Abstract
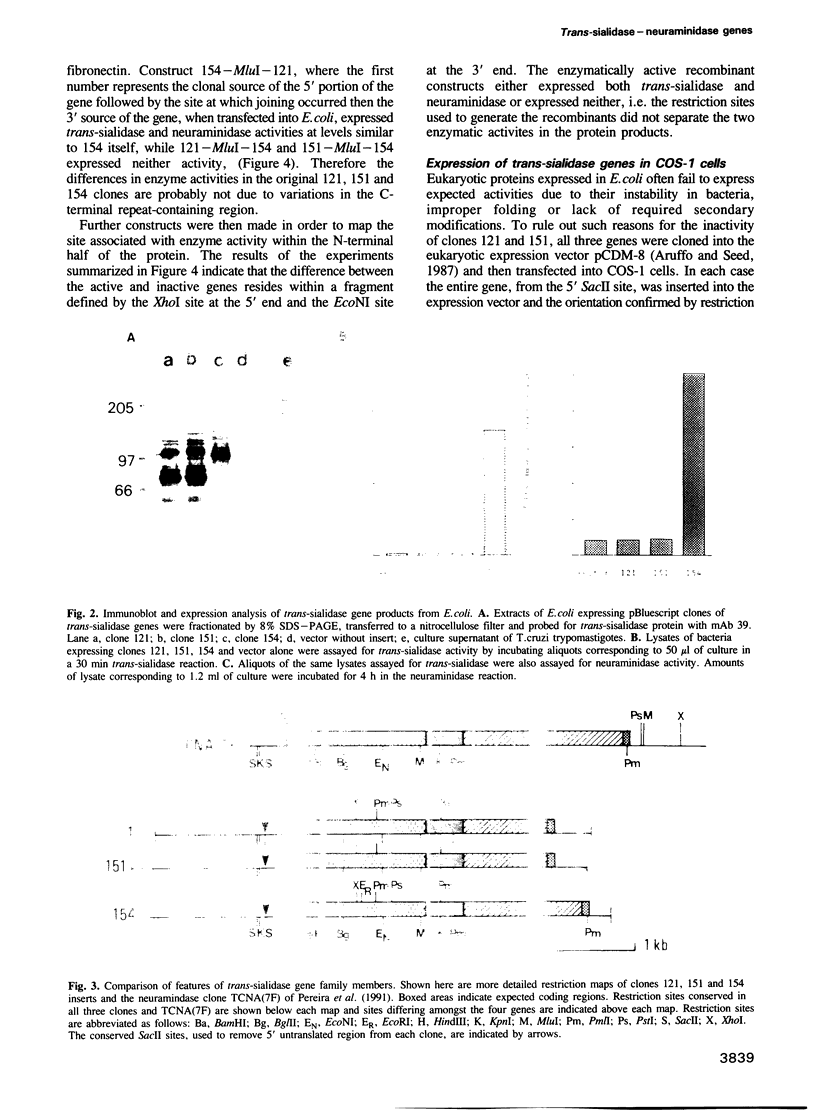
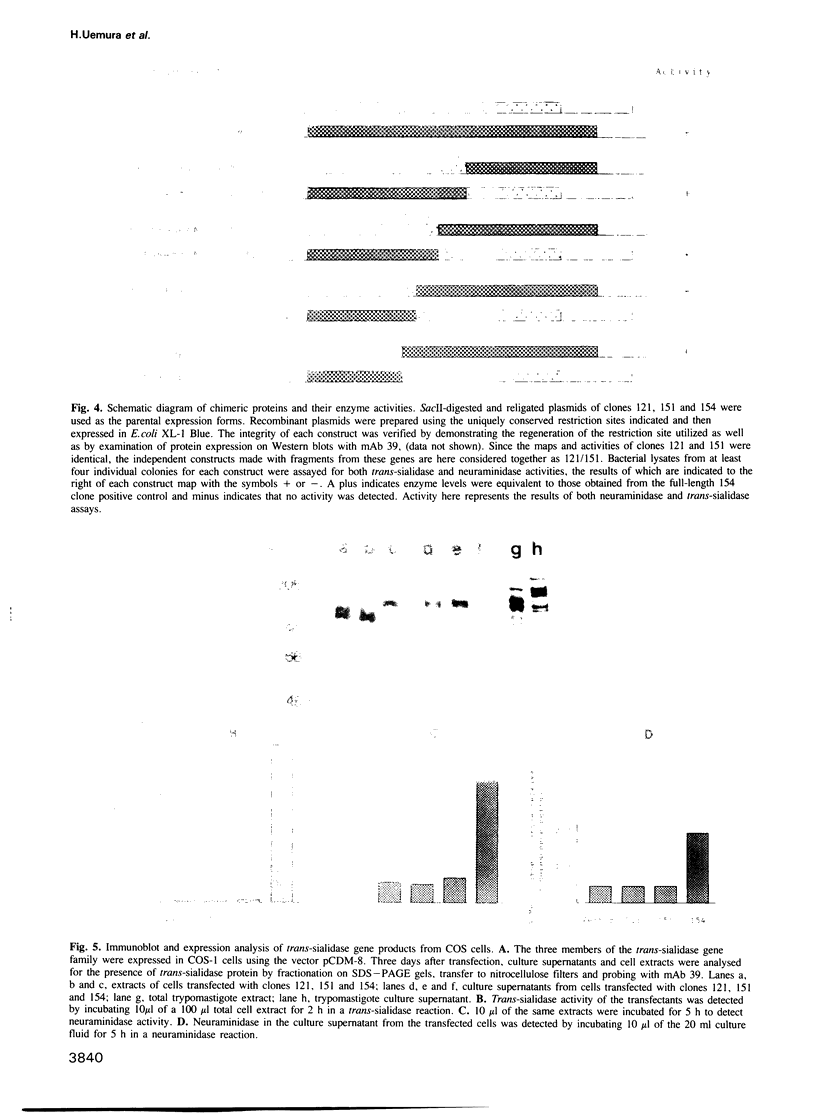
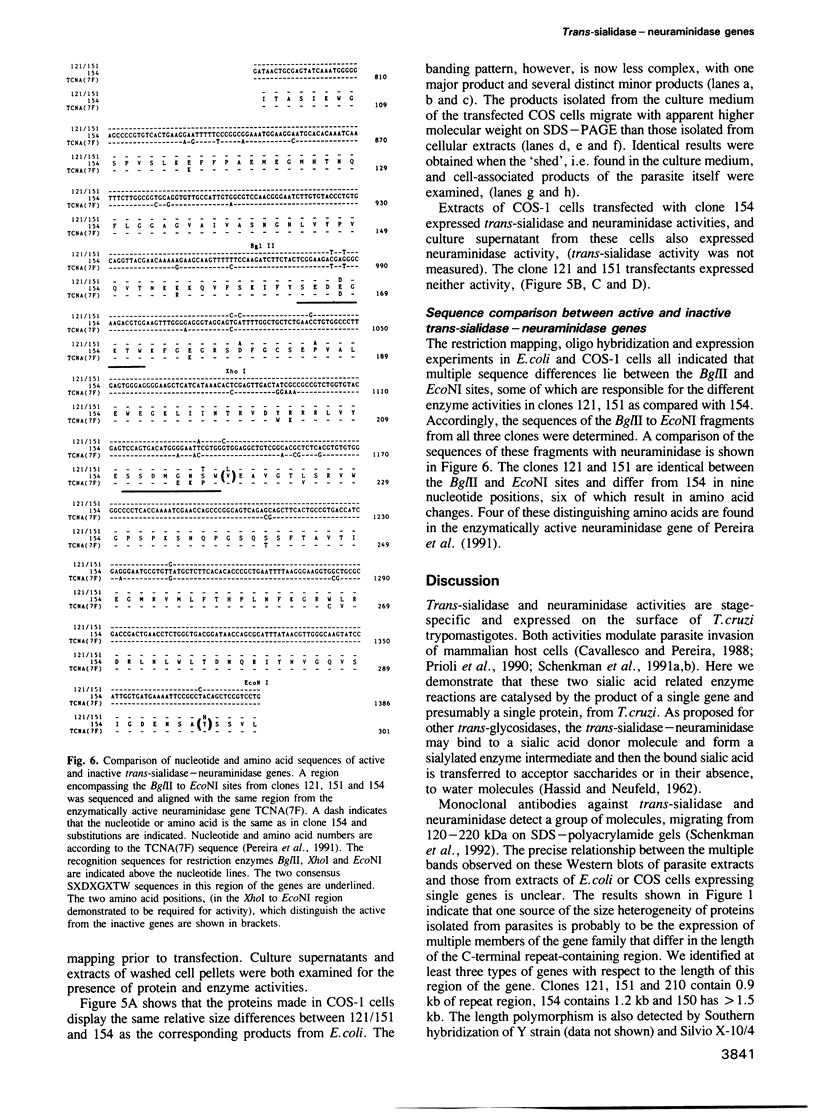
Trypomastigotes, the blood stage form of the human parasite Trypanosoma cruzi, contain an enzyme on their surface, trans-sialidase, which catalyses the transfer of sialic acid from host glycoconjugates to acceptors on its own cell surface. At least a subset of the sialic acid-bearing acceptor molecules are involved in parasite invasion of host cells, an essential step in the life cycle of the parasite. Another trypomastigote surface enzyme that affects host cell invasion is neuraminidase and recent evidence suggests that both trans-sialidase and neuraminidase activities may be expressed by the same proteins on the parasite surface. We describe here the isolation and expression of several members of a trans-sialidase--neuraminidase gene family from T.cruzi. One of the isolated genes does indeed encode a protein with both trans-sialidase and neuraminidase activities, while other members of the gene family encode closely related proteins that express neither enzymatic activity. Chimeric protein constructs combining different portions of active and inactive genes identified a region of the gene necessary for enzymatic activity. Sequence analysis of this portion of the gene revealed a limited number of amino acid differences between the predicted active and inactive gene products.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Hong K. S., Robbins E. S., Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987 Dec;64(3):474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschetti M. A., Piras M. M., Henríquez D., Piras R. The interaction of a Trypanosoma cruzi surface protein with Vero cells and its relationship with parasite adhesion. Mol Biochem Parasitol. 1987 Jun;24(2):175–184. doi: 10.1016/0166-6851(87)90104-6. [DOI] [PubMed] [Google Scholar]
- Campetella O., Henriksson J., Aslund L., Frasch A. C., Pettersson U., Cazzulo J. J. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol Biochem Parasitol. 1992 Feb;50(2):225–234. doi: 10.1016/0166-6851(92)90219-a. [DOI] [PubMed] [Google Scholar]
