010;29:2147–60. doi: 10.1038/emboj.2010.106. Provides a comprehensive analysis of in vitro derived DNA binding sequences for vertebrate ETS proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang CY, Petryniak B, Ho IC, Thompson CB, Leiden JM. Evolutionarily conserved Ets family members display distinct DNA binding specificities. J Exp Med. 1992;175:1391–9. doi: 10.1084/jem.175.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosselut R, Levin J, Adjadj E, Ghysdael J. A single amino-acid substitution in the Ets domain alters core DNA binding specificity of Ets1 to that of the related transcription factors Elf1 and E74. Nucleic Acids Res. 1993;21:5184–91. doi: 10.1093/nar/21.22.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollenhorst PC, Chandler KJ, Poulsen RL, Johnson WE, Speck NA, Graves BJ. DNA specificity determinants associate with distinct transcription factor functions. PLoS Genet. 2009;5:e1000778. doi: 10.1371/journal.pgen.1000778. Shows redundant ETS protein binding at housekeeping promoters and genome-wide evidence for ETS1/RUNX partnership. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oettgen P, Finger E, Sun Z, Akbarali Y, Thamrongsak U, et al. PDEF, a novel prostate epithelium specific Ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem. 2000;275:1216–25. doi: 10.1074/jbc.275.2.1216. [DOI] [PubMed] [Google Scholar]
- 67.Donaldson LW, Petersen JM, Graves BJ, McIntosh LP. Solution structure of the ETS domain from murine Ets-1: a winged helix-turn-helix DNA binding motif. EMBO J. 1996;15:125–34. [PMC free article] [PubMed] [Google Scholar]
- 68.Wasylyk C, Bradford AP, Gutierrez-Hartmann A, Wasylyk B. Conserved mechanisms of Ras regulation of evolutionary related transcription factors, Ets1 and Pointed P2. Oncogene. 1997;14:899–913. doi: 10.1038/sj.onc.1200914. [DOI] [PubMed] [Google Scholar]
- 69.Pufall MA, Graves BJ. Autoinhibitory domains: modular effectors of cellular regulation. Annu Rev Cell Dev Biol. 2002;18:421–62. doi: 10.1146/annurev.cellbio.18.031502.133614. [DOI] [PubMed] [Google Scholar]
- 70.Hagman J, Grosschedl R. An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of ETS family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci USA. 1992;89:8889–93. doi: 10.1073/pnas.89.19.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wasylyk C, Kerckaert JP, Wasylyk B. A novel modulator domain of Ets transcription factors. Genes Dev. 1992;6:965–74. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- 72.Lim F, Kraut N, Framptom J, Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992;11:643–52. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lionneton F, Lelievre E, Baillat D, Stehelin D, Soncin F. Characterization and functional analysis of the p42Ets-1 variant of the mouse Ets-1 transcription factor. Oncogene. 2003;22:9156–64. doi: 10.1038/sj.onc.1207241. [DOI] [PubMed] [Google Scholar]
- 74.Rabault B, Ghysdael J. Calcium-induced phosphorylation of ETS1 inhibits its specific DNA binding activity. J Biol Chem. 1994;269:28143–51. [PubMed] [Google Scholar]
- 75.Cowley DO, Graves BJ. Phosphorylation represses Ets-1 DNA binding by reinforcing autoinhibition. Genes Dev. 2000;14:366–76. [PMC free article] [PubMed] [Google Scholar]
- 76.Petersen JM, Skalicky JJ, Donaldson LW, Mcintosh LP, Alber T, Graves BJ. Modulation of transcription factor ETS-1 DNA-binding—DNA-induced unfolding of an alpha-helix. Science. 1995;269:1866–69. doi: 10.1126/science.7569926. [DOI] [PubMed] [Google Scholar]
- 77.Kalodimos CG, Biris N, Bonvin AM, Levandoski MM, Guennuegues M, et al. Structure and flexibility adaptation in nonspecific and specific protein-DNA complexes. Science. 2004;305:386–89. doi: 10.1126/science.1097064. [DOI] [PubMed] [Google Scholar]
- 78.Kalodimos CG, Boelens R, Kaptein R. Toward an integrated model of protein-DNA recognition as inferred from NMR studies on the Lac repressor system. Chem Rev. 2004;104:3567–86. doi: 10.1021/cr0304065. [DOI] [PubMed] [Google Scholar]
- 79.Pufall MA, Lee GM, Nelson ML, Kang HS, Velyvis A, et al. Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science. 2005;309:142–45. doi: 10.1126/science.1111915. Demonstrates role of dynamics in regulation of ETS1 DNA binding by a phosphorylated unstructured region. [DOI] [PubMed] [Google Scholar]
