Abstract
Background
An important area of research in childhood obesity is the identification of factors that predict or moderate the responses to obesity intervention programs, yet few studies have examined the impact of self-esteem and family functioning on obesity treatment outcomes.
Objectives
We sought to determine whether baseline self-esteem and family functioning predicted or moderated childhood obesity intervention outcomes at six months.
Methods
From 2009–2011, seventy-five 10–16 year old, racially/ethnically-diverse obese youths with abnormal glucose tolerance were randomized to six months of an intensive family-based obesity lifestyle intervention (Bright Bodies) or routine outpatient Clinic Care. We examined youth self-esteem/self-concept, parent-rated family functioning, and 6-month outcomes (youths’ glucose tolerance, weight, body mass index, and percent fat). We set the significance threshold as P ≤ 0.05 for moderator and predictor analyses.
Results
Baseline poor family functioning and self-concept scores indicating high anxiety and low self-esteem predicted poor six-month outcomes overall (Bright Bodies and Clinic Care groups combined). Additionally, baseline self-esteem and family functioning moderated treatment effects–Bright Bodies outperformed Clinic Care in youths with low self-esteem and poorly functioning families, whereas youths with high self-esteem and high-functioning families did similarly well with either intervention.
Discussion
Our findings suggest intensive family-based lifestyle programs are particularly beneficial for youth with low self-esteem and poorly functioning families.
ClinicalTrials.gov Identifier
Keywords: pediatrics, obesity, diabetes mellitus type 2, clinical trial, adolescents, children
Introduction
According to the Centers for Disease Control and Prevention, 17% of 2 to 19 year olds were obese in 2011–2012 in the United States.1 The cut-off for childhood obesity in the USA is ≥ 95th percentile for gender and age based on the Centers for Disease Control and Prevention growth charts.2 Pediatric obesity has several negative sequelae including increased risk of diabetes, cardiovascular impairment, musculoskeletal disorders, fatty liver disease, and poor quality of life.3–5 While family-based obesity treatment programs that include a combination of behavior modification, nutrition education, and physical activity have been characterized as the “gold standard” in pediatric obesity treatment, some children do better in these programs than others.6,7 Consequently, an important area of research in childhood obesity is the identification of factors that predict or moderate the responses to obesity intervention programs. Predictors are baseline patient characteristics that affect outcomes for all treatments; whereas, moderators are baseline patient characteristics that affect how much a patient benefits from receiving a specific treatment.
Prior research has suggested that younger age, female sex, parental weight loss, better impulse control, good parental mental health, social support, and living in a neighborhood with more parkland are predictors of positive outcomes in most childhood obesity interventions.8 On the other hand, baseline characteristics that moderate response to specific obesity interventions have not been identified.6,8 While pediatric weight management programs have been linked to improvements in self-esteem,9 it has not been established whether baseline self-esteem or family functioning predict or moderate outcomes in family-based childhood obesity interventions.6,8,10,11
The Yale Bright Bodies (BB) Weight Management Program is a family-based childhood obesity intervention designed for urban youth and was recently shown to improve anthropometric and metabolic profiles in comparison to standard Clinic Care (CC) in a randomized trial of obese children and adolescents with impaired glucose tolerance.12,13 Important outcomes of the study were that BB improved metabolic outcomes relative to CC after six months of treatment.13 Herein we report the results of baseline self-esteem and family functioning data that were analyzed to determine whether they predicted or moderated anthropometric and glucoregulatory outcomes in these obese youths.
Methods
The Bright Bodies participants, study design, treatment groups, and anthropometric and glucoregulatory outcome measures have been detailed previously.12–14 In this manuscript, we provide a brief overview of the study.
Participants
Participants were referred to the study by four urban pediatric clinics in New Haven, Connecticut, USA from 2009–2011. Eligible children and adolescents were 10–16 years old with a 2-hour plasma glucose of 130–199 mg/dL on oral glucose tolerance test (OGTT), body mass index (BMI) > 95th percentile for age and gender, Tanner stage ≥ 2, and an interest in participating in an intensive lifestyle program. Exclusion criteria included having diabetes, a psychiatric disorder, or an unstable medical condition. Youths participating in another lifestyle program and youths taking medications affecting weight, insulin sensitivity, or glucose metabolism were excluded. Of the 577 children assessed, 432 did not meet the primary inclusion criteria (a 2-hour blood glucose of 130–199 mg/dl), 53 did not meet other study criteria, and 17 declined participation in the study, leaving a total of 75 children who were randomized. The Yale Human Investigation Committee approved the study, and written informed assent and consent were obtained from youths and parents.
