Abstract
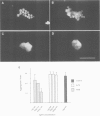
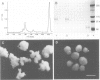
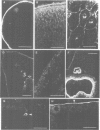
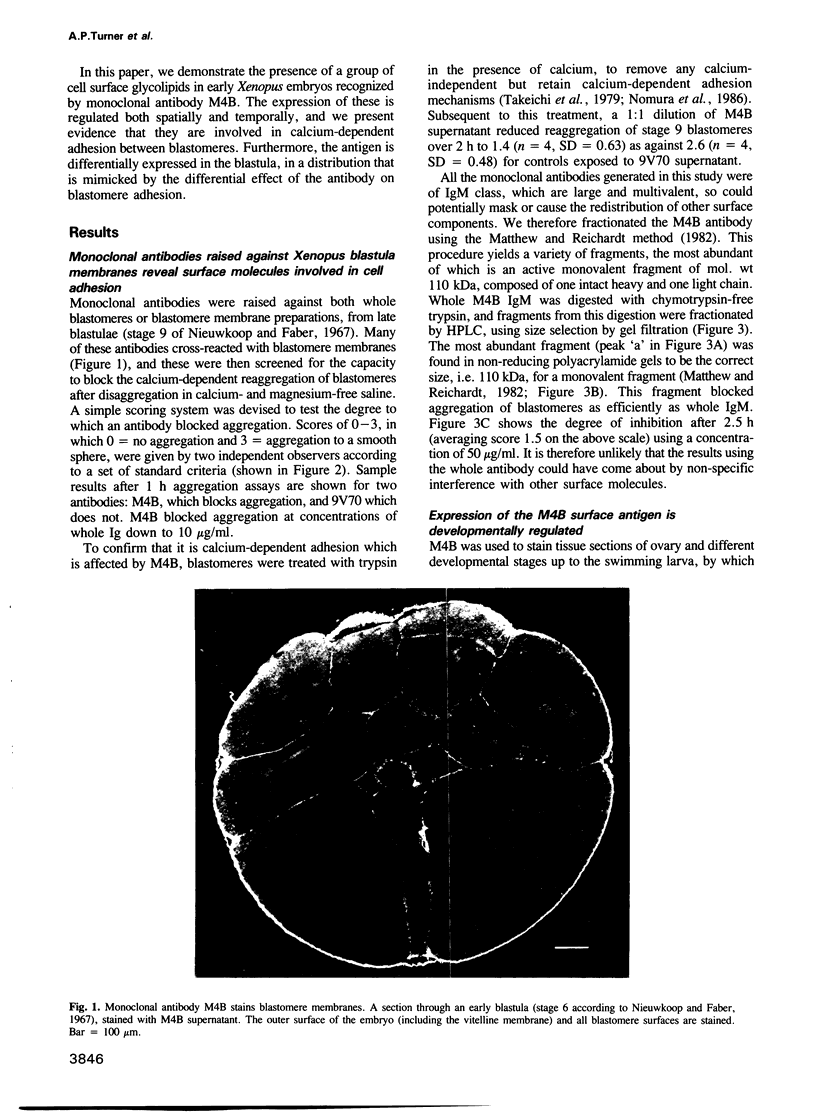
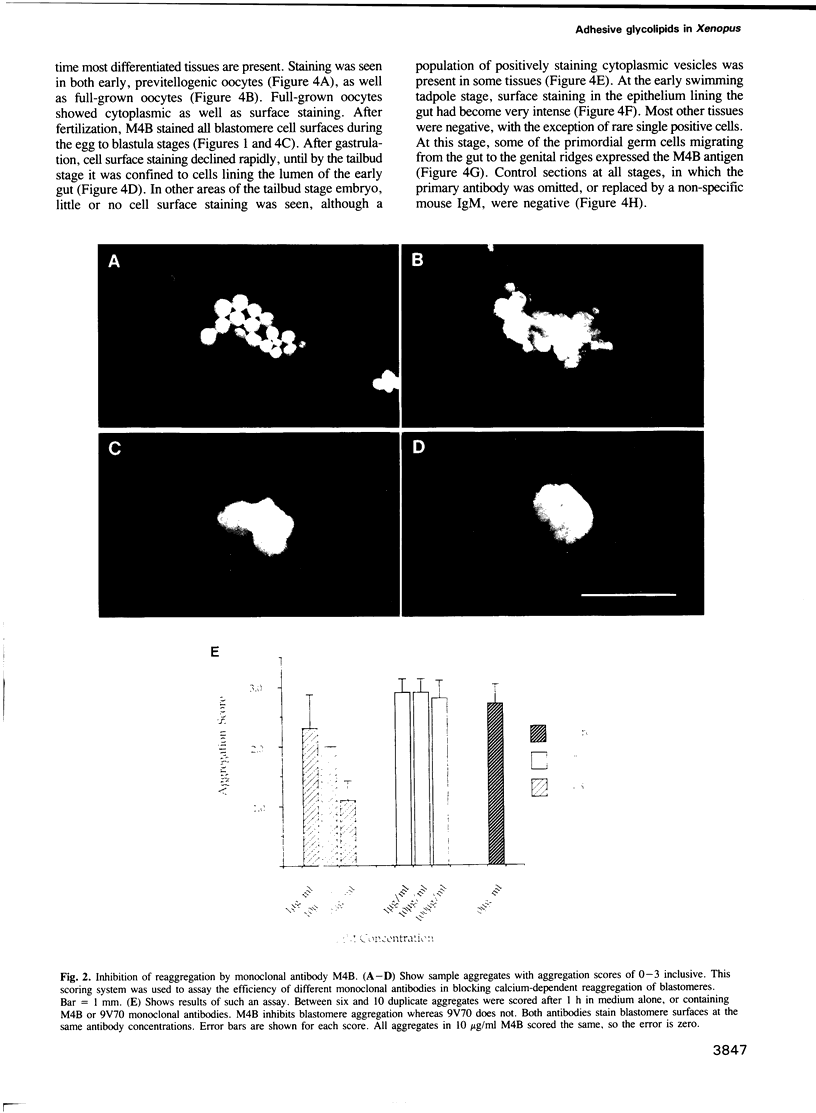
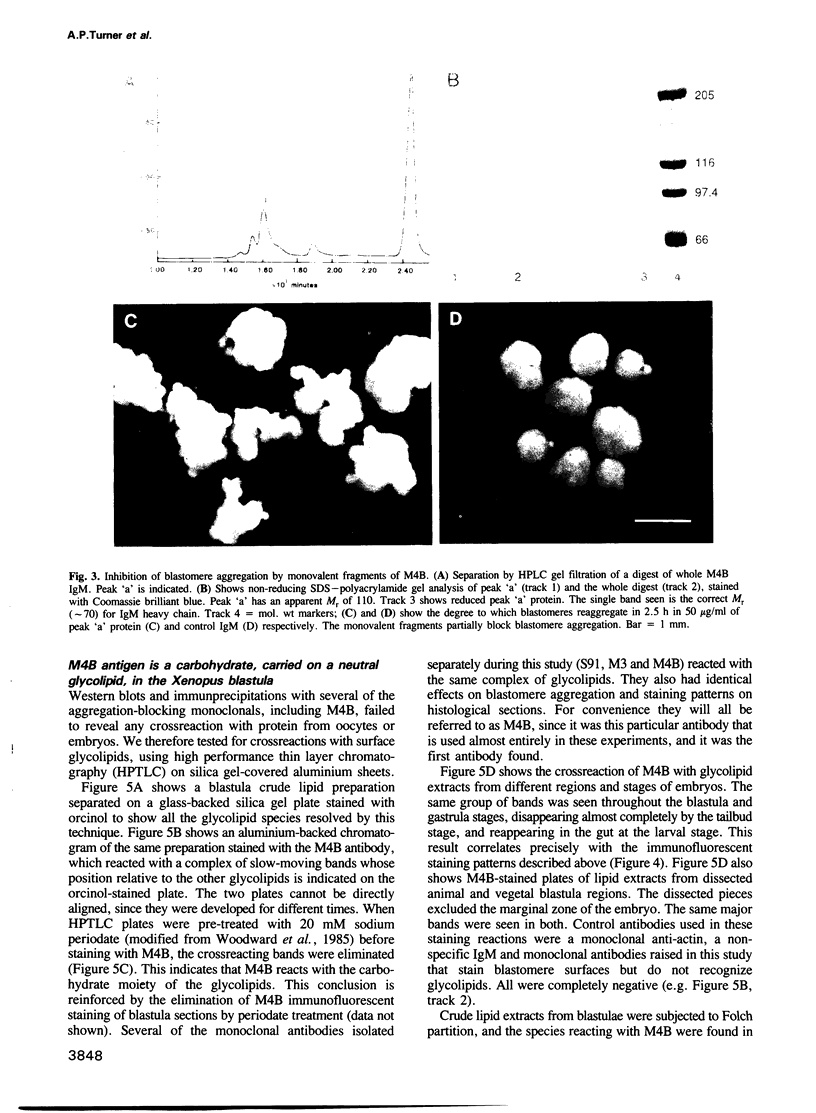
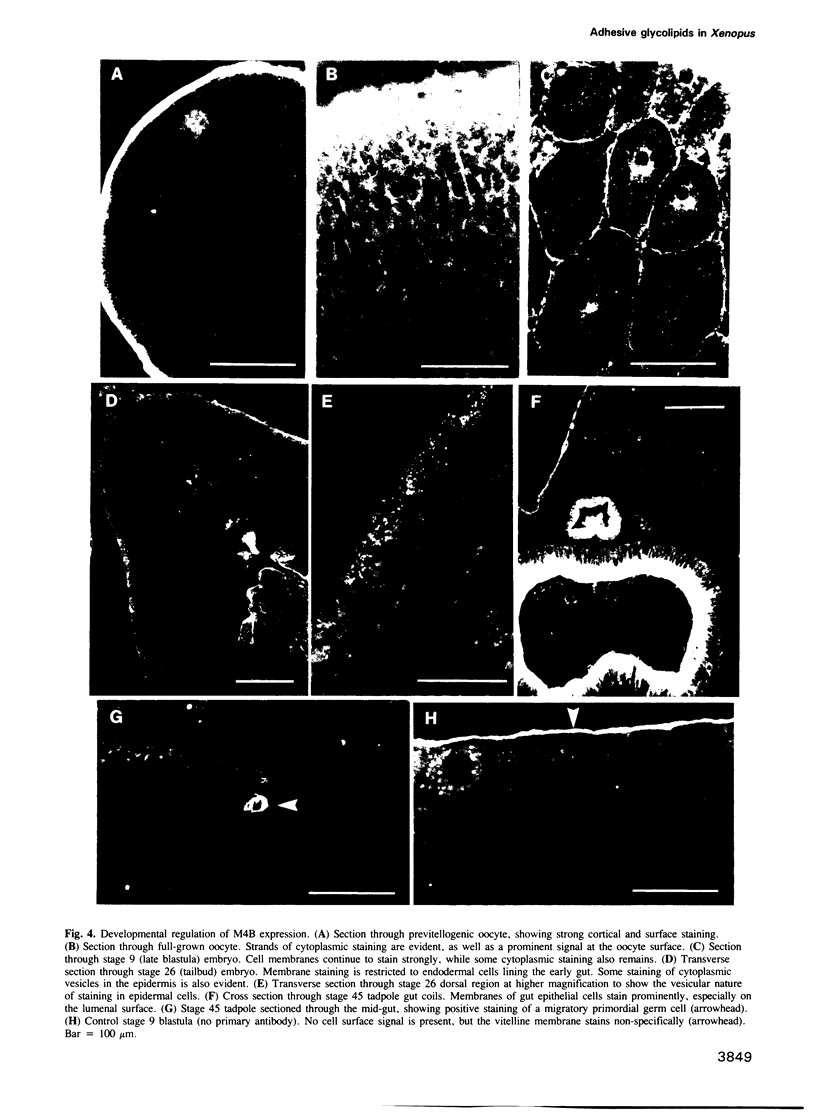
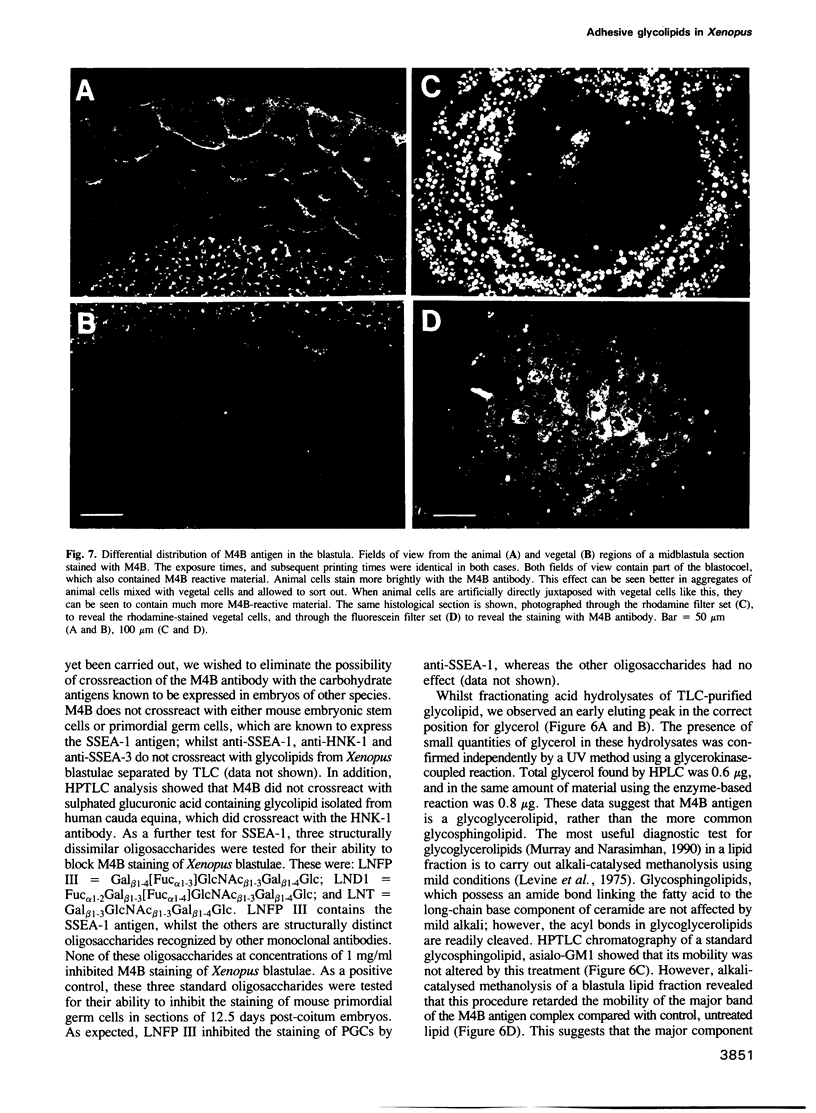
Many different molecular species mediate cell adhesion during embryonic development. These can have either protein or carbohydrate functional groups, which can act in either a homophilic or a heterophilic manner, and often in concert. We report here that a monoclonal antibody, M4B, raised against Xenopus blastomere membranes, inhibits the calcium-dependent adhesion of dissociated blastomeres. M4B maintains its inhibitory effect on adhesion when converted into univalent fragments, and specifically affects calcium-dependent adhesion. The antigen is regulated in both space and time during early development. It is found on cell surfaces throughout the egg to blastula stages, but is more concentrated on cells in the animal and marginal zones of the blastula. It is dramatically downregulated during gastrulation, and becomes largely restricted to gut epithelium by the larval stages. We show also that M4B function is spatially differentiated at the blastula stage, since it inhibits the aggregation of dissociated animal cells to a greater extent than vegetal cells. This membrane antigen may therefore play a role in the differential adhesion observed between different regions of