- 80.Lee GM, Pufall MA, Meeker CA, Kang HS, Graves BJ, McIntosh LP. The affinity of Ets-1 for DNA is modulated by phosphorylation through transient interactions of an unstructured region. J Mol Biol. 2008;382:1014–30. doi: 10.1016/j.jmb.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu H, Grundstrom T. Calcium regulation of GM-CSF by calmodulin-dependent kinase II phosphorylation of Ets1. Mol Biol Cell. 2002;13:4497–507. doi: 10.1091/mbc.E02-03-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gardner KH, Montminy M. Can you hear me now? Regulating transcriptional activators by phosphorylation. Sci STKE. 2005;2005:pe44. doi: 10.1126/stke.3012005pe44. [DOI] [PubMed] [Google Scholar]
- 83.Kamberaj H, van der Vaart A. Correlated motions and interactions at the onset of the DNA-induced partial unfolding of Ets-1. Biophys J. 2009;96:1307–17. doi: 10.1016/j.bpj.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greenall A, Willingham N, Cheung E, Boam DS, Sharrocks AD. DNA binding by the ETS-domain transcription factor PEA3 is regulated by intramolecular and intermolecular protein-protein interactions. J Biol Chem. 2001;276:16207–15. doi: 10.1074/jbc.M011582200. [DOI] [PubMed] [Google Scholar]
- 85.de Launoit Y, Baert JL, Chotteau A, Monte D, Defossez PA, et al. Structure-function relationships of the PEA3 group of Ets-related transcription factors. Biochem Mol Med. 1997;61:127–35. doi: 10.1006/bmme.1997.2605. [DOI] [PubMed] [Google Scholar]
- 86.Kopp JL, Wilder PJ, Desler M, Kinarsky L, Rizzino A. Different domains of the transcription factor ELF3 are required in a promoter-specific manner and multiple domains control its binding to DNA. J Biol Chem. 2007;282:3027–41. doi: 10.1074/jbc.M609907200. [DOI] [PubMed] [Google Scholar]
- 87.Oettgen P, Kas K, Dube A, Gu X, Grall F, et al. Characterization of ESE-2, a novel ESE-1-related Ets transcription factor that is restricted to glandular epithelium and differentiated keratinocytes. J Biol Chem. 1999;274:29439–52. doi: 10.1074/jbc.274.41.29439. [DOI] [PubMed] [Google Scholar]
- 88.Sharrocks AD. Complexities in ETS-domain transcription factor function and regulation: lessons from the TCF (ternary complex factor) subfamily. The Colworth Medal Lecture. Biochem Soc Trans. 2002;30:1–9. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 89.Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. doi: 10.1016/j.gene.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 90.Stinson J, Inoue T, Yates P, Clancy A, Norton JD, Sharrocks AD. Regulation of TCF ETS-domain transcription factors by helix-loop-helix motifs. Nucleic Acids Res. 2003;31:4717–28. doi: 10.1093/nar/gkg689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang SH, Shore P, Willingham N, Lakey JH, Sharrocks AD. Themechanism of phosphorylation inducible activation of the ETS-domain transcription factor Elk-1. EMBO J. 1999;18:5666–74. doi: 10.1093/emboj/18.20.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slupsky CM, Gentile LN, Donaldson LW, Mackereth CD, Seidel JJ, et al. Structure of the Ets-1 pointed domain and mitogen-activated protein kinase phosphorylation site. Proc Natl Acad Sci USA. 1998;95:12129–34. doi: 10.1073/pnas.95.21.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim CA, Phillips ML, Kim W, Gingery M, Tran HH, et al. Polymerization of the SAM domain of TEL in leukemogenesis and transcriptional repression. EMBO J. 2001;20:4173–82. doi: 10.1093/emboj/20.15.4173. Discovery of the polymerization properties of the PNT domain of ETV6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tran HH, Kim CA, Faham S, Siddall M-C, Bowie JU. Native interface of the SAM domain polymer of TEL. BMC Struct Biol. 2002;2:5. doi: 10.1186/1472-6807-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mackereth CD, Scharpf M, Gentile LN, MacIntosh SE, Slupsky CM, McIntosh LP. Diversity in structure and function of the Ets family PNT domains. J Biol Mol. 2004;342:1249–64. doi: 10.1016/j.jmb.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 96.Qiao F, Song HY, Kim CA, Sawaya MR, Hunter JB, et al. Derepression by depolymerization: Structural insights into the regulation of Yan by Mae. Cell. 2004;118:163–73. doi: 10.1016/j.cell.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 97.Nelson ML, Kang HS, Lee GM, Blaszczak AG, Lau DKW, et al. Ras signaling requires dynamic properties of Ets1 for phosphorylation-enhanced binding to coactivator CBP. Proc Natl Acad Sci USA. 2010;107:10026–31. doi: 10.1073/pnas.0915137107. Demonstrates mechanism for phosphorylation-dependent binding of CBP and ETS1 via charge complementarity and conformational dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim CA, Bowie JU. SAM domains: uniform structure, diversity of function. Trends Biochem Sci. 2003;28:625–28. doi: 10.1016/j.tibs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Klambt C. The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development. 1993;117:163–76. doi: 10.1242/dev.117.1.163. [DOI] [PubMed] [Google Scholar]