Of the 75 youths who enrolled in the study, 77% (58, 31 BB and 27 CC) completed the study and are included in this secondary analysis.13 At baseline, there were no significant demographic or outcome measure differences between completers and drop-outs or by intervention group. For the completers, the mean age was 13 years old with a standard deviation of 2 years (range 10–16 years), 66% were girls, 40% were Hispanic White, 33% were non-Hispanic White, and 24% were Black (race and ethnicity were based on parent-report). The annual household income was less than $30,000 for 80% of participants, and parent reports were completed by female caregivers 90% of the time.13
Study Design
In this parallel-group trial, eligible youths were randomized (1:1) to Bright Bodies (BB) or standard Clinic Care (CC) using electronic randomization with permuted blocks maintained by the study statistician to assure concealment. At the end of the six-month study, youths randomized to CC were offered entry into BB.
Treatment Groups
Bright Bodies (BB) Program
The BB lifestyle program was conducted at two New Haven public schools in the evenings. One site implemented the program in Spanish with bilingual (English/Spanish) instructors. BB youths attended the program twice a week for 6 months – once for a 50-minute workout and once for a 90-minute session (50-minute workout and 40-minute class on either nutrition or behavior modification). Study dietitians emphasized a non-diet approach and used the Smart Moves Workbook offered in English and Spanish.12,14 Parents attended the program weekly for nutrition classes and other classes that focused on how to help their children make healthy lifestyle changes.
Standard of Care - Clinic Care (CC)
At the start of the study, CC youths attended one session with a study dietitian fluent in English and Spanish. During the session, the dietitian provided basic nutrition and exercise guidance, identified physical activities the youth enjoyed, and developed goals with the child and family. A copy of the written goal sheet was mailed to the youth’s usual clinician. CC youths followed up with their usual clinician every 2–3 months during the study. To ensure all clinicians could provide sound weight management guidance at follow-up visits, the study dietitian conducted training workshops for clinicians at the four referral clinics and supplied the clinics with educational materials that were distributed to patients.
Anthropometric and Glucoregulatory Outcome Variables
Independent evaluators blinded to treatment condition obtained anthropometric (after shoes were removed) and glucoregulatory assessments at baseline and six months at the Yale Center for Clinical Investigation. Outcomes examined were change in each of the variables listed below at six months. The primary outcome of the randomized control trial was change in 2-hour blood glucose during OGTT. Of note, in a previous analysis we found weight change only explained 13% of the change in 2-hour glucose.13
Anthropometrics: Weight (kg), BMI (kg/m2), BMI z-score,2,15 percent body fat determined by a body fat analyzer (TBF 300, Tanita Corp, Arlington Heights, Il, USA), body fat mass calculated by multiplying percent body fat by the youth’s total weight (kg).16
OGTT: Fasting and 2-hour blood glucose (mg/dL) and insulin (mIU/L) (OGTT procedure: 1.75 g/kg body weight (max 75 g) of flavored glucose (Orangedex; Custom Laboratories, Baltimore, MD, USA) was given orally and blood samples were obtained for glucose and insulin every 30 minutes for 2 hours).
Insulin Sensitivity: Homeostasis model assessment of insulin resistance (HOMA-IR)17 and whole-body insulin sensitivity index (WBISI)18 were calculated using fasting and mean glucoses and insulins from the OGTT. These formulas were described previously.13
Psychosocial Assessments
Piers-Harris Children’s Self-Concept Scale: measured self-esteem and how participants viewed themselves.19 The Piers-Harris is internally consistent (Cronbach alpha for subscales 0.71–0.80), reliable (test-retest reliability coefficient 0.71 at four months), and correlated with other measures of self-concept like the Gordon How I See Myself Scale and Sears Self-Concept Inventory.19 Participants completed the scale at baseline and six months. The Piers-Harris has a total of 80 yes/no questions and the Total Score encompasses six subscales: Behavioral Adjustment (appropriate behavior, 16 questions), Intellectual and School Status (17 questions), Physical Appearance and Attributes (13 questions), Freedom From Anxiety (14 questions), Popularity (social functioning, 12 questions), Happiness and Satisfaction (overall happiness with oneself and with life, 10 questions). Some questions were included in multiple subscales. T-scores for each subscale are standardized for youth 7–18 years old. Higher scores indicate higher self-esteem.
