Abstract
The sirtuins are a family of proteins that comprise Class III of the histone deacetylases. These NAD+ dependent proteins have been found to be intricately involved in a variety of important and skin-relevant cellular functions and processes, including aging, UV damage response, oxidative stress, and wound repair. In addition, recent research is unraveling the role of sirtuins in a variety of skin diseases, including melanoma and non-melanoma skin cancers. In this review, we provide a discussion on the potential roles and implications of different sirtuins in skin-specific cellular processes, which may have relevance to skin health and skin diseases. Based on the available literature, the sirtuins appear to be important targets in the management of a variety of skin diseases from cosmetic conditions (e.g. skin aging) to fatal conditions (e.g. melanoma).
Keywords: Sirtuins, Skin, HDACs
INTRODUCTION
Sirtuins (SIRTs) comprise one of four classes of histone deacetylases (HDACs; I–IV) that play important roles in a variety of cellular functions. This class III of HDACs is entirely dedicated to the SIRTs, based on their homology to the yeast protein SIR2 (silent information regulator 2), their conserved catalytic domain, and nicotinamide adenine dinucleotide (NAD+) dependence [1, 2]. Seven members of the sirtuin family (SIRTs 1–7) have been identified so far. Although their core domain is conserved, they differ in their N- and C-terminal domains (see Figure 1) [3]. SIRTs are evolutionarily conserved from prokaryotic through eukaryotic cells, and although they are classified as HDACs, the family is responsible for several types of post-translational modifications in both histone and non-histone proteins, which are important for a variety of cellular processes. Some of the non-deacetylase activities for each sirtuin are outlined in Figure 1. Additionally, despite structural similarities, each sirtuin has its own biological niche, performing unique functions via regulating critical mechanisms in the cell.
Figure 1. Sirtuin Function and Localization.
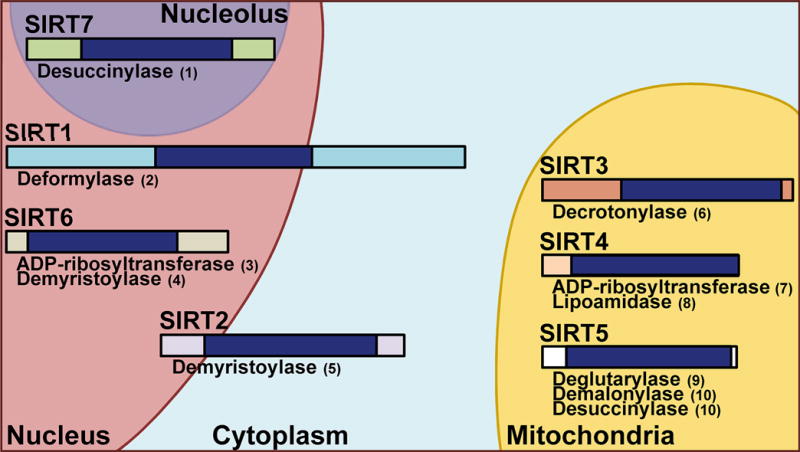
A visual representation of the seven mammalian sirtuins. Relative size and non-deacetylation activities are shown, as well as subcellular location. SIRTs 1 and 2 function in both the nuclear and cytoplasm, with SIRT1 showing more nuclear functions and SIRT2 more cytoplasmic. SIRT 6 is found in the nucleus and SIRT7 is associated with the nucleolus, while SIRTs 3–5 are primarily mitochondrial. Dark blue bands represent the conserved core catalytic domains while surrounding bands indicate the N- and C-terminal regions of each sirtuin. References for the non-deacetylation activities are as follows: (1) [87]; (2) [88]; (3) [89]; (4) [90]; (5) [91]; (6) [92]; (7) [93]; (8) [94]; (9) [95]; (10) [96].
As an indication of the different roles they play biologically, the different sirtuin members have been found to be located in relatively discrete cellular compartments (shown in Figure 1). For example, SIRTs 1, 6 and 7 are located mostly in the nucleus, consistent with their roles in transcription facilitation and epigenetic regulation [4]. SIRT2 is found mainly in the cytoplasm, enabling it to interact with proteins involved in gluconeogenesis, the immune and inflammatory response, and microtubule stabilization [5, 6]. SIRTs 3, 4 and 5 are most commonly found within the mitochondria, which is in accordance with their importance in cellular metabolism (reviewed in [7]). Interestingly, some of these sirtuins have been shown to be both nuclear and cytoplasmic, suggesting nuclear-cytoplasmic shuttling, and intracellular locations and roles which could be dependent on tissue and cell type (discussed in [8]).
While the roles of sirtuins have been explored extensively in other systems, their roles in the skin and skin cancers are less well defined (discussed in [9]). Here, we discuss the function of sirtuins in the skin as it pertains to aging, ultraviolet (UV) radiation damage, and oxidative stress, as well as several skin diseases, with an emphasis on skin cancers.
The skin and the impact of sirtuins on chronological aging
As the largest organ in the body, the skin performs many different and vital functions. Through its complex structure, it acts to both protect the body from damage as well as to provide a surface for external interactions. The functions of the skin range from simple barrier activities to complex endocrine signaling and biomolecule synthesis activities [10]. Damage to the skin can disrupt or alter its ability to fulfill these crucial roles, and can come in many forms, including environmental stresses and physical injuries, and can even be exacerbated by psychosocial stress [10, 11]. Each of these stressors can have different effects on the skin, and some may not show any visible effects for a long time, such as damage from UV exposure and oxidative stress. To complicate matters, some potentially damaging agents like UV radiation may actually be beneficial or even essential to a healthy body when used in moderation (discussed in [12]). To maintain the necessary balance of protection and selective penetrance, the skin must tightly regulate its activities via a complex network of cells, nerves, chemicals, and lipids that are structured in a way to best accomplish its varied roles.