- 100.Seidel JJ, Graves BJ. An ERK2 docking site in the Pointed domain distinguishes a subset of ETS transcription factors. Genes Dev. 2002;16:127–37. doi: 10.1101/gad.950902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waas WF, Dalby KN. Transient protein-protein interactions and a random-ordered kinetic mechanism for the phosphorylation of a transcription factor by extracellular-regulated protein kinase 2. J Biol Chem. 2002;277:12532–40. doi: 10.1074/jbc.M110523200. [DOI] [PubMed] [Google Scholar]
- 102.Qiao F, Harada B, Song HY, Whitelegge J, Courey AJ, Bowie JU. Mae inhibits Pointed-P2 transcriptional activity by blocking its MAPK docking site. EMBO J. 2006;25:70–79. doi: 10.1038/sj.emboj.7600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharrocks AD, Yang SH, Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem Sci. 2000;25:448–53. doi: 10.1016/s0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 105.Rainey MA, Callaway K, Barnes R, Wilson B, Dalby KN. Proximity-induced catalysis by the protein kinase ERK2. J Am Chem Soc. 2005;127:10494–95. doi: 10.1021/ja052915p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goldsmith EJ, Akella R, Min X, Zhou T, Humphreys JM. Substrate and docking interactions in serine/threonine protein kinases. Chem Rev. 2007;107:5065–81. doi: 10.1021/cr068221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akella R, Moon TM, Goldsmith EJ. Unique MAP Kinase binding sites. Biochim Biophys Acta. 2008;1784:48–55. doi: 10.1016/j.bbapap.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callaway K, Waas WF, Rainey MA, Ren PY, Dalby KN. Phosphorylation of the transcription factor ETS-1 by ERK2: rapid dissociation of ADP and phospho-ETS-1. Biochemistry. 2010;49:3619–30. doi: 10.1021/bi100199q. Presents a kinetic and structural mechanism for the docking of ETS1 and MAP kinase ERK2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Callaway KA, Rainey MA, Riggs AF, Abramczyk O, Dalby KN. Properties and regulation of a transiently assembled ERK2·Ets-1 signaling complex. Biochemistry. 2006;45:13719–33. doi: 10.1021/bi0610451. [DOI] [PubMed] [Google Scholar]
- 110.Abramczyk O, Rainey MA, Barnes R, Martin L, Dalby KN. Expanding the repertoire of an ERK2 recruitment site: cysteine footprinting identifies the D-recruitment site as a mediator of Ets-1 binding. Biochemistry. 2007;46:9174–86. doi: 10.1021/bi7002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 112.Foulds CE, Nelson ML, Blaszczak AG, Graves BJ. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol. 2004;24:10954–64. doi: 10.1128/MCB.24.24.10954-10964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wojciak JM, Martinez-Yamout MA, Dyson HJ, Wright PE. Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. EMBO J. 2009;28:948–58. doi: 10.1038/emboj.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown LA, Yang SH, Hair A, Galanis A, Sharrocks AD. Molecular characterization of a zebrafish TCF ETS-domain transcription factor. Oncogene. 1999;18:7985–93. doi: 10.1038/sj.onc.1203197. [DOI] [PubMed] [Google Scholar]
- 115.Li QJ, Yang SH, Maeda Y, Sladek FM, Sharrocks AD, Martins-Green M. MAP kinase phosphorylation-dependent activation of Elk-1 leads to activation of the co-activator p300. EMBO J. 2003;22:281–91. doi: 10.1093/emboj/cdg028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang SH, Jaffray E, Hay RT, Sharrocks AD. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol Cell. 2003;12:63–74. doi: 10.1016/s1097-2765(03)00265-x. [DOI] [PubMed] [Google Scholar]
- 117.Bai Y, Srinivasan L, Perkins L, Atchison ML. Protein acetylation regulates both PU.1 transactivation and Ig kappa 3′ enhancer activity. J Immunol. 2005;175:5160–69. doi: 10.4049/jimmunol.175.8.5160. [DOI] [PubMed] [Google Scholar]
- 118.Aikawa Y, Katsumoto T, Zhang P, Shima H, Shino M, et al. PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2. Nat Med. 2010;16:580–85. doi: 10.1038/nm.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goel A, Janknecht R. Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J Biol Chem. 2004;279:14909–16. doi: 10.1074/jbc.M400036200. [DOI] [PubMed] [Google Scholar]