the blastula, and which we presume to underlie the segregation of the primary germ layers during gastrulation. M4B recognizes a complex of plasma membrane glycolipids. Periodate treatment destroys the ability of these glycolipids to react with the antibody, indicating that the epitope resides in the carbohydrate moiety of the glycolipids. Chemical characterization shows that it is a neutral glycolipid, and that the major component is of the glycoglycerolipid, rather than the more common glycosphingolipid class. Blocking experiments with oligosaccharides of defined structure, and antibody crossreactivity show that the M4B antibody does not recognize several known embryonic carbohydrate antigens. These results demonstrate that M4B antibody recognizes a novel group of developmentally regulated glycolipids which function in calcium-dependent cell--cell adhesion in the Xenopus blastula.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angres B., Müller A. H., Kellermann J., Hausen P. Differential expression of two cadherins in Xenopus laevis. Development. 1991 Mar;111(3):829–844. doi: 10.1242/dev.111.3.829. [DOI] [PubMed] [Google Scholar]
- Bird J. M., Kimber S. J. Oligosaccharides containing fucose linked alpha(1-3) and alpha(1-4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev Biol. 1984 Aug;104(2):449–460. doi: 10.1016/0012-1606(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Pennington J., Uemura K., Scudder P., Goodfellow P. N., Evans M. J., Feizi T. High-molecular-weight glycoproteins