Keratinocytes, melanocytes, Langerhans cells, and Merkel cells make up the majority of the cells in the skin. The importance of each of these cells, along with the structure, function, and key molecules present in the skin are discussed in depth and explained in an article on the integumentary system by McLafferty et al [10]. Briefly, the skin consists of a series of layers, ranging from the external epidermis to the dermis. The outermost layer of the skin, the epidermis, consists mainly of keratinocytes and is responsible for the bulk of its barrier functions. (reviewed in [13]). Underneath the epidermis is the dermis. This layer contains the structural and nutritive components necessary to support a healthy epidermis, as well as the various vessels, glands, and nerves that make the skin so complex [10]. The close proximity of the skin to the environment facilitates aging, cellular and tissue damage, wounds, and diseases, all of which appear to be linked to the sirtuins.
From the time of their discovery, the sirtuins have been implicated in the process of aging. Preceding their identification in mammals, lifespan extension via caloric restriction was widely studied as one of the primary functions of the yeast homolog protein Sir2 (reviewed in[14]). It was initially thought that this would translate to mammals, and although many reports have found similar results, several other studies have found no effects of sirtuins on lifespan [15–20]. There was more consistent success when using small molecule activators, but initial successes, as with some of the initial yeast and fly studies [21], were likely due to off-target effects [22, 23]. Thus, the final verdict on the ability of sirtuins to directly affect aging remains an ongoing quest (reviewed in [19, 24]). However, beyond direct effects, sirtuins have been found to interact with other major longevity factors, including AMP-activated protein kinase (AMPK), phosphatidylinositol-3-OH kinase (PI3K), insulin like growth factor 1 (IGF1), and mechanistic target of rapamycin (mTOR) (reviewed in [25]). Indeed, the process of aging itself is still not clearly understood and includes aspects of lifespan, cellular changes (such as apoptosis, protein modifications, and increased senescence), and cosmetic changes. Prevalent theories for these changes include decreased telomere length from repeated cell division, exhaustion of a limited neuronal or stem cell population, accumulation of cellular toxins over time, or repeated DNA damage from environmental stresses [26–29]. Because of findings supporting their role in longevity, there is abundant evidence regarding the link between sirtuins and aging throughout the body (reviewed in [30]).
In the skin, the physical characteristics associated with aging are visually apparent. Aged skin becomes thin and shows an increase in wrinkles, as well as a decrease in hydration and skin elasticity [31, 32]. Interestingly, researchers have found that several sirtuins have a changed expression profile depending on the age of the person or cell line being studied. For example, a 2014 study found that SIRT1 levels decreased with age in dermal fibroblasts isolated from female donors who ranged from 20–67 years old [33]. Another study found that both SIRT1 and SIRT6 levels decreased in human dermal fibroblasts with higher passage numbers, and that these levels were associated with aging biomarkers found in the same cells [34]. Since fibroblasts are involved in production of the extracellular matrix in the skin, these studies suggest that decreasing SIRT levels in aged fibroblasts may have an effect on the chronological aging processes. To take this a step further, Sharma et al found that there was a greater resistance in human dermal fibroblasts from older human subjects to reprogramming using classical Yamanaka factors, as well as higher SIRT6 levels in younger cells [35]. However, they found that adding SIRT6 while reprogramming allowed the aged cells to have an increased reprogramming efficiency. These findings taken together suggest that both SIRT1 and SIRT6 are important molecules in skin cell aging, and warrant further exploration, especially because skin health is considered a factor representing overall “good health”.
The role of sirtuins in UV radiation and photoaging
Premature development of the physiological changes observed in chronological skin aging, as well as uneven pigmentation, a deepening of wrinkles, and a rough texture can occur as the result of the process known as photoaging [31, 32]. Photoaging is strongly correlated with sun exposure, with both UVA (320–400 nm) and UVB (290–320 nm) radiation contributing to its progression [36]. Most UVB is absorbed in the epidermis, where it causes sunburn and damages cellular DNA through the formation of cyclobutane pyrimidine dimers and pyrimidine (6–4) pyrimidone photoproducts [37, 38]. DNA damage that is not adequately repaired can lead to increased cellular senescence, apoptosis, and carcinogenesis [37]. UVA penetrates deeper into the dermal layer, damaging DNA, proteins, and lipids indirectly through the generation of reactive oxygen species (ROS), as well as breaking down collagen through the activation of the tissue remodeling matrix metalloproteinases (MMPs) [38]. Sirtuins play a role in both UVA and UVB-mediated events, suggesting that they could be key participants in photoaging.
SIRT1 has been shown to play a role in photoaging, especially through the inhibition of MMPs and subsequent collagen degradation. The SIRT1 activators resveratrol and metformin have been shown to inhibit MMP-9 and prevent collagen degradation when applied to human fibroblast cells or mouse skin prior to UV radiation exposure [39]. Resveratrol has similarly been shown to inhibit MMP-1 expression, whereas SIRT1 knockdown increases MMP-1 and -3 levels [40]. This protective role of SIRT1 in UV-induced photoaging has also been found in clinical samples, as SIRT1 and MMP-1 expression has been shown to increase in response to UVA radiation in both human skin in vivo, as well as human fibroblasts in vitro [41, 42]. Together, this suggests that SIRT1 activators have therapeutic potential in photoaging prevention.