- 120.Papoutsopoulou S, Janknecht R. Phosphorylation of ETS transcription factor ER81 in a complex with its coactivators CREB-binding protein and p300. Mol Cell Biol. 2000;20:7300–10. doi: 10.1128/mcb.20.19.7300-7310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balamotis MA, Pennella MA, Stevens JL, Wasylyk B, Belmont AS, Berk AJ. Complexity in transcription control at the activation domain-mediator interface. Sci Signal. 2009;2:RA20. doi: 10.1126/scisignal.1164302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cantin GT, Stevens JL, Berk AJ. Activation domain-mediator interactions promote transcription preinitiation complex assembly on promoter DNA. Proc Natl Acad Sci USA. 2003;100:12003–8. doi: 10.1073/pnas.2035253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stevens JL, Cantin GT, Wang G, Shevchenko A, Berk AJ. Transcription control by E1A and MAP kinase pathway via Sur2 Mediator subunit. Science. 2002;296:755–58. doi: 10.1126/science.1068943. [DOI] [PubMed] [Google Scholar]
- 124.Wang W, Huang L, Huang Y, Yin JW, Berk AJ, et al. Mediator MED23 links insulin signaling to the adipogenesis transcription cascade. Dev Cell. 2009;16:764–71. doi: 10.1016/j.devcel.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Potter MD, Buijs A, Kreider B, van Rompaey L, Grosveld GC. Identification and characterization of a new human ETS-family transcription factor, TEL2, that is expressed in hematopoietic tissues and can associate with TEL1/ETV6. Blood. 2000;95:3341–48. [PubMed] [Google Scholar]
- 126.Meruelo AD, Bowie JU. Identifying polymer-forming SAM domains. Proteins. 2009;74:1–5. doi: 10.1002/prot.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vivekanand P, Rebay I. Intersection of signal transduction pathways and development. Annu Rev Genet. 2006;40:139–57. doi: 10.1146/annurev.genet.40.110405.090555. [DOI] [PubMed] [Google Scholar]
- 128.Baker DA, Mille-Baker B, Wainwright SM, Ish-Horowicz D, Dibb NJ. Mae mediates MAP kinase phosphorylation of Ets transcription factors in Drosophila. Nature. 2001;411:330–34. doi: 10.1038/35077122. [DOI] [PubMed] [Google Scholar]
- 129.Tootle TL, Lee PS, Rebay I. CRM1-mediated nuclear export and regulated activity of the receptor tyrosine kinase antagonist YAN require specific interactions with MAE. Development. 2003;130:845–57. doi: 10.1242/dev.00312. [DOI] [PubMed] [Google Scholar]
- 130.Farnham PJ. Insights from genomic profiling of transcription factors. Nat Rev Genet. 2009;10:605–16. doi: 10.1038/nrg2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barski A, Zhao K. Genomic location analysis by ChIP-Seq. J Cell Biochem. 2009;107:11–18. doi: 10.1002/jcb.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hollenhorst PC, Shah AA, Hopkins C, Graves BJ. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 2007;21:1882–94. doi: 10.1101/gad.1561707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gangwal K, Sankar S, Hollenhorst PC, Kinsey M, Haroldsen SC, et al. Microsatellites as EWS/FLI response elements in Ewing’s sarcoma. Proc Natl Acad Sci USA. 2008;105:10149–54. doi: 10.1073/pnas.0801073105. Genome-wide EWS-FLI1 occupancy shows route to specificity for oncogenic ETS protein via use of microsatellites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boros J, Donaldson IJ, O’Donnell A, Odrowaz ZA, Zeef L, et al. Elucidation of the ELK1 target gene network reveals a role in the coordinate regulation of core components of the gene regulation machinery. Genome Res. 2009;19:1963–73. doi: 10.1101/gr.093047.109. Genome-wide ELK1 occupancy displays redundant and specific binding patterns and evidence for ELK1/SRF partnership. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kubosaki A, Lindgren G, Tagami M, Simon C, Tomaru Y, et al. The combination of gene perturbation assay and ChIP-chip reveals functional direct target genes for IRF8 in THP-1 cells. Mol Immunol. 2010;47:2295–302. doi: 10.1016/j.molimm.2010.05.289. [DOI] [PubMed] [Google Scholar]
- 136.Valouev A, Johnson DS, Sundquist A, Medina C, Anton E, et al. Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nat Methods. 2008;5:829–34. doi: 10.1038/nmeth.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guillon N, Tirode F, Boeva V, Zynovyev A, Barillot E, Delattre O. The oncogenic EWS-FLI1 protein binds in vivo GGAA microsatellite sequences with potential transcriptional activation function. PLoS One. 2009;4:e4932. doi: 10.1371/journal.pone.0004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–28. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 139.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–54. doi: 10.1016/j.ccr.2010.03.018. Proposes a mechanistic route for ERG-driven oncogenesis through effects on an epigenetics program. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–53. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boros J, O’Donnell A, Donaldson IJ, Kasza A, Zeef L, Sharrocks AD. Overlapping promoter targeting by Elk-1 and other divergent ETS-domain transcription factor family members. Nucleic Acids Res. 2009;37:7368–80. doi: 10.1093/nar/gkp804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–18. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 143.Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, et al. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–22. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fitzgerald PC, Shlyakhtenko A, Mir AA, Vinson C. Clustering of DNA sequences in human promoters. Genome Res. 2004;14:1562–74. doi: 10.1101/gr.1953904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bina M, Wyss P, Ren W, Szpankowski W, Thomas E, et al. Exploring the characteristics of sequence elements in proximal promoters of human genes. Genomics. 2004;84:929–40. doi: 10.1016/j.ygeno.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 146.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, et al. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–45. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pio F, Assa-Munt N, Yguerabide J, Maki RA. Mutants of ETS domain PU.1 and GGAA/T recognition: free energies and kinetics. Protein Sci. 1999;8:2098–109. doi: 10.1110/ps.8.10.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yokomori N, Kobayashi R, Moore R, Sueyoshi T, Negishi M. A DNA methylation site in the male-specific P450 (Cyp 2 d-9) promoter and binding of the heteromeric transcription factor GABP. Mol Cell Biol. 1995;15:5355–62. doi: 10.1128/mcb.15.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gaston K, Fried M. CpG methylation has differential effects on the binding of YY1 and ETS proteins to the bi-directional promoter of the Surf-1 and Surf-2 genes. Nucleic Acids Res. 1995;23:901–9. doi: 10.1093/nar/23.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Massie CE, Mills IG. Chromatin immunoprecipitation (ChIP) methodology and readouts. Methods Mol Biol. 2009;505:123–37. doi: 10.1007/978-1-60327-575-0_7. [DOI] [PubMed] [Google Scholar]
- 151.Li R, Pei H, Watson DK. Regulation of Ets function by protein-protein interactions. Oncogene. 2000;19:6514–23. doi: 10.1038/sj.onc.1204035. [DOI] [PubMed] [Google Scholar]
- 152.Verger A, Duterque-Coquillaud M. When Ets transcription factors meet their partners. Bioessays. 2002;24:362–70. doi: 10.1002/bies.10068. [DOI] [PubMed] [Google Scholar]
- 153.Cooper SJ, Trinklein ND, Nguyen L, Myers RM. Serum response factor binding sites differ in three human cell types. Genome Res. 2007;17:136–44. doi: 10.1101/gr.5875007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brass AL, Zhu AQ, Singh H. Assembly requirements of PU.1-Pip (IRF-4) activator complexes: inhibiting function in vivo using fused dimers. EMBO J. 1999;18:977–91. doi: 10.1093/emboj/18.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yee AA, Yin P, Siderovski DP, Mak TW, Litchfield DW, Arrowsmith CH. Cooperative interaction between the DNA-binding domains of PU.1 and IRF4. J Mol Biol. 1998;279:1075–83. doi: 10.1006/jmbi.1998.1838. [DOI] [PubMed] [Google Scholar]
- 156.Goetz TL, Gu TL, Speck NA, Graves BJ. Auto-inhibition of Ets-1 is counteracted by DNA binding cooperativity with core-binding factor alpha 2. Mol Cell Biol. 2000;20:81–90. doi: 10.1128/mcb.20.1.81-90.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gu TL, Goetz TL, Graves BJ, Speck NA. Auto-inhibition and partner proteins, core-binding factor beta (CBF beta) and Ets-1, modulate DNA finding by CBF alpha 2 (AML1) Mol Cell Biol. 2000;20:91–103. doi: 10.1128/mcb.20.1.91-103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wotton D, Ghysdael J, Wang S, Speck NA, Owen MJ. Cooperative binding of Ets-1 and core binding factor to DNA. Mol Cell Biol. 1994;14:840–50. doi: 10.1128/mcb.14.1.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muthusamy N, Barton K, Leiden JM. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature. 1995;377:639–42. doi: 10.1038/377639a0. [DOI] [PubMed] [Google Scholar]
