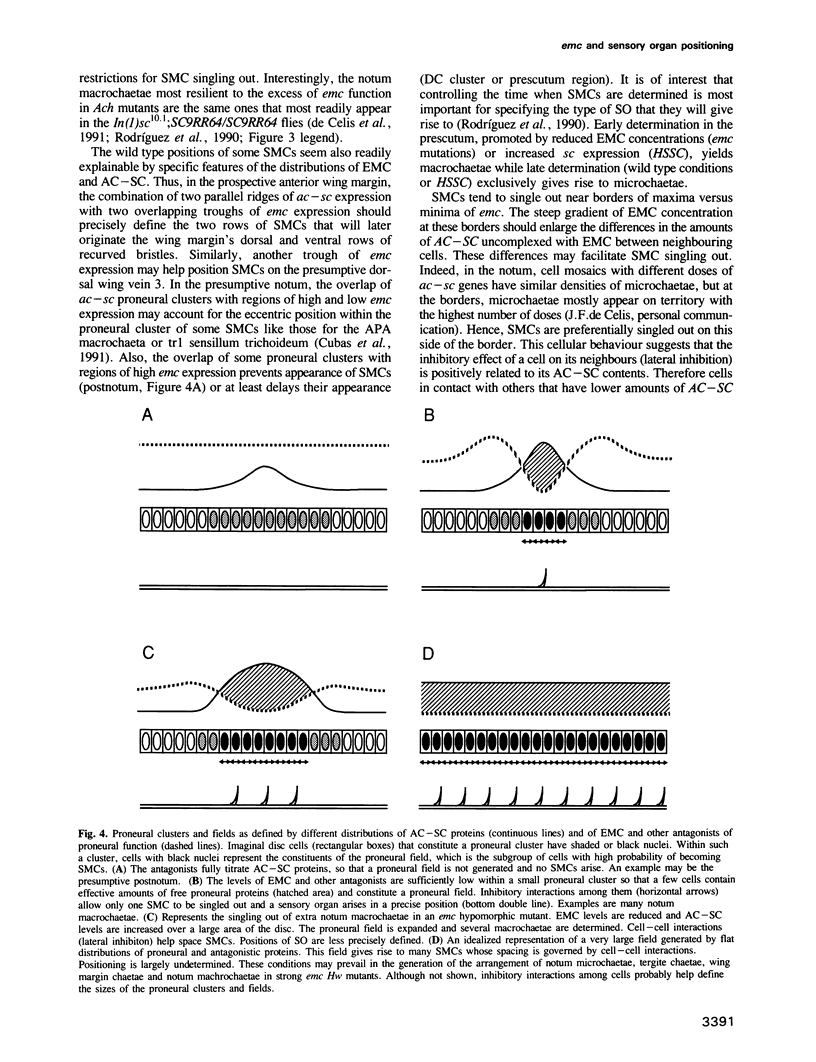
Abstract
The Drosophila adult epidermis displays a stereotyped pattern of bristles and other types of sensory organs (SOs). Its generation requires the proneural achaete (ac) and scute (sc) genes. In the imaginal wing disc, the anlage for most of the thoracic and wing epidermis, their products accumulate in groups of cells, the proneural clusters, whose distribution prefigures the adult pattern of SOs. These proteins then induce the emergence of SO mother cells (SMCs). Here, we show that the extramacrochaetae (emc) gene, an antagonist of the proneural function, is another agent that contributes to SO positioning. In the wing disc, emc is expressed in a complex and evolving pattern. SMCs appear not only within proneural clusters but also within minima of emc expression. When one of these spatial restrictions is eliminated, by ubiquitously expressing ac-sc, SMCs still emerge within minima of emc. When in addition, the other spatial restriction is reduced by decreasing emc expression, many ectopic SMCs emerge in a relatively even spaced and less constant pattern. Thus, the heterogeneous distribution of the emc product is one of the elements that define the positions where SMCs arise. emc probably refines SMC (and SO) positioning by reducing both the size of proneural clusters and the number of cells within clusters that can become SMCs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcells L., Modolell J., Ruiz-Gómez M. A unitary basis for different Hairy-wing mutations of Drosophila melanogaster. EMBO J. 1988 Dec 1;7(12):3899–3906. doi: 10.1002/j.1460-2075.1988.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R., Carretto R., Jan Y. N. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989 Jul;3(1):21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Botas J., Moscoso del Prado J., García-Bellido A. Gene-dose titration analysis in the search of trans-regulatory genes in Drosophila. EMBO J. 1982;1(3):307–310. doi: 10.1002/j.1460-2075.1982.tb01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant P. J. Pattern formation in the imaginal wing disc of Drosophila melanogaster: fate map, regeneration and duplication. J Exp Zool. 1975 Jul;193(1):49–77. doi: 10.1002/jez.1401930106. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Jan Y. N. Genetic and molecular bases of neurogenesis in Drosophila melanogaster. Annu Rev Neurosci. 1991;14:399–420. doi: 10.1146/annurev.ne.14.030191.002151. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992 Jun;8(6):202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Cubas P., de Celis J. F., Campuzano S., Modolell J. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 1991 Jun;5(6):996–1008. doi: 10.1101/gad.5.6.996. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Spann D. R., Posakony J. W. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990 Apr 6;61(1):27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- García-Bellido A. Genetic Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1979 Mar;91(3):491–520. doi: 10.1093/genetics/91.