Abstract
Objectives
Elucidating the biological mechanisms involved in Attention-deficit/hyperactivity disorder (ADHD) has been challenging. Relatively unexplored is the fact that these mechanisms can differ with age.
Methods
We present an overview on the major differences between children and adults with ADHD, describing several studies from genomics to metabolomics performed in ADHD children and in adults. A systematic search (up until February, 2016) was conducted.
Results
From a PRISMA flow-chart, a total of eligibility 350 studies from genomics and metabolomics were found for cADHD and 91 for aADHD. For children, associations were found for genes belonging to dopaminergic (SLC6A3, DRD4, MAOA) and neurodevelopmental (LPHN3, DIRAS2) systems and OPRM1 (Yates Corrected p=0.016; OR=2.27 95%CI:1.15–4.47). Studies of adults have implicated circadian rhythms genes, HTR2A, MAOB and a more generic neurodevelopmental/neurite outgrowth network (BCHE, SNAP25, BAIAP2, NOS1/NO, KCNIP4, SPOCK3; Yates Corrected p=0.007; OR= 3.30 95%CI:1.33–8.29). In common among cADHD and aADHD, the most significant findings are for oxidative stress proteins (MAD, SOD, PON1, ARES, TOS, TAS, OSI), and, in the second level, DISC1, DBH, DDC, microRNA and adiponectin.
Conclusions
Through a convergent functional genomics, this review contributes to clarify which genetic/biological mechanisms differ with age. The effects of some genes do not change throughout lifetime, whereas others are linked to age specific stages. Additional research and further studies are needed to generate firmer conclusions that might someday be useful for predicting the remission and persistence of the disorder. Although the limitations, some of these genes/proteins could be potential useful biomarkers to discriminate cADHD versus aADHD.
Keywords: Attention-deficit/hyperactivity disorder, children, adults, genomics, metabolomics
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common syndrome characterized by excessive inattention, hyperactivity and impulsivity (Faraone et al. 2015). Although it had long been considered a disorder of childhood, 65% of affected children continue manifesting symptoms into adulthood (Faraone, Biederman, Mick 2006).
The ADHD prevalence was estimated 5.3% in childhood (Polanczyk et al. 2007; Polanczyk et al. 2014; Willcutt 2012) and 2.5–4.9% in adulthood (Ramos-Quiroga et al. 2014a; Simon et al. 2009). Although many clinical features are similar in ADHD children and adults, adults have less symptoms of hyperactivity and impulsivity and more inattentive symptoms (Volkow and Swanson 2013). Furthermore some structural brain findings differentiate ADHD in children and adults (Shaw et al. 2013). These data suggest potential differential etiological pathways for children and adults.
Data about the heritability of ADHD in children and adults had been inconsistent, with some suggestion that heritability is higher in children (75–90%) (Faraone and Mick 2010) compared with adults (30%–50%) (Boomsma et al. 2010; Kan et al. 2013; Larsson et al. 2013) and other studies supporting greater heritability in adults (Biederman et al. 1995; Biederman et al. 1996; Faraone, Biederman, Monuteaux 2000; Faraone et al. 2000). A more recent review suggests that the heritability of ADHD in adults and in childhood is better explained by rater effects (Brikell, Kuja-Halkola, Larsson 2015).
From the linkage and candidate gene (CGA) to genome-wide association (GWA) studies, the ADHD community has made real breakthroughs. 40% of ADHD’s heritability is due to a polygenic liability consisting of many common variants (Single Nucleotide Polymorphisms SNPs) (Lee et al. 2013) along with rare deletions and insertions (Copy Number Variants CNVs) (Williams et al. 2012; Martin et al. 2015).
The identification of biomarkers may facilitate the differential diagnosis of ADHD. The results of a recent meta-analysis of childhood ADHD studies identified some potential peripheral biomarkers linked to monoaminergic pathways and to the HPA axis (Scassellati et al. 2012).
To date, relatively unexplored is the fact that the biological mechanisms involved in ADHD can differ with age. By using a convergent functional genomics, Here we present an overview on the major significant differences between child ADHD (cADHD) and adult ADHD (aADHD), describing several studies from genomics (linkage and CGA, SNPs/CNVs-GWA, expression studies) to metabolomics carried out in cADHD and in aADHD. We sought to delineate which genes and proteins are common and distinct to these two age groups of ADHD persons.
This review presents the results structured in two main paragraphs: -Genomics subdivided in a) linkage studies, b) in candidate gene association studies where each specific pathway was evidenced, c) in genome-wide association studies according to SNPs and CNVs, d) expression studies in peripheral systems and -Metabolomics where biochemical studies in peripheral tissues were described. Each of them performed in ADHD children and adults.
Methods
Detailed information on literature search strategy, inclusion/exclusion criteria and statistical analyses are reported in supplementary material.
Results