- 160.Mao S, Frank RC, Zhang J, Miyazaki Y, Nimer SD. Functional and physical interactions between AML1 proteins and an ETS protein, MEF: implications for the pathogenesis of t(8;21)-positive leukemias. Mol Cell Biol. 1999;19:3635–44. doi: 10.1128/mcb.19.5.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang H, Yu M, Akie TE, Moran TB, Woo AJ, et al. Differentiation-dependent interactions between RUNX-1 and FLI-1 during megakaryocyte development. Mol Cell Biol. 2009;29:4103–15. doi: 10.1128/MCB.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fitzsimmons D, Lutz R, Wheat W, Chamberlin HM, Hagman J. Highly conserved amino acids in Pax and Ets proteins are required for DNA binding and ternary complex assembly. Nucleic Acids Res. 2001;29:4154–65. doi: 10.1093/nar/29.20.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fitzsimmons D, Lukin K, Lutz R, Garvie CW, Wolberger C, Hagman J. Highly cooperative recruitment of Ets-1 and release of autoinhibition by Pax5. J Mol Biol. 2009;392:452–64. doi: 10.1016/j.jmb.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Golub TR, Barker GF, Stegmaier K, Gilliland DG. The TEL gene contributes to the pathogenesis of myeloid and lymphoid leukemias by diverse molecular genetic mechanisms. Curr Top Microbiol Immunol. 1997;220:67–79. doi: 10.1007/978-3-642-60479-9_5. [DOI] [PubMed] [Google Scholar]
- 165.Cazzaniga G, Daniotti M, Tosi S, Giudici G, Aloisi A, et al. The paired box domain gene PAX5 is fused to ETV6/TEL in an acute lymphoblastic leukemia case. Cancer Res. 2001;61:4666–70. [PubMed] [Google Scholar]
- 166.Fazio G, Palmi C, Rolink A, Biondi A, Cazzaniga G. PAX5/TEL acts as a transcriptional repressor causing down-modulation of CD19, enhances migration to CXCL12, and confers survival advantage in pre-BI cells. Cancer Res. 2008;68:181–89. doi: 10.1158/0008-5472.CAN-07-2778. [DOI] [PubMed] [Google Scholar]
- 167.Baillat D, Begue A, Stehelin D, Aumercier M. ETS-1 transcription factor binds cooperatively to the palindromic head to head ETS-binding sites of the stromelysin-1 promoter by counteracting autoinhibition. J Biol Chem. 2002;277:29386–98. doi: 10.1074/jbc.M200088200. [DOI] [PubMed] [Google Scholar]
- 168.Leprivier G, Baillat D, Begue A, Hartmann B, Aumercier M. Ets-1 p51 and p42 isoforms differentially modulate Stromelysin-1 promoter according to induced DNA bend orientation. Nucleic Acids Res. 2009;37:4341–52. doi: 10.1093/nar/gkp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gotea V, Visel A, Westlund JM, Nobrega MA, Pennacchio LA, Ovcharenko I. Homotypic clusters of transcription factor binding sites are a key component of human promoters and enhancers. Genome Res. 2010;20:565–77. doi: 10.1101/gr.104471.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Helgeson BE, Tomlins SA, Shah N, Laxman B, Cao Q, et al. Characterization of TMPRSS2: ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008;68:73–80. doi: 10.1158/0008-5472.CAN-07-5352. [DOI] [PubMed] [Google Scholar]
- 171.Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396–400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- 172.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–99. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 173.Cai C, Hsieh CL, Omwancha J, Zheng Z, Chen SY, et al. ETV1 is a novel androgen receptorregulated gene that mediates prostate cancer cell invasion. Mol Endocrinol. 2007;21:1835–46. doi: 10.1210/me.2006-0480. [DOI] [PubMed] [Google Scholar]
- 174.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–88. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci USA. 2009;106:12465–70. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, et al. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8:871–78. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang M, Magit D, Sager R. Expression of maspin in prostate cells is regulated by a positive Ets element and a negative hormonal responsive element site recognized by androgen receptor. Proc Natl Acad Sci USA. 1997;94:5673–78. doi: 10.1073/pnas.94.11.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schneikert J, Peterziel H, Defossez PA, Klocker H, Launoit Y, Cato AC. Androgen receptor–Ets protein interaction is a novel mechanism for steroid hormone-mediated down-modulation of matrix metalloproteinase expression. J Biol Chem. 1996;271:23907–13. doi: 10.1074/jbc.271.39.23907. [DOI] [PubMed] [Google Scholar]
- 180.Rickman DS, Chen YB, Banerjee S, Pan Y, Yu J, et al. ERG cooperates with androgen receptor in regulating trefoil factor 3 in prostate cancer disease progression. Neoplasia. 2010;12:1031–40. doi: 10.1593/neo.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shin S, Kim TD, Jin F, van Deursen JM, Dehm SM, et al. Induction of prostatic intraepithelial neoplasia and modulation of androgen receptor by ETS variant 1/ETS-related protein 81. Cancer Res. 2009;69:8102–10. doi: 10.1158/0008-5472.CAN-09-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.May WA, Denny CT. Biology of EWS/FLI and related fusion genes in Ewing’s sarcoma and primitive neuroectodermal tumor. Curr Top Microbiol Immunol. 1997;220:143–50. [PubMed] [Google Scholar]