are the major carriers of the carbohydrate differentiation antigens I, i and SSEA-1 of mouse teratocarcinoma cells. Biochem J. 1983 Dec 1;215(3):491–503. doi: 10.1042/bj2150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Eggens I., Fenderson B., Toyokuni T., Dean B., Stroud M., Hakomori S. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem. 1989 Jun 5;264(16):9476–9484. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Feizi T. Carbohydrate differentiation antigens: probable ligands for cell adhesion molecules. Trends Biochem Sci. 1991 Mar;16(3):84–86. doi: 10.1016/0968-0004(91)90038-w. [DOI] [PubMed] [Google Scholar]
- Fenderson B. A., Eddy E. M., Hakomori S. Glycoconjugate expression during embryogenesis and its biological significance. Bioessays. 1990 Apr;12(4):173–179. doi: 10.1002/bies.950120406. [DOI] [PubMed] [Google Scholar]
- Francis D., Toda K., Merkl R., Hatfield T., Gerisch G. Mutants of Polysphondylium pallidum altered in cell aggregation and in the expression of a carbohydrate epitope on cell surface glycoproteins. EMBO J. 1985 Oct;4(10):2525–2532. doi: 10.1002/j.1460-2075.1985.tb03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg D., DeSimone D., Geiger B. Expression of a novel cadherin (EP-cadherin) in unfertilized eggs and early Xenopus embryos. Development. 1991 Feb;111(2):315–325. doi: 10.1242/dev.111.2.315. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Feizi T., Kapadia A., Knowles B. B., Solter D., Evans M. J. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981 Jul 9;292(5819):156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Methods for nuclear transplantation in amphibia. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- Heasman J., Wylie C. C., Hausen P., Smith J. C. Fates and states of determination of single vegetal pole blastomeres of X. laevis. Cell. 1984 May;37(1):185–194. doi: 10.1016/0092-8674(84)90314-3. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Hynes M. A., Dodd J. Carbohydrates and carbohydrate-binding proteins in the nervous system. Annu Rev Neurosci. 1990;13:227–255. doi: 10.1146/annurev.ne.13.030190.001303. [DOI] [PubMed] [Google Scholar]
- Kapadia A., Feizi T., Evans M. J. Changes in the expression and polarization of blood group I and i antigens in post-implantation embryos and teratocarcinomas of mouse associated with cell differentiation. Exp Cell Res. 1981 Jan;131(1):185–195. doi: 10.1016/0014-4827(81)90418-3. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Melton D. A. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development. 1987 Mar;99(3):311–325. doi: 10.1242/dev.99.3.311. [DOI] [PubMed] [Google Scholar]
- Künemund V., Jungalwala F. B., Fischer G., Chou D. K., Keilhauer G., Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988 Jan;106(1):213–223. doi: 10.1083/jcb.106.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen E., Palabrica T., Sajer S., Gilbert G. E., Wagner D. D., Furie B. C., Furie B. PADGEM-dependent adhesion of platelets to monocytes and neutrophils is mediated by a lineage-specific carbohydrate, LNF III (CD15). Cell. 1990 Nov 2;63(3):467–474. doi: 10.1016/0092-8674(90)90443-i. [DOI] [PubMed] [Google Scholar]