McMaster Family Assessment Device (FAD): measured family functioning and was completed by parents at baseline and at six months.20 FAD had 53 statements about family dynamics divided into seven subscales. Parents rated how well each statement described their family using one of four responses: strongly agree, agree, disagree, or strongly disagree. Each statement was scored 1–4, with lower scores indicating superior family functioning. The subscale scores were an average of the individual items within the subscale. Prior studies have demonstrated FAD subscales are internally consistent (Cronbach’s alpha range 0.72–0.92), reliable (test-retest reliability coefficient 0.76 at six months), and significantly correlated with other measures of family functioning, like the Family Adaptability and Cohesion Evaluation Scales. FAD subscales:
Problem Solving (5 statements) assessed ability to resolve problems in the family.
Communication (6 statements) assessed exchange of clear and direct verbal information.
Roles (8 statements) assessed division of responsibility for completing tasks in the family.
Affective Responsiveness (6 statements) assessed expression of love and other feelings.
Affective Involvement (7 statements) assessed interest in one another.
Behavior Control (9 statements) assessed how behavioral standards were expressed and maintained.
General Functioning (12 statements) assessed family functioning overall.
Statistical Analysis
Participants’ demographics and clinical characteristics did not significantly differ between randomized groups, as shown in a previous publication.13 We compared baseline psychosocial assessments between BB and CC groups using the two-sample t-test. We used analysis of covariance (ANCOVA) to determine whether either intervention resulted in Piers-Harris or FAD change. Pearson’s Correlation Coefficients (r) were calculated between baseline Piers-Harris/FAD subscales and anthropometric/metabolic change to investigate their association. We examined the moderating effects of baseline FAD or Piers-Harris subscales by including a group by baseline FAD/Piers-Harris subscale interaction in the model. A significant interaction indicated that the group difference (treatment effect) depended on baseline subscale level. The moderation effect was demonstrated by plotting the size of treatment effect vs level of baseline subscale with 95% CI indicating the significance of treatment effect corresponding to each level of baseline subscale. We used SAS 9.3 (Cary, NC, USA), performed log transformation for normality when indicated, and set the level of significance as a two-tailed P value ≤ 0.05 for all analyses.
Results
Baseline Patient Psychosocial Characteristics and Change with Treatment
Baseline Piers-Harris and FAD scores did not differ between study completers and dropouts (data not shown). Supplementary Table S1 displays baseline psychosocial characteristics for youths who completed BB and CC. With the exception of FAD Communication, neither Piers-Harris nor FAD means (M) differed by intervention group at baseline. Baseline FAD Communication scores were higher (indicating poorer communication) in CC (M 2.2, SD 0.4) than in BB (M 2.0, SD 0.3) (P = 0.05). Piers-Harris Subscale T-score means for the obese participants in our sample ranged from 49.3–55.3 (relative to standardized T-score of 50 with standard deviation of 10 for general youth populations). Neither BB nor CC significantly changed FAD or Piers-Harris subscale scores at six months (data not shown).
Predictors of Outcomes
Table 1 displays relationships between baseline Piers-Harris/FAD Subscale scores and change in glucoregulatory/anthropometric outcomes at six months. High baseline scores on two Piers-Harris Subscales were associated with improved secondary outcomes: Freedom From Anxiety was correlated with a reduction in fasting insulin; Happiness and Satisfaction was correlated with a reduction in BMI z-score and fasting glucose. Other baseline Piers-Harris Subscales were not associated with glucoregulatory/anthropometric outcomes. There was a significant association between better baseline family functioning (in Problem Solving, Communication, Affective Responsiveness, Affective Involvement, and General Functioning FAD domains) and better glucoregulatory outcomes and reduced fat mass. Only healthy family Affective Responsiveness was correlated with improvement in the primary outcome, 2-hour blood glucose (r = 0.41, P < 0.001).