- 183.Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–65. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 184.Janknecht R. EWS-ETS oncoproteins: the linchpins of Ewing tumors. Gene. 2005;363:1–14. doi: 10.1016/j.gene.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 185.May WA, Lessnick SL, Braun BS, Klemsz M, Lewis BC, et al. The Ewing’s sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol. 1993;13:7393–98. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luo W, Gangwal K, Sankar S, Boucher KM, Thomas D, Lessnick SL. GSTM4 is a microsatellite-containing EWS/FLI target involved in Ewing’s sarcoma oncogenesis and therapeutic resistance. Oncogene. 2009;28:4126–32. doi: 10.1038/onc.2009.262. [DOI] [PubMed] [Google Scholar]
- 187.Gangwal K, Close D, Enriquez CA, Hill CP, Lessnick SL. Emergent properties of EWS/FLI regulation via GGAA microsatellites in Ewing’s sarcoma. Genes Cancer. 2010;1:177–87. doi: 10.1177/1947601910361495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, et al. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS One. 2010;5:e10547. doi: 10.1371/journal.pone.0010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188a.Hollenhorst PC, Paul L, Ferris MW, Graves BJ. The ETS gene ETV4 is required for anchorage independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer. 2011;1:1044–52. doi: 10.1177/1947601910395578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jane-Valbuena J, Widlund HR, Perner S, Johnson LA, Dibner AC, et al. An oncogenic role for ETV1 in melanoma. Cancer Res. 2010;70:2075–84. doi: 10.1158/0008-5472.CAN-09-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jothi R, Cuddapah S, Barski A, Cui K, Zhao K. Genome-wide identification of in vivo protein-DNA binding sites from ChIP-Seq data. Nucleic Acids Res. 2008;36:5221–31. doi: 10.1093/nar/gkn488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wain HM, Bruford EA, Lovering RC, Lush MJ, Wright MW, Povey S. Guidelines for human gene nomenclature. Genomics. 2002;79:464–70. doi: 10.1006/geno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 192.Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 194.Loughran SJ, Kruse EA, Hacking DF, de Graaf CA, Hyland CD, et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol. 2008;9:810–19. doi: 10.1038/ni.1617. [DOI] [PubMed] [Google Scholar]
- 195.Garrett-Sinha LA, Su GH, Rao S, Kabak S, Hao Z, et al. PU.1 and Spi-B are required for normal B cell receptor-mediated signal transduction. Immunity. 1999;10:399–408. doi: 10.1016/s1074-7613(00)80040-0. [DOI] [PubMed] [Google Scholar]
- 196.Zhang Z, Verheyden JM, Hassell JA, Sun X. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell. 2009;16:607–13. doi: 10.1016/j.devcel.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kamath MB, Houston IB, Janovski AJ, Zhu X, Gowrisankar S, et al. Dose-dependent repression of T-cell and natural killer cell genes by PU.1 enforces myeloid and B-cell identity. Leukemia. 2008;22:1214–25. doi: 10.1038/leu.2008.67. [DOI] [PubMed] [Google Scholar]
- 199.Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat Genet. 2006;38:27–37. doi: 10.1038/ng1679. [DOI] [PubMed] [Google Scholar]
- 200.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med. 2005;201:1487–502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–58. [PMC free article] [PubMed] [Google Scholar]
- 202.Houston IB, Kamath MB, Schweitzer BL, Chlon TM, DeKoter RP. Reduction in PU.1 activity results in a block to B-cell development, abnormal myeloid proliferation, and neonatal lethality. Exp Hematol. 2007;35:1056–68. doi: 10.1016/j.exphem.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–77. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 204.Su GH, Chen HM, Muthusamy N, Garrett-Sinha LA, Baunoch D, et al. Defective B cell receptor mediated responses in mice lacking the Ets protein, Spi-B. EMBO J. 1997;16:7118–29. doi: 10.1093/emboj/16.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–21. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the Ets-related factor TEL. EMBO J. 1997;16:4374–83. doi: 10.1093/emboj/16.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, et al. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–41. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ng AY, Waring P, Ristevski S, Wang C, Wilson T, et al. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology. 2002;122:1455–66. doi: 10.1053/gast.2002.32990. [DOI] [PubMed] [Google Scholar]