The case for SIRT1 involvement in UVB-mediated DNA damage has been demonstrated in several in vitro experiments using human fibroblasts. UVB radiation has been shown to decrease SIRT1 protein levels in these cells [43]. Interestingly, the natural compound juglone (5-hydroxy-1,4-napthalenedione) which is found in several plants, has been shown to restore SIRT1 to normal levels after UVB treatment, suggesting that SIRT1 might play a role in preventing UVB-induced carcinogenesis [43]. Overexpressing SIRT1 in human fibroblasts reinforces this possibility, as it results in protection from UVB-induced cellular senescence and oxidative stress, presumably through the suppression of p53 acetylation [44]. However, in vivo studies show that the SIRT1 story is more complex, and the level of SIRT1 expression is critical for its role in UVB protection. Contrary to the in vitro findings in fibroblasts, keratinocyte-specific homozygous SIRT1 deletion suppresses skin cancer development in mice via p53 activation and UVB-induced apoptosis, whereas heterozygous SIRT1 deletion promotes UVB-induced skin carcinogenesis [45]. Thus, as has been seen in many of the cancer studies to date, the role of SIRT1 as a tumor promoter or suppressor in UVB-induced cancer initiation is unclear, and might vary with cell/tissue type or protein levels.
Research on the role of the remaining sirtuins in UV-damage response is limited. Lang et al have shown that SIRT4 levels increase in fibroblasts exposed to UVB radiation in vitro, and this correlates with an increase in cellular senescence [46]. This finding was corroborated in vivo by an observed elevation in SIRT4 levels in naturally photoaged human skin samples [46]. Benavente et al have shown that solar simulated light (containing both UVA and UVB) induces upregulation of both SIRTs 1 and 4 mRNAs, which appear to play roles in resistance to photodamage [47]. SIRT6 has also been shown to increase in human keratinocytes in response to UVB exposure, and silencing its expression results in increased UVB-induced apoptosis in these cells [48]. This suggests that SIRTs 4 and 6 play protective roles in the UVB damage response.
Connections between oxidative stress and sirtuins in the skin
The relationship between aging and UV exposure in the skin is closely intertwined with oxidative stress, as thoroughly reviewed by Kammeyer and Rinnerthaler [37, 49]. Briefly, ROS are generated in UVA-exposed skin through the excitation of photosensitizers, which then transfer energy to molecular oxygen to produce superoxide anions, hydroxyl radicals, or singlet oxygen. These ROS have the capability to cause significant cellular damage, but also have a functional role in molecular signaling pathways. Endogenous controls for cellular damage include the conversion of ROS into less reactive species such as when superoxide dismutase (SOD) reacts with superoxide (SOX) anions to form hydrogen peroxide (H2O2). Although H2O2 is less reactive than other ROS, it still has the capability to cause oxidative stress through subsequent conversion to more harmful compounds, and due to its increased stability, it is frequently used to induce oxidative stress experimentally. Studies have shown that H2O2-induced oxidative stress correlates with a decrease in SIRT1 levels in keratinocytes [50]. Treatment with the SIRT1 activator resveratrol has been shown to prevent H2O2-induced cell death, decreased proliferation, and suppresses senescence, whereas SIRT1 inhibitors sirtinol and nicotinamide enhance H2O2-induced cell death [50, 51]. Keratinocytes can also be protected from H2O2-damage and autophagy via melatonin treatment, an effect that is reversed through SIRT1 siRNA or sirtinol treatment [52]. Together, these data suggest that SIRT1 is an active player in the prevention of H2O2-induced cell damage, although the mechanism is complex. Mechanistically, JNK signaling has been implicated upstream of SIRT1, and p53 has been shown to function downstream in H2O2-induced keratinocyte death [50, 53]. Studies have also suggested a coordinating role between SIRT1 and AMPK in the downstream activation of FOXO3 that affects H2O2-induced cellular senescence and proliferation, as well as interactions between SIRT1 and FOXO3a in UVB-induced oxidative stress resistance. [44, 51]. Interestingly, SIRT2 has also been shown to target FOXO3a in mouse fibroblasts, thereby regulating manganese superoxide dismutase (MnSOD), decreasing H2O2-induced ROS, and promoting cell death [54]. Thus, multiple signaling pathways, and multiple sirtuins seem to be involved in the cellular response to oxidative stress in the skin.
SIRT3 has been shown to play a role in skin maintenance through oxidative stress-induced keratinocyte differentiation, a process that is crucial for skin regeneration, maintenance, and is important in skin disease. Bause et al have shown that the process is strongly linked to SIRT3 expression, as its knockdown induces differentiation through increased ROS, and increases mitochondrial SOX generation in response to H2O2 treatment [55]. This role for SIRT3 in oxidative stress regulation might extend to stress induced by environmental stressors, as a recent study showed that ozone exposure results in decreased SIRT3 levels, correlating with increased cellular H2O2, reduced SOD2, and increased DNA damage [56]. Thus, SIRT3 may be involved in the management of oxidative stress in more than one process that is critical for normal skin maintenance.
Role of sirtuins in wound healing and other skin diseases
In addition to their roles in aging, UV damage, and oxidative stress, the sirtuins affect skin health in a number of different disease backgrounds. In 2013, Serravallo et al wrote a very thorough, detailed review on the topic, covering inflammatory, autoimmune, and hyperproliferative skin diseases, as well as cutaneous infections, inherited diseases, and cancer [9]. Since then, a number of studies have expanded on the role of sirtuins in these areas.