- Levi G., Crossin K. L., Edelman G. M. Expression sequences and distribution of two primary cell adhesion molecules during embryonic development of Xenopus laevis. J Cell Biol. 1987 Nov;105(5):2359–2372. doi: 10.1083/jcb.105.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Kornblatt M. J., Murray R. K. Isolation and partial characterization of a sulfogalactoglycerolipid from rat brain. Can J Biochem. 1975 Jun;53(6):679–689. doi: 10.1139/o75-094. [DOI] [PubMed] [Google Scholar]
- Lloyd C. W. Sialic acid and the social behaviour of cells. Biol Rev Camb Philos Soc. 1975 Aug;50(3):325–350. doi: 10.1111/j.1469-185x.1975.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Spitalnik S. L., Ginsburg V. Antibodies against cell surface carbohydrates: determination of antigen structure. Methods Enzymol. 1987;138:195–207. doi: 10.1016/0076-6879(87)38016-4. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Reichardt L. F. Development and application of an efficient procedure for converting mouse IgM into small, active fragments. J Immunol Methods. 1982;50(3):239–253. doi: 10.1016/0022-1759(82)90162-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. B., Edidin M., Orr C. W., Roseman S. An L-glutamine requirement for intercellular adhesion. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1395–1402. doi: 10.1073/pnas.63.