- Cavallesco R., Pereira M. E. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J Immunol. 1988 Jan 15;140(2):617–625. [PubMed] [Google Scholar]
- Henriksson J., Aslund L., Macina R. A., Franke de Cazzulo B. M., Cazzulo J. J., Frasch A. C., Pettersson U. Chromosomal localization of seven cloned antigen genes provides evidence of diploidy and further demonstration of karyotype variability in Trypanosoma cruzi. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):213–223. doi: 10.1016/0166-6851(90)90164-h. [DOI] [PubMed] [Google Scholar]
- Kahn S., Colbert T. G., Wallace J. C., Hoagland N. A., Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S., Van Voorhis W. C., Eisen H. The major 85-kD surface antigen of the mammalian form of Trypanosoma cruzi is encoded by a large heterogeneous family of simultaneously expressed genes. J Exp Med. 1990 Aug 1;172(2):589–597. doi: 10.1084/jem.172.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macina R. A., Affranchino J. L., Pollevick G. D., Jazin E. E., Frasch A. C. Variable number of repeat units in genes encoding Trypanosoma cruzi antigens. FEBS Lett. 1989 Nov 6;257(2):365–368. doi: 10.1016/0014-5793(89)81573-x. [DOI] [PubMed] [Google Scholar]
- Meirelles M. N., Martinez-Palomo A., Souto-Padron T., De Souza W. Participation of concanavalin A binding sites in the interaction between Trypanosoma cruzi and macrophages. J Cell Sci. 1983 Jul;62:287–299. doi: 10.1242/jcs.62.1.287. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Pollevick G. D., Mautner M., Buschiazzo A., Sanchez D. O., Frasch A. C. Identification of the gene(s) coding for the trans-sialidase of Trypanosoma cruzi. EMBO J. 1992 May;11(5):1705–1710. doi: 10.1002/j.1460-2075.1992.tb05221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Fouts D. L., Manning J. E. The 85-kd surface antigen gene of Trypanosoma cruzi is telomeric and a member of a multigene family. EMBO J. 1989 Dec 1;8(12):3911–3916. doi: 10.1002/j.1460-2075.1989.tb08571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollevick G. D., Affranchino J. L., Frasch A. C., Sánchez D. O. The complete sequence of a shed acute-phase antigen of Trypanosoma cruzi. Mol Biochem Parasitol. 