Wound healing in the skin is impaired by knockdown or accelerated by activation of several different sirtuins. Activation of SIRTs 1, 2, and 3 through treatment with MC2562, or SIRT1 activation via resveratrol accelerate wound repair in a mouse model through increased keratinocyte proliferation [57]. In addition, SIRT1 knockdown at the wound site via shRNA results in dense, disordered collagen fibers during healing similar to those seen in hypertrophic scar formation, whereas collagen fibers similar to those seen in normal wound healing are observed after resveratrol treatment at the site [58]. SIRT6 knockdown in a diabetic mouse background further exacerbates the impaired wound healing associated with the db/db phenotype [25]. SIRT7−/− mice also show impaired wound healing in an otherwise wild type background [59]. Thus, it is likely that further exploration of sirtuins could lead to new treatments for disease-induced impairment of wound healing, or aid in the minimization of scar formation.
In addition to its role in wound healing, research has recently linked SIRT1 to several different skin diseases. Its activation via resveratrol has been shown to improve psoriasis in humans [60] and an induced psoriasis-like inflammation in mice [61]. SIRT1 is downregulated in systemic sclerosis and likely plays a role in the regulation of fibroblast activation in the disease via TGF-P signaling [62, 63]. SIRT1 also appears to play a protective role in vitiligo [64], and aids in maintaining skin barrier integrity [65]. These recent findings underscore the importance of sirtuins in skin diseases and provide new avenues of treatment for these common ailments. Finally, advances regarding the role of SIRT1, as well as several other sirtuins, have been made in both melanoma and non-melanoma skin cancers as discussed in the following section.
Influences of sirtuins in skin cancer
Skin cancer is one of the major health problems in the world, as 3.5 million cases of non-melanoma skin cancer (NMSC) are estimated to be diagnosed in 2016 in the United States alone, along with more than 76,000 cases of melanoma [66]. Melanoma develops solely from melanocytes while NMSCs arise from other cells in the skin. NMSCs such as basal cell and cutaneous squamous cell carcinomas (BCC and SCC, respectively) are unlikely to metastasize and can generally be removed by a dermatologist, whereas melanoma can be more dangerous because it can rapidly metastasize if not diagnosed and removed in its early stages. To date, surgical and pharmacological treatment of melanoma has not been sufficiently effective. Currently, the most widely used melanoma treatments are immunotherapy and BRAF/MAPK pathway inhibitors [67, 68]. While immunotherapies are quite encouraging, they are successful in only a subset of melanoma patients and are associated with the induction of autoimmune and pro-inflammatory side effects [68]. BRAF/MAPK pathway inhibitors fail to control melanomas in the long term, with most patients acquiring resistance after approximately twelve months of treatment and subsequent cancer recurrence (reviewed in [69]). Melanoma also develops resistance to other commonly used antineoplastic agents, such as doxorubicin, that are extremely successful in other cancers. Thus, the development of novel therapeutics for melanoma treatment is critical, and sirtuins are among the proteins being investigated as potential targets in both melanoma and NMSCs.
Melanoma
Targeting sirtuins as therapeutics for melanoma treatment is complicated by the fact that sirtuins have been found to work as both tumor suppressors and promoters depending on several factors, including cell and tissue type (reviewed in [70–73]). Research on sirtuins in melanoma is still in an early enough stage that their function as tumor suppressors or promoters cannot be defined with certainty, but studies to date support the latter. The first three studies on sirtuin function in melanoma were published concurrently in 2014. Kunimoto et al showed that SIRT1 is involved in lamellipodium extension and Akt-dependent melanoma cell migration [74]. This could indicate potential for SIRT1 inhibition in the development of therapeutics to limit melanoma metastasis. Data from the remaining two studies showed SIRT1 overexpression and increased activity in melanoma cells and tissues [75, 76]. Inhibition via siRNA or tenovin-1 decreased proliferation and clonogenic survival, and increased G0/G1 cell cycle arrest and senescence-like properties, suggesting a role for SIRT1 as an oncogene. Mechanistically, MITF was shown to be an upstream regulator of SIRT1, and p53 and p21 were shown to be activated downstream upon SIRT1 inhibition. However, the network of proteins involved is complex, and a further study found that there may be a link between SIRT1 inhibition and the BUB family of cell cycle regulating proteins [77]. This suggests that SIRT1 acts through several pathways in its promotion of cancer growth and more research into this network is needed.
Currently, limited information is available regarding the role of other sirtuins in melanoma. SIRT2 has shown potential as a regulator of several cancer progression genes in a study by Karwaciak et al, and may be a good candidate for dual therapy when combined with doxorubicin as it decreases resistance to the antineoplastic agent and reduces the dosage needed for effect [78]. Interestingly, it was recently shown that loss of SIRT2 led to drug resistance in melanoma through BRAF and MEK inhibitors [79]. It was shown that loss of SIRT2 lead to resistance of the BRAF inhibitor vemurafenib in A375 melanoma cells. In addition, inhibition of SIRT2 in melanoma was found to decrease colony formation ability. This suggests that SIRT2 may be a key contributor in the process of drug resistance in melanoma, as well as a regulator of cell growth. Taken together, these studies suggest a role for SIRT2 in melanoma progression and drug resistance.
The role of SIRT3 in melanoma has only been assessed in a recently published study from our laboratory, where we found that SIRT3 is overexpressed in both human melanoma cell lines and clinical tissue samples. Knockdown of SIRT3 in highly expressing melanoma lines via shRNA decreased cell growth and migration, colony formation, and induced senescence in vitro, as well as reduced tumor growth in a melanoma xenograft mouse model [80]. These anti-proliferative changes were accompanied by a G1-phase cell cycle arrest, as well as decreased expression of several cyclins and cyclin-dependent kinases. Interestingly, forced overexpression of SIRT3 in a melanoma line that had lower endogenous levels of SIRT3 had the opposite impact, leading to increased proliferation. Taken together, these data suggest that SIRT3 plays an oncogenic role in melanoma. This makes it likely that targeting SIRT3, as well as the other sirtuins, may lead to potential melanoma treatments.