Table 1.
Pearson Correlations of Baseline Self-Esteem and Family Functioning With Metabolic/Anthropometric Change At Six Months (Bright Bodies and Clinic Care Groups Combined)
| Baseline Predictors | Metabolic/Anthropometric Variable | Pearson r | P Value | N |
|---|---|---|---|---|
| Piers-Harris Self-Concept Subscale (T-Score) | ||||
| Physical Appearance/Attributes | Body fat (%) | 0.26 | 0.07 | 50 |
| Body fat mass | 0.24 | 0.09 | 50 | |
| Freedom From Anxiety | Fasting insulina | −0.27 | 0.05 | 52 |
| Happiness and Satisfaction | BMI z-score | −0.17 | 0.05 | 56 |
| Fasting glucose | −0.32 | 0.02 | 55 | |
| Family Assessment Device Subscaleb | ||||
| Poor Problem Solving | 2-hour insulina | 0.35 | 0.02 | 46 |
| Insulin sensitivity (WBISI) | −0.27 | 0.08 | 42 | |
| Poor Communication | Fasting glucose | 0.29 | 0.04 | 49 |
| 2-hour glucose | 0.26 | 0.07 | 50 | |
| 2-hour insulina | 0.36 | 0.01 | 46 | |
| Insulin sensitivity (WBISI) | −0.36 | 0.02 | 42 | |
| Poor Affective Responsiveness | Fasting glucose | 0.33 | 0.02 | 49 |
| 2-hour glucose | 0.41 | <0.001 | 50 | |
| 2-hour insulina | 0.53 | <0.001 | 46 | |
| Insulin sensitivity (WBISI) | −0.39 | 0.01 | 42 | |
| Poor Affective Involvement | Body fat mass | 0.32 | 0.04 | 44 |
| Poor Behavioral Control | BMI | 0.25 | 0.08 | 50 |
| Body fat mass | 0.28 | 0.07 | 44 | |
| Fasting glucose | 0.27 | 0.06 | 49 | |
| Fasting insulina | 0.26 | 0.08 | 46 | |
| 2-hour insulina | 0.25 | 0.09 | 46 | |
| Insulin resistance (HOMA-IR)a | 0.28 | 0.06 | 45 | |
| Poor General Functioning | Fasting glucose | 0.38 | 0.01 | 49 |
| 2-hour insulina | 0.37 | 0.01 | 46 | |
| Insulin sensitivity (WBISI) | −0.27 | 0.08 | 42 |
Log transformed
For the McMaster Family Assessment Device, higher scores indicate lower functioning, and “poor” is added as a prefix to the Subscales to make the table easier to understand.
Note: Correlations with P value < 0.10 are listed. Changes were calculated as (6 months – baseline), so positive correlation indicates the predictor was associated with an increase (or less of a decrease) in outcome measure. Ns differ due to missing data.
Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; WBISI, whole body insulin sensitivity index.