- 209.Zhou J, Chehab R, Tkalcevic J, Naylor MJ, Harris J, et al. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 2005;24:635–44. doi: 10.1038/sj.emboj.7600538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Donnison M, Beaton A, Davey HW, Broadhurst R, L’Huillier P, Pfeffer PL. Loss of the extraembryonic ectoderm in Elf5 mutants leads to defects in embryonic patterning. Development. 2005;132:2299–308. doi: 10.1242/dev.01819. [DOI] [PubMed] [Google Scholar]
- 211.Lacorazza HD, Miyazaki Y, Di Cristofano A, Deblasio A, Hedvat C, et al. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity. 2002;17:437–49. doi: 10.1016/s1074-7613(02)00422-3. [DOI] [PubMed] [Google Scholar]
- 212.Garrett-Sinha LA, Dahl R, Rao S, Barton KP, Simon MC. PU.1 exhibits partial functional redundancy with Spi-B, but not with Ets-1 or Elf-1. Blood. 2001;97:2908–12. doi: 10.1182/blood.v97.9.2908. [DOI] [PubMed] [Google Scholar]
- 213.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Investig. 2009;119:2914–24. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, et al. The Ets-domain transcription factor SPDEF promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–45. e1–3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 215.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–98. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 216.Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–34. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, et al. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–92. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 218.Laing MA, Coonrod S, Hinton BT, Downie JW, Tozer R, et al. Male sexual dysfunction in mice bearing targeted mutant alleles of the PEA3 Ets gene. Mol Cell Biol. 2000;20:9337–45. doi: 10.1128/mcb.20.24.9337-9345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ayadi A, Zheng H, Sobieszczuk P, Buchwalter G, Moerman P, et al. Net-targeted mutant mice develop a vascular phenotype and up-regulate Egr-1. EMBO J. 2001;20:5139–52. doi: 10.1093/emboj/20.18.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Costello PS, Nicolas RH, Watanabe Y, Rosewell I, Treisman R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nat Immunol. 2004;5:289–98. doi: 10.1038/ni1038. [DOI] [PubMed] [Google Scholar]
- 221.Cesari F, Brecht S, Vintersten K, Vuong LG, Hofmann M, et al. Mice deficient for the Ets transcription factor Elk-1 show normal immuneresponses and mildly impaired neuronal gene activation. Mol Cell Biol. 2004;24:294–305. doi: 10.1128/MCB.24.1.294-305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bories JC, Willerford DM, Grevin D, Davidson L, Camus A, et al. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature. 1995;377:635–38. doi: 10.1038/377635a0. [DOI] [PubMed] [Google Scholar]
- 223.Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–63. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- 224.Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, et al. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–26. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee D, Park C, Lee H, Lugus JJ, Kim SH, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, et al. Nkx2–5 transactivates the Etsrelated protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci USA. 2009;106:814–19. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Papadaki C, Alexiou M, Cecena G, Verykokakis M, Bilitou A, et al. Transcriptional repressor Erf determines extraembryonic ectoderm differentiation. Mol Cell Biol. 2007;27:5201–13. doi: 10.1128/MCB.02237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang XK, Moussa O, LaRue A, Bradshaw S, Molano I, et al. The transcription factor Fli-1 modulates marginal zone and follicular B cell development in mice. J Immunol. 2008;181:1644–54. doi: 10.4049/jimmunol.181.3.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hart A, Melet F, Grossfeld P, Chien K, Jones C, et al. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–77. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 230.Melet F, Motro B, Rossi DJ, Zhang L, Bernstein A. Generation of a novel Fli-1 protein by gene targeting leads to a defect in thymus development and a delay in Friend virus-induced erythroleukemia. Mol Cell Biol. 1996;16:2708–18. doi: 10.1128/mcb.16.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–52. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 233.Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–98. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ristevski S, O’Leary DA, Thornell AP, Owen MJ, Kola I, Hertzog PJ. The ETS transcription factor GABPalpha is essential for early embryogenesis. Mol Cell Biol. 2004;24:5844–49. doi: 10.1128/MCB.24.13.5844-5849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.