Squamous Cell Carcinoma
While most studies on sirtuins in skin cancer focus on melanoma, recent research has also investigated their role in skin cancer’s less deadly forms. Cutaneous squamous cell carcinomas (SCCs) are generally less aggressive than melanoma, but can become metastatic if left untreated [81]. Thus, new therapeutic targets are needed to counter these neoplasms. Recent research suggests that sirtuins could prove useful in this respect, as several studies support oncogenic sirtuin function in SCC. All seven sirtuins have been shown to be overexpressed at the mRNA level in SCC tissue samples, as well as several sirtuins in an SCC cell line (A431; SIRTs 1 and 3) and actinic keratosis (SIRTs 2, 3, 5–7) [47]. At the protein level, overexpression of SIRT6 has also been observed, and skin-specific deletion of SIRT6 in mice inhibits skin tumorigenesis via a mechanism involving COX-2 suppression [48]. However, not all evidence supports oncogenic sirtuin function in SCC. Contrary to the findings of SIRT2 overexpression at the mRNA level, a separate study has shown that SIRT2 protein is downregulated in SCC, and further, that SIRT2 knockdown by siRNA increases the risk of cancer [82]. Thus, the role of the sirtuins in SCC of the skin is unclear, and further elucidation of the mechanisms involved is necessary to assess their potential as novel therapeutics in the treatment of SCC.
Basal Cell Carcinoma
Although it is the most common skin cancer, BCC is rarely fatal. In most cases, BCCs are successfully treated with surgery or radiation therapy. However, some patients cannot undergo these treatments for various reasons, including location and complexity of the tumor [83]. Without proper treatment, BCCs may become more aggressive, invading surrounding tissues and even metastasizing [84, 85]. Therefore, novel molecular targets are needed to treat complex cases of BCC, and a recent study suggests sirtuins as potential candidates. The study shows expression of all seven sirtuins in BCC patient samples, with SIRTs 2 and 3 showing downregulation relative to patient-matched normal tissue [86]. This finding suggests that SIRTs 2 and 3 may play a role in BCC pathogenesis, and further investigation could lead to their use as therapeutic targets or prognostic markers.
CONCLUSION
Since Sir2 was discovered in yeast, an abundance of research has shown a very important role of this class of HDACs in a variety of physiological functions and disease conditions. In the skin, several sirtuins have been found to play important roles in aging as well as in UV damage and oxidative stress responses. Additionally, recent research has implicated sirtuins in many skin conditions, including psoriasis and skin malignancies. Although the study of skin-based roles of sirtuins is relatively new, exploring this family of proteins further may lead to the development of novel therapeutics in skin disorders, as well as in both melanoma and non-melanoma skin cancer management.
Acknowledgments
This work was partially supported by funding from the National Institutes of Health (R01AR059130 and R01CA176748) and the Department of Veterans Affairs (VA Merit Review Awards I01BX001008 and I01CX00144; and a Research Career Scientist Award IK6BX003780 to NA).
ABBREVIATIONS
- Akt
v-akt murine thymoma viral oncogene homolog 1
- AMPK
5′-prime-AMP-activated protein kinase
- BCC
basal cell carcinoma
- BRAF
v-raf murine sarcoma viral oncogene homolog B
- BUB
budding uninhibited by benzimidazoles (yeast homolog)
- COX-2
cytochrome c oxidase subunit II
- H2O2
hydrogen peroxide
- HDAC
histone deacetylase
- IGF1
Insulin-like growth factor
- JNK
JUN N-terminal kinase
- MAPK
mitogen activated kinase-like protein
- MEK
Mitogen-activated protein kinase kinase
- MITF
microphthalmia-associated transcription factor
- MMP
matrix metalloproteinase
- MnSOD
manganese superoxide dismutase
- mTOR
Mechanistic target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- NMSC
non-melanoma skin cancer
- PI3K
Phosphatidylinositol-3-OH kinase
- ROS
reactive oxygen species
- SCC
squamous cell carcinoma
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SIRT
Sirtuin
- SOD
superoxide dismutase
- SOD2
superoxide dismutase 2
- SOX
superoxide
- TGF-β
transforming growth factor beta
- UV
ultraviolet
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest
References
- 1.Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 2.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 3.Kleszcz R, Paluszczak J, Baer-Dubowska W. Targeting aberrant cancer metabolism - The role of sirtuins. Pharmacol Rep. 2015;67:1068–1080. doi: 10.1016/j.pharep.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Kim W, Kim JE. SIRT7 an emerging sirtuin: deciphering newer roles. J Physiol Pharmacol. 2013;64:531–534. [PubMed] [Google Scholar]
- 5.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Zhu Y, Ozden O, Kim HS, Jiang H, Deng CX, Gius D, Vassilopoulos A. SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis. Transl Cancer Res. 2012;1:15–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 9.Serravallo M, Jagdeo J, Glick SA, Siegel DM, Brody NI. Sirtuins in dermatology: applications for future research and therapeutics. Arch Dermatol Res. 2013;305:269–282. doi: 10.1007/s00403-013-1320-2. [DOI] [PubMed] [Google Scholar]
- 10.McLafferty E, Hendry C, Alistair F. The integumentary system: anatomy, physiology and function of skin. Nurs Stand. 2012;27:35–42. doi: 10.7748/ns2012.10.27.7.35.c9358. [DOI] [PubMed] [Google Scholar]