Moderation of Treatment Effects
Piers-Harris Children’s Self-Concept Scale
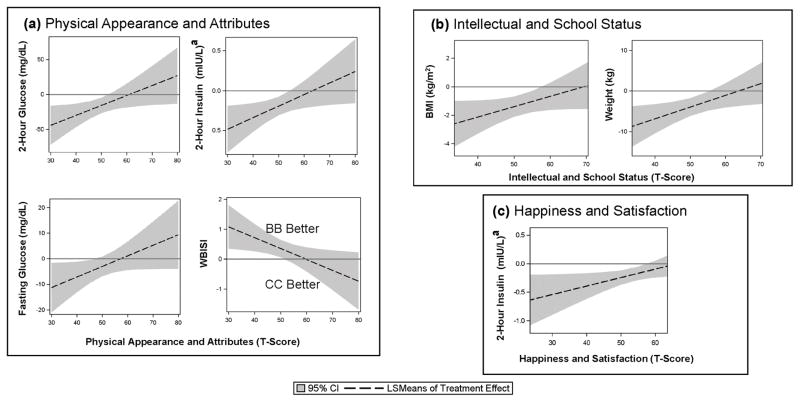
Fig. 1 displays Piers-Harris Subscales that significantly moderated treatment effects (Pinteraction ≤ 0.05). Low baseline self-esteem regarding Physical Appearance and Attributes moderated treatment effects on 2-hour OGTT glucose (the primary outcome) at six months (Pinteraction = 0.03), such that BB outperformed CC by −27 mg/dL (95% CI, −42 to −12) in youths with physical self-esteem one standard deviation (SD) below the mean but not at one SD above the mean (BB treatment effect, −1 mg/dL; 95% CI, −18 to 16) (Fig. 1(a)). BB also outperformed CC in terms of other glucoregulatory and anthropometric outcomes at six months in youths with lower Piers-Harris Self-Concept scores in three domains: Physical Appearance and Attributes for fasting glucose, 2-hour glucose, 2-hour insulin, and insulin sensitivity (Fig. 1(a)); Intellectual and School Status for weight and BMI (Fig. 1(b)); Happiness and Satisfaction for 2-hour insulin (Fig. 1(c)). That is, youths with lower self-appraisals of their physical appearance, intellect, and/or overall happiness particularly benefitted from BB. BB and CC treatment effects did not significantly differ in youths with positive self-concept of their physical appearance, intellect, and/or overall happiness with themselves and life.
Figure 1.
Moderation of Treatment Effects by Piers-Harris Children’s Self-Concept Subscales: (a) Physical Appearance and Attributes, (b) Intellectual and School Status, (c) Happiness and Satisfaction. When the 95% CI is below zero, Bright Bodies (BB) outperformed Clinic Care (CC), except for WBISI (whole-body insulin sensitivity index) because an increase in WBISI is good, whereas increases in all other outcomes are bad.
aLog transformed. Abbreviations: BMI, body mass index; CI, confidence interval; LSMeans, least square means.
McMaster Family Assessment Device
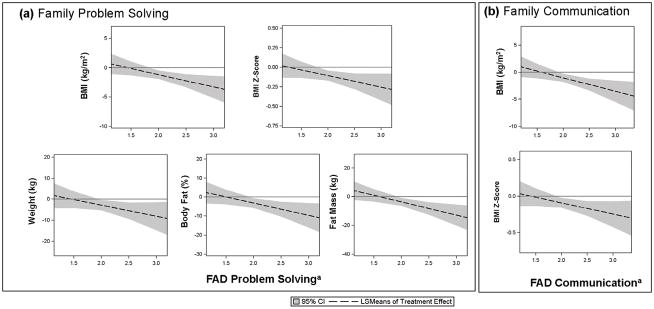
Fig. 2 displays FAD Subscales that significantly moderated effects (Pinteraction ≤ 0.05). BB especially outperformed CC in terms of anthropometric outcomes at six months in youths with poor family functioning (high FAD score) in two domains: Problem Solving for weight, BMI, BMI z-score, percent body fat, and body fat mass (Fig. 2(a)); Communication for BMI and BMI z-score (Fig. 2(b)). That is, youths in families with poor problem solving and communication especially benefitted from BB. BB and CC treatment effects did not significantly differ in youths from families with good family problem solving and/or communication.
Figure 2.
Moderation of Treatment Effects by McMaster Family Assessment Device (FAD) Subscales:a (a) Family Problem Solving and (b) Family Communication. When the 95% CI is below zero, Bright Bodies (BB) outperformed Clinic Care (CC).
aHigher scores indicate poorer family functioning. Abbreviations: BMI, body mass index; CI, confidence interval; LSMeans, least square means.
Discussion
Moderators help clinicians match particular patients with particular treatments. We found that self-esteem and family functioning moderated obesity intervention treatment effects at six months. Specifically, we found that in terms of glucoregulatory and anthropometric outcomes, youths with low self-esteem and poor family functioning especially benefitted from the intensive family-based obesity intervention (Bright Bodies, BB) relative to routine outpatient treatment (Clinic Care, CC). For participants with high self-esteem and high-functioning families, BB and CC treatment effects did not significantly differ. Our results build on work published by Braet and Beyers in 2009 which found that inpatient obesity treatment increased unhealthy dietary restraint in a subgroup of children and adolescents with anxiety and depression symptoms.21 Braet and Beyers surmised that baseline psychopathology and other baseline patient characteristics could be used to tailor obesity treatments to fit a particular child (i.e. personalized medicine).21 Our study findings suggest baseline self-esteem and family functioning can help identify ideal treatment modalities for obese youth.