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell J., Campuzano S. The helix-loop-helix domain: a common motif for bristles, muscles and sex. Bioessays. 1991 Oct;13(10):493–498. doi: 10.1002/bies.950131002. [DOI] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. Genesis of the Drosophila peripheral nervous system. Trends Genet. 1989 Aug;5(8):251–255. doi: 10.1016/0168-9525(89)90097-8. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudière C. From DNA to form: the achaete-scute complex. Genes Dev. 1988 May;2(5):495–501. doi: 10.1101/gad.2.5.495. [DOI] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989 Oct;107(2):389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Huang F., Dambly-Chaudière C., Ghysen A. The emergence of sense organs in the wing disc of Drosophila. Development. 1991 Apr;111(4):1087–1095. doi: 10.1242/dev.111.4.1087. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Genes required for specifying cell fates in Drosophila embryonic sensory nervous system. Trends Neurosci. 1990 Dec;13(12):493–498. doi: 10.1016/0166-2236(90)90083-m. [DOI] [PubMed] [Google Scholar]
- Martínez C., Modolell J. Cross-regulatory interactions between the proneural achaete and scute genes of Drosophila. Science. 1991 Mar 22;251(5000):1485–1487. doi: 10.1126/science.1900954. [DOI] [PubMed] [Google Scholar]
- Richelle J., Ghysen A. Determination of sensory bristles and pattern formation in Drosophila. I. A model. Dev Biol. 1979 Jun;70(2):418–437. doi: 10.1016/0012-1606(79)90036-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez I., Hernández R., Modolell J., Ruiz-Gómez M. Competence to develop sensory organs is temporally and spatially regulated in Drosophila epidermal primordia. EMBO J. 1990 Nov;9(11):3583–3592. doi: 10.1002/j.1460-2075.1990.tb07569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Macagno E. R., Modolell J. Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 1989 Jul;3(7):997–1007. doi: 10.1101/gad.3.7.997. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Modolell J. Deletion analysis of the achaete-scute locus of Drosophila melanogaster. Genes Dev. 1987 Dec;1(10):1238–1246. doi: 10.1101/gad.1.10.1238. [DOI] [PubMed] [Google Scholar]
- SMITH J. M., SONDHI K. C. The arrangement of bristles in Drosophila. J Embryol Exp Morphol. 1961 Dec;9:661–672. [PubMed] [Google Scholar]
- Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development. 1990 Jul;109(3):509–519. doi: 10.1242/dev.109.3.509. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991 Jun;5(6):984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., White J. G. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 1980 Aug;78(2):577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Ellis H. M., Posakony J. W. The Drosophila extramacrochaetae protein antagonizes sequence-specific DNA binding by daughterless/achaete-scute protein complexes. Development. 1991 Sep;113(1):245–255. doi: 10.1242/dev.113.1.245. [DOI] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]