- 11.Hunter HJ, Momen SE, Kleyn CE. The impact of psychosocial stress on healthy skin. Clin Exp Dermatol. 2015;40:540–546. doi: 10.1111/ced.12582. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Sunlight, ultraviolet radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16. [PubMed] [Google Scholar]
- 13.Boer M, Duchnik E, Maleszka R, Marchlewicz M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol Alergol. 2016;33:1–5. doi: 10.5114/pdia.2015.48037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 18.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, de Cabo R, Cohen HY. Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett. 2008;582:543–548. doi: 10.1016/j.febslet.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longo VD. Linking sirtuins, IGF-I signaling, and starvation. Exp Gerontol. 2009;44:70–74. doi: 10.1016/j.exger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–1634. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang W. The controversial world of sirtuins. Drug Discov Today Technol. 2014;12:e9–e17. doi: 10.1016/j.ddtec.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thandavarayan RA, Garikipati VN, Joladarashi D, Suresh Babu S, Jeyabal P, Verma SK, Mackie AR, Khan M, Arumugam S, Watanabe K, Kishore R, Krishnamurthy P. Sirtuin-6 deficiency exacerbates diabetes-induced impairment of wound healing. Exp Dermatol. 2015;24:773–778. doi: 10.1111/exd.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passarino G, De Rango F, Montesanto A. Human longevity: Genetics or Lifestyle? It takes two to tango. Immun Ageing. 2016;13:12. doi: 10.1186/s12979-016-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillin A, Gottschling DE, Nystrom T. The good and the bad of being connected: the integrons of aging. Curr Opin Cell Biol. 2014;26:107–112. doi: 10.1016/j.ceb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watroba M, Szukiewicz D. The role of sirtuins in aging and age-related diseases. Adv Med Sci. 2016;61:52–62. doi: 10.1016/j.advms.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Durai PC, Thappa DM, Kumari R, Malathi M. Aging in elderly: chronological versus photoaging. Indian J Dermatol. 2012;57:343–352. doi: 10.4103/0019-5154.100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richey ML, Richey HK, Fenske NA. Aging-related skin changes: development and clinical meaning. Geriatrics. 1988;43:49–52. 57–59, 63–64. [PubMed] [Google Scholar]
- 33.Kalfalah F, Sobek S, Bornholz B, Gotz-Rosch C, Tigges J, Fritsche E, Krutmann J, Kohrer K, Deenen R, Ohse S, Boerries M, Busch H, Boege F. Inadequate mito-biogenesis in primary dermal fibroblasts from old humans is associated with impairment of PGC1A-independent stimulation. Exp Gerontol. 2014;56:59–68. doi: 10.1016/j.exger.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Kim KS, Park HK, Lee JW, Kim YI, Shin MK. Investigate correlation between mechanical property and aging biomarker in passaged human dermal fibroblasts. Microsc Res Tech. 2015;78:277–282. doi: 10.1002/jemt.22472. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, Chua KF, Wu JC. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem. 2013;288:18439–18447. doi: 10.1074/jbc.M112.405928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han A, Chien AL, Kang S. Photoaging. Dermatol Clin. 2014;32:291–299, vii. doi: 10.1016/j.det.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Baron ED, Suggs AK. Introduction to photobiology. Dermatol Clin. 2014;32:255–266, vii. doi: 10.1016/j.det.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Lee JS, Park KY, Min HG, Lee SJ, Kim JJ, Choi JS, Kim WS, Cha HJ. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Exp Dermatol. 2010;19:1060–1066. doi: 10.1111/j.1600-0625.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 40.Ohguchi K, Itoh T, Akao Y, Inoue H, Nozawa Y, Ito M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br J Dermatol. 2010;163:689–694. doi: 10.1111/j.1365-2133.2010.09825.x. [DOI] [PubMed] [Google Scholar]
- 41.Niu T, Tian Y, Ren Q, Wei L, Li X, Cai Q. Red light interferes in UVA-induced photoaging of human skin fibroblast cells. Photochem Photobiol. 2014;90:1349–1358. doi: 10.1111/php.12316. [DOI] [PubMed] [Google Scholar]
- 42.Tian Y, Liu W, Niu T, Dai C, Li X, Cui C, Zhao X, E Y, Lu H. The injury and cumulative effects on human skin by UV exposure from artificial fluorescence emission. Photochem Photobiol. 2014;90:1433–1438. doi: 10.1111/php.12315. [DOI] [PubMed] [Google Scholar]
- 43.Wahedi HM, Lee TH, Moon EY, Kim SY. Juglone up-regulates sirt1 in skin cells under normal and UVB irradiated conditions. J Dermatol Sci. 2016;81:210–212. doi: 10.1016/j.jdermsci.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Chung KW, Choi YJ, Park MH, Jang EJ, Kim DH, Park BH, Yu BP, Chung HY. Molecular Insights into SIRT1 Protection Against UVB-Induced Skin Fibroblast Senescence by Suppression of Oxidative Stress and p53 Acetylation. J Gerontol A Biol Sci Med Sci. 2015;70:959–968. doi: 10.1093/gerona/glu137. [DOI] [PubMed] [Google Scholar]