Our finding that participants with low self-esteem (in the domains of intellect, physical characteristics, and overall happiness) and poor family functioning (in communication and problem solving domains) especially benefitted from the BB program (relative to CC) is likely related to BB counseling sessions involving the youngster that addressed issues related to self-esteem like “Loving Yourself Inside and Out” and “Bullies, Teasers, and Other Annoying People.” BB also helped parents identify non-weight-related character strengths of their child. Additionally, BB had components that addressed family functioning; for instance, parent sessions were designed to facilitate communication between parent and youth and also to help parents collaborate with their child to overcome weight loss difficulties. While low self-esteem11 and poor family functioning10 are associated with poor glucoregulatory outcomes in the absence of specialized treatment, our findings demonstrate that intensive family-based treatments can lessen the impact of low self-esteem and poor family functioning on outcomes.
While patients with low self-esteem and poor family functioning particularly benefitted from the weight management program, at the end of the intervention youths who began with higher scores on self-esteem and family functioning measures still had better outcomes overall. Specifically, when examining outcomes regardless of intervention type, we found that self-concept scores indicating greater overall happiness with oneself and/or less anxiety were associated with better BMI z-score, fasting glucose, and fasting insulin outcomes. These findings underscore the importance of addressing mental health when treating childhood obesity and highlight an opportunity for interventions that simultaneously emphasize psychological and physical wellbeing. For instance, an effective family-based obesity intervention developed by Danielsen et al. included techniques like emotion regulation and examination of automatic thoughts, which are core components of cognitive behavioral therapy for anxiety and depression.22 Successful use of psychological interventions as a means to enhance medical outcomes have been documented in pediatric asthma, type 1 diabetes mellitus, and cystic fibrosis,23 and our work demonstrates the relationship between psychological wellbeing and intervention efficacy in childhood obesity and abnormal glucose tolerance.
Additionally, when looking at intervention outcomes overall, we found better family functioning was associated with better glucoregulatory outcomes and reduced fat mass. How a family dealt with adversity, communicated, expressed love, and showed interest in one another affected how successful obesity intervention was. Our results fit well with a study that demonstrated four sessions of family therapy focused on communication, appropriate limit setting, expressing feelings, and consistency significantly lowered BMI z-score in obese children.24 Similarly, a prior study found that obese teenagers who rated their families as high functioning were more likely to sustain weight loss one year after a summer camp obesity intervention.25
Bright Bodies did not significantly affect self-esteem or family functioning. Of note, prior studies have found that weight management programs increase self-esteem in children,9 and the evidence is particularly robust regarding the mental health benefits of physical activity.26 However, because the primary outcome of the present study was glucose tolerance, it was not powered to detect change in self-esteem or family functioning, which may explain why we did not find a relationship between intervention and improvement on psychosocial measures. Furthermore, family functioning and self-esteem were surprisingly within normal limits at baseline, and it may be harder to improve these measures when they are already normal (ceiling effect). A strength of our study is that many participants were low-income African Americans and Hispanics, making our results more generalizable to low-income African-American and Hispanic communities in the USA, which often have high childhood obesity rates1 and distinct cultural traditions that affect obestiy.27–29 On the other hand, our study has some limitations. Family functioning was measured using parent ratings on the FAD, which may have overestimated levels of healthy family functioning given that parent reports of family functioning are often more positive than the reports given by their children.30 Moreover, we had limited information on the children’s family structures (e.g. lacked marital status of parents and involvement of extended family members as caregivers). Another limitation is that youth with psychiatric disorders and youth treated with pharmacotherapies for obesity were excluded from the study; therefore, our findings may not be generalizable to obese youth with psychiatric comorbidity or youth taking commonly prescribed medications like metformin. Additionally, we did not adjust for multiple comparisons. However, because of the exploratory feature of this study that aimed to be hypothesis-generating, we prioritized reducing type II error so that we did not overlook important findings. Although several findings are quite robust as evidenced by not only p values but also magnitude of effects, further investigation is still warranted.