1991 Aug;47(2):247–250. doi: 10.1016/0166-6851(91)90185-9. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Mejia J. S., Pereira M. E. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J Immunol. 1990 Jun 1;144(11):4384–4391. [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Kaper J. B., Galen J., Lawrisuk L., Vimr E. R., Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- Rosenberg I., Prioli R. P., Ortega-Barria E., Pereira M. E. Stage-specific phospholipase C-mediated release of Trypanosoma cruzi neuraminidase. Mol Biochem Parasitol. 1991 Jun;46(2):303–305. doi: 10.1016/0166-6851(91)90054-a. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R., Reuter G., Mühlpfordt H., Andrade A. F., Pereira M. E. The occurrence of N-acetyl- and N-glycoloylneuraminic acid in Trypanosoma cruzi. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):1053–1057. doi: 10.1515/bchm2.1983.364.2.1053. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Diaz C., Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol. 1991 Jan;72(1):76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Jiang M. S., Hart G. W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991 Jun 28;65(7):1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Pontes de Carvalho L., Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J Exp Med. 1992 Feb 1;175(2):567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takle G. B., Cross G. A. An 85-kilodalton surface antigen gene family of Trypanosoma cruzi encodes polypeptides homologous to bacterial neuraminidases. Mol Biochem Parasitol. 1991 Oct;48(2):185–198. doi: 10.1016/0166-6851(91)90114-l. [DOI] [PubMed] [Google Scholar]
- Takle G. B., Young A., Snary D., Hudson L., Nicholls S. C. Cloning and expression of a trypomastigote-specific 85-kilodalton surface antigen gene from Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Nov;37(1):57–64. doi: 10.1016/0166-6851(89)90102-3. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Milhausen M., Rutter W. J., Agabian N. Tubulin genes are tandemly linked and clustered in the genome of trypanosoma brucei. Cell. 1983 Jan;32(1):35–43. doi: 10.1016/0092-8674(83)90494-4. [DOI] [PubMed] [Google Scholar]