- 45.Ming M, Soltani K, Shea CR, Li X, He YY. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene. 2015;34:357–363. doi: 10.1038/onc.2013.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang A, Grether-Beck S, Singh M, Kuck F, Jakob S, Kefalas A, Altinoluk-Hambuchen S, Graffmann N, Schneider M, Lindecke A, Brenden H, Felsner I, Ezzahoini H, Marini A, Weinhold S, Vierkotter A, Tigges J, Schmidt S, Stuhler K, Kohrer K, Uhrberg M, Haendeler J, Krutmann J, Piekorz RP. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging (Albany NY) 2016;8:484–509. doi: 10.18632/aging.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benavente CA, Schnell SA, Jacobson EL. Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin. PLoS One. 2012;7:e42276. doi: 10.1371/journal.pone.0042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ming M, Han W, Zhao B, Sundaresan NR, Deng CX, Gupta MP, He YY. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014;74:5925–5933. doi: 10.1158/0008-5472.CAN-14-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5:545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, Kouttab N, Xu A, Wan Y. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009;13:3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ido Y, Duranton A, Lan F, Weikel KA, Breton L, Ruderman NB. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One. 2015;10:e0115341. doi: 10.1371/journal.pone.0115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JH, Moon JH, Nazim UM, Lee YJ, Seol JW, Eo SK, Park SY. Melatonin protects skin keratinocyte from hydrogen peroxide-mediated cell death via the SIRT1 pathway. Oncotarget. 2016;7:12075–12088. doi: 10.18632/oncotarget.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johns RA. Endothelium-derived relaxing factor: basic review and clinical implications. J Cardiothorac Vasc Anesth. 1991;5:69–79. doi: 10.1016/1053-0770(91)90099-f. [DOI] [PubMed] [Google Scholar]
- 54.Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 55.Bause AS, Matsui MS, Haigis MC. The protein deacetylase SIRT3 prevents oxidative stress-induced keratinocyte differentiation. J Biol Chem. 2013;288:36484–36491. doi: 10.1074/jbc.M113.472324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarthy JT, Pelle E, Dong K, Brahmbhatt K, Yarosh D, Pernodet N. Effects of ozone in normal human epidermal keratinocytes. Exp Dermatol. 2013;22:360–361. doi: 10.1111/exd.12125. [DOI] [PubMed] [Google Scholar]
- 57.Spallotta F, Cencioni C, Straino S, Nanni S, Rosati J, Artuso S, Manni I, Colussi C, Piaggio G, Martelli F, Valente S, Mai A, Capogrossi MC, Farsetti A, Gaetano C. A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J Biol Chem. 2013;288:11004–11012. doi: 10.1074/jbc.M112.441816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai XZ, Liu JQ, Yang LL, Fan L, He T, Su LL, Shi JH, Tang CW, Zheng Z, Hu DH. Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. Br J Pharmacol. 2016;173:1589–1601. doi: 10.1111/bph.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araki S, Izumiya Y, Rokutanda T, Ianni A, Hanatani S, Kimura Y, Onoue Y, Senokuchi T, Yoshizawa T, Yasuda O, Koitabashi N, Kurabayashi M, Braun T, Bober E, Yamagata K, Ogawa H. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-beta Signaling Pathway. Circulation. 2015;132:1081–1093. doi: 10.1161/CIRCULATIONAHA.114.014821. [DOI] [PubMed] [Google Scholar]
- 60.Krueger JG, Suarez-Farinas M, Cueto I, Khacherian A, Matheson R, Parish LC, Leonardi C, Shortino D, Gupta A, Haddad J, Vlasuk GP, Jacobson EW. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLoS One. 2015;10:e0142081. doi: 10.1371/journal.pone.0142081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kjaer TN, Thorsen K, Jessen N, Stenderup K, Pedersen SB. Resveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in mice. PLoS One. 2015;10:e0126599. doi: 10.1371/journal.pone.0126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei J, Ghosh AK, Chu H, Fang F, Hinchcliff ME, Wang J, Marangoni RG, Varga J. The Histone Deacetylase Sirtuin 1 Is Reduced in Systemic Sclerosis and Abrogates Fibrotic Responses by Targeting Transforming Growth Factor beta Signaling. Arthritis Rheumatol. 2015;67:1323–1334. doi: 10.1002/art.39061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zerr P, Palumbo-Zerr K, Huang J, Tomcik M, Sumova B, Distler O, Schett G, Distler JH. Sirt1 regulates canonical TGF-beta signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis. 2016;75:226–233. doi: 10.1136/annrheumdis-2014-205740. [DOI] [PubMed] [Google Scholar]
- 64.Becatti M, Fiorillo C, Barygina V, Cecchi C, Lotti T, Prignano F, Silvestro A, Nassi P, Taddei N. SIRT1 regulates MAPK pathways in vitiligo skin: insight into the molecular pathways of cell survival. J Cell Mol Med. 2014;18:514–529. doi: 10.1111/jcmm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ming M, Zhao B, Shea CR, Shah P, Qiang L, White SR, Sims DM, He YY. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. J Allergy Clin Immunol. 2015;135:936–945 e934. doi: 10.1016/j.jaci.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 67.Shelledy L, Roman D. Vemurafenib: First-in-Class BRAF-Mutated Inhibitor for the Treatment of Unresectable or Metastatic Melanoma. J Adv Pract Oncol. 2015;6:361–365. doi: 10.6004/jadpro.2015.6.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaufman HL, Kirkwood JM, Hodi FS, Agarwala S, Amatruda T, Bines SD, Clark JI, Curti B, Ernstoff MS, Gajewski T, Gonzalez R, Hyde LJ, Lawson D, Lotze M, Lutzky J, Margolin K, McDermott DF, Morton D, Pavlick A, Richards JM, Sharfman W, Sondak VK, Sosman J, Steel S, Tarhini A, Thompson JA, Titze J, Urba W, White R, Atkins MB. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol. 2013;10:588–598. doi: 10.1038/nrclinonc.2013.153. [DOI] [PubMed] [Google Scholar]