Despite these limitations, our study is the first to identify moderators that can help clinicians and families decide between two common childhood obesity treatments: a brief health education intervention with standard outpatient follow-up (CC) and an intensive family-based lifestyle intervention (BB). Our findings suggest that intensive, family-based interventions are especially beneficial for youth with low self-esteem and poorly functioning families.
Supplementary Material
What Is Already Known About This Subject
Younger age and parental weight loss are robust predictors of positive outcomes in most childhood obesity interventions.
An important area of research in childhood obesity is the identification of factors that predict or moderate the responses to obesity intervention programs (moderators are treatment-specific predictors that can help a clinician match a particular intervention to a child based on the child’s characteristics).
What This Study Adds
Low self-esteem and poor family functioning predicted poor glucoregulatory and anthropometric treatment outcomes overall in obese youths.
Intensive family-based treatment (relative to standard clinic care) lessened the impact of low self-esteem and family dysfunction on outcomes. Our findings suggest intensive family-based lifestyle programs are particularly beneficial for youth with low self-esteem and poorly functioning families.
Acknowledgments
Author Contributions: M Savoye, PN, and FL conceived the study. M Savoye and M Shaw collected the data. YX, FL, and JT conducted the data analysis. All authors assisted with data interpretation and writing the manuscript.
Funding/Support: The authors gratefully acknowledge support from the National Institutes of Health (NIH) - National Institute of Mental Health grant 5T32MH018268 (Dr Taylor); the American Psychiatric Association/Substance Abuse and Mental Health Services Administration Minority Fellowship Program (Dr Taylor); NIH/American Recovery and Reinvestment Act grant 3UL1 RR024139-04S2 (Ms Savoye); Yale Clinical and Translational Science Award grant UL1TR000142 from NIH National Center for Advancing Translational Science (Dr Tamborlane); the Tegger Foundation (Dr Nowicka); the Vinnova Marie Curie International Qualification 2011-03443 (Dr Nowicka).
Role of the Funders/Sponsors: Funders/sponsors did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations
- BB
Yale Bright Bodies Weight Management Program
- CC
standard Clinic Care
- OGTT
oral glucose tolerance test
- BMI
body mass index
- PH
Piers-Harris Children’s Self-Concept Scale
- FAD
McMaster Family Assessment Device
Footnotes
Conflict of Interest Statement: There are no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014 Feb 26;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey. 2002;(246):1–190. [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004 Jun 3;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Farpour-Lambert NJ, Baker JL, Hassapidou M, et al. Childhood Obesity Is a Chronic Disease Demanding Specific Health Care-a Position Statement from the Childhood Obesity Task Force (COTF) of the European Association for the Study of Obesity (EASO) Obesity facts. 2015;8(5):342–349. doi: 10.1159/000441483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slyper A, Rosenberg H, Kabra A, Huang WM, Blech B, Matsumura M. Fatty liver disease, glucose tolerance and insulin resistance in obese adolescents. Pediatr Obes. 2015 doi: 10.1111/ijpo.279. [DOI] [PubMed] [Google Scholar]
- 6.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. The Cochrane Library. 2009 doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Kokkvoll A, Grimsgaard S, Steinsbekk S, Flægstad T, Njølstad I. Health in overweight children: 2-year follow-up of Finnmark Activity School—a randomised trial. Arch Dis Child. 2015;100(5):441–448. doi: 10.1136/archdischild-2014-307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altman M, Wilfley DE. Evidence update on the treatment of overweight and obesity in children and adolescents. J Clin Child Adolesc Psychol. 2015;44(4):521–537. doi: 10.1080/15374416.2014.963854. [DOI] [PubMed] [Google Scholar]
- 9.Lowry KW, Sallinen BJ, Janicke DM. The effects of weight management programs on self-esteem in pediatric overweight populations. J Pediatr Psychol. 2007;32(10):1179–1195. doi: 10.1093/jpepsy/jsm048. [DOI] [PubMed] [Google Scholar]