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Roberson M. M., Armstrong P. B. Carbohydrate-binding component of amphibian embryo cell surfaces: restriction to surface regions capable of cell adhesion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3460–3463. doi: 10.1073/pnas.77.6.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting G. A., Jungalwala F. B., Chou D. K., Boyer A. M., Yamamoto M. Sulfated glucuronic acid-containing glycoconjugates are temporally and spatially regulated antigens in the developing mammalian nervous system. Dev Biol. 1987 Mar;120(1):65–76. doi: 10.1016/0012-1606(87)90104-7. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem. 1965 Jul;12(7):629–638. doi: 10.1111/j.1471-4159.1965.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Takeichi M., Ozaki H. S., Tokunaga K., Okada T. S. Experimental manipulation of cell surface to affect cellular recognition mechanisms. Dev Biol. 1979 May;70(1):195–205. doi: 10.1016/0012-1606(79)90016-2. [DOI] [PubMed] [Google Scholar]
- Turner A., Snape A. M., Wylie C. C., Heasman J. Regional identity is established before gastrulation in the Xenopus embryo. J Exp Zool. 1989 Aug;251(2):245–252. doi: 10.1002/jez.1402510212. [DOI] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Willison K. R., Karol R. A., Suzuki A., Kundu S. K., Marcus D. M. Neutral glycolipid antigens as developmental markers of mouse teratocarcinoma and early embryos: an immunologic and chemical analysis. J Immunol. 1982 Aug;129(2):603–609. [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]