- 69.Welsh SJ, Rizos H, Scolyer RA, Long GV. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: Where to next? Eur J Cancer. 2016;62:76–85. doi: 10.1016/j.ejca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Wilking MJ, Ahmad N. The role of SIRT1 in cancer: the saga continues. Am J Pathol. 2015;185:26–28. doi: 10.1016/j.ajpath.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, Fu LL, Wen X, Wang XY, Liu J, Cheng Y, Huang J. Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis. 2014;5:e1047. doi: 10.1038/cddis.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stunkel W, Campbell RM. Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen. 2011;16:1153–1169. doi: 10.1177/1087057111422103. [DOI] [PubMed] [Google Scholar]
- 73.Fang Y, Nicholl MB. Sirtuin 1 in malignant transformation: friend or foe? Cancer Lett. 2011;306:10–14. doi: 10.1016/j.canlet.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 74.Kunimoto R, Jimbow K, Tanimura A, Sato M, Horimoto K, Hayashi T, Hisahara S, Sugino T, Hirobe T, Yamashita T, Horio Y. SIRT1 regulates lamellipodium extension and migration of melanoma cells. J Invest Dermatol. 2014;134:1693–1700. doi: 10.1038/jid.2014.50. [DOI] [PubMed] [Google Scholar]
- 75.Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014;563:94–100. doi: 10.1016/j.abb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohanna M, Bonet C, Bille K, Allegra M, Davidson I, Bahadoran P, Lacour JP, Ballotti R, Bertolotto C. SIRT1 promotes proliferation and inhibits the senescence-like phenotype in human melanoma cells. Oncotarget. 2014;5:2085–2095. doi: 10.18632/oncotarget.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh CK, George J, Nihal M, Sabat G, Kumar R, Ahmad N. Novel downstream molecular targets of SIRT1 in melanoma: a quantitative proteomics approach. Oncotarget. 2014;5:1987–1999. doi: 10.18632/oncotarget.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karwaciak I, Gorzkiewicz M, Ryba K, Dastych J, Pulaski L, Ratajewski M. AC-93253 triggers the downregulation of melanoma progression markers and the inhibition of melanoma cell proliferation. Chem Biol Interact. 2015;236:9–18. doi: 10.1016/j.cbi.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 79.Bajpe PK, Prahallad A, Horlings H, Nagtegaal I, Beijersbergen R, Bernards R. A chromatin modifier genetic screen identifies SIRT2 as a modulator of response to targeted therapies through the regulation of MEK kinase activity. Oncogene. 2015;34:531–536. doi: 10.1038/onc.2013.588. [DOI] [PubMed] [Google Scholar]
- 80.George J, Nihal M, Singh CK, Zhong W, Liu X, Ahmad N. Pro-Proliferative Function of Mitochondrial Sirtuin Deacetylase SIRT3 in Human Melanoma. J Invest Dermatol. 2016;136:809–818. doi: 10.1016/j.jid.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazareth V. Management of non-melanoma skin cancer. Semin Oncol Nurs. 2013;29:182–194. doi: 10.1016/j.soncn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Ming M, Qiang L, Zhao B, He YY. Mammalian SIRT2 inhibits keratin 19 expression and is a tumor suppressor in skin. Exp Dermatol. 2014;23:207–209. doi: 10.1111/exd.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohan SV, Chang AL. Advanced Basal Cell Carcinoma: Epidemiology and Therapeutic Innovations. Curr Dermatol Rep. 2014;3:40–45. doi: 10.1007/s13671-014-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maroun C, Alam E, Khalifeh I, Abbas O, Moukarbel RV. Nasal Basal Cell Carcinoma with Matrical Differentiation: Risk of Metastasis and Impact on Management. Head Neck Pathol. 2016 doi: 10.1007/s12105-016-0739-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lear JT, Corner C, Dziewulski P, Fife K, Ross GL, Varma S, Harwood CA. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476–1481. doi: 10.1038/bjc.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Temel M, Koc MN, Ulutas S, Gogebakan B. The expression levels of the sirtuins in patients with BCC. Tumour Biol. 2016;37:6429–6435. doi: 10.1007/s13277-015-4522-8. [DOI] [PubMed] [Google Scholar]
- 87.Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, Liang J, Cheng Z, Shang Y, Yu W. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun. 2016;7:12235. doi: 10.1038/ncomms12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seidel J, Klockenbusch C, Schwarzer D. Investigating Deformylase and Deacylase Activity of Mammalian and Bacterial Sirtuins. Chembiochem. 2016;17:398–402. doi: 10.1002/cbic.201500611. [DOI] [PubMed] [Google Scholar]
- 89.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 90.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, Lin H. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496:110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teng YB, Jing H, Aramsangtienchai P, He B, Khan S, Hu J, Lin H, Hao Q. Efficient demyristoylase activity of SIRT2 revealed by kinetic and structural studies. Sci Rep. 2015;5:8529. doi: 10.1038/srep08529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bao X, Wang Y, Li X, Li XM, Liu Z, Yang T, Wong CF, Zhang J, Hao Q, Li XD. Identification of ‘erasers’ for lysine crotonylated histone marks using a chemical proteomics approach. Elife. 2014;3 doi: 10.7554/eLife.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 94.Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T, Cristea IM. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. 2014;159:1615–1625. doi: 10.1016/j.cell.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, Ro J, Wagner GR, Green MF, Madsen AS, Schmiesing J, Peterson BS, Xu G, Ilkayeva OR, Muehlbauer MJ, Braulke T, Muhlhausen C, Backos DS, Olsen CA, McGuire PJ, Pletcher SD, Lombard DB, Hirschey MD, Zhao Y. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]