- 10.Halliday JA, Palma CL, Mellor D, Green J, Renzaho AMN. The relationship between family functioning and child and adolescent overweight and obesity: a systematic review. Int J Obes. 2014 Apr;38(4):480–493. doi: 10.1038/ijo.2013.213. [DOI] [PubMed] [Google Scholar]
- 11.Wardle J, Cooke L. The impact of obesity on psychological well-being. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19(3):421–440. doi: 10.1016/j.beem.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007 Jun 27;297(24):2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 13.Savoye M, Caprio S, Dziura J, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014 Feb;37(2):317–324. doi: 10.2337/dc13-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw M, Savoye M, Cali A, Dziura J, Tamborlane WV, Caprio S. Effect of a successful intensive lifestyle program on insulin sensitivity and glucose tolerance in obese youth. Diabetes Care. 2009;32(1):45–47. doi: 10.2337/dc08-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer K, Marcus M, Ogden C, Foster G. Cardio-metabolic risk screening among adolescents: understanding the utility of body mass index, waist circumference and waist to height ratio. Pediatr Obes. 2014 doi: 10.1111/ijpo.267. [DOI] [PMC free article] [PubMed]
- 16.Javed A, Jumean M, Murad M, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes. 2015;10(3):234–244. doi: 10.1111/ijpo.242. [DOI] [PubMed] [Google Scholar]
- 17.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 19.Piers EV, Harris DB. Age and other correlates of self-concept in children. J Educ Psychol. 1964;55(2):91. [Google Scholar]
- 20.Epstein NB, Baldwin LM, Bishop DS. The McMaster family assessment device. J Marital Fam Ther. 1983;9(2):171–180. [Google Scholar]
- 21.Braet C, Beyers W. Subtyping children and adolescents who are overweight: Different symptomatology and treatment outcomes. J Consult Clin Psychol. 2009;77(5):814. doi: 10.1037/a0016304. [DOI] [PubMed] [Google Scholar]
- 22.Danielsen YS, Nordhus IH, Júlíusson PB, Mæhle M, Pallesen S. Effect of a family-based cognitive behavioural intervention on body mass index, self-esteem and symptoms of depression in children with obesity (aged 7–13): A randomised waiting list controlled trial. Obes Res Clin Pract. 2013;7(2):e116–e128. doi: 10.1016/j.orcp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- 24.Nowicka P, Pietrobelli A, Flodmark C-E. Low-intensity family therapy intervention is useful in a clinical setting to treat obese and extremely obese children. Int J Pediatr Obes. 2007;2(4):211–217. doi: 10.1080/17477160701379810. [DOI] [PubMed] [Google Scholar]
- 25.Sampat S, Kirschenbaum DS, Gierut KJ, Germann JN, Krawczyk R. Ya gotta have friends: Social support and self-efficacy predict success following immersion treatment. Obesity. 2014;22(12):2579–2585. doi: 10.1002/oby.20863. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Wu L, Ming Q. How does physical activity intervention improve self-esteem and self-concept in children and adolescents? Evidence from a meta-analysis. PLoS One. 2015;10(8):e0134804. doi: 10.1371/journal.pone.0134804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans A, Chow S, Jennings R, et al. Traditional foods and practices of Spanish-speaking Latina mothers influence the home food environment: implications for future interventions. J Am Diet Assoc. 2011;111(7):1031–1038. doi: 10.1016/j.jada.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 28.James D. Factors influencing food choices, dietary intake, and nutrition-related attitudes among African Americans: application of a culturally sensitive model. Ethn Health. 2004;9(4):349–367. doi: 10.1080/1355785042000285375. [DOI] [PubMed] [Google Scholar]
- 29.Taylor J, Jr, Belay B, Park S, Onufrak S, Dietz W. Association of church-sponsored activity participation and prevalence of overweight and obesity in African American Protestants, National Survey of American Life, 2001–2003. Ethn Dis. 2012;23(3):322–328. [PMC free article] [PubMed] [Google Scholar]
- 30.Sawyer MG, Sarris A, Baghurst PA, Cross DG, Kalucy RS. Family Assessment Device: Reports from mothers, fathers, and adolescents in community and clinic families. J Marital Fam Ther. 1988;14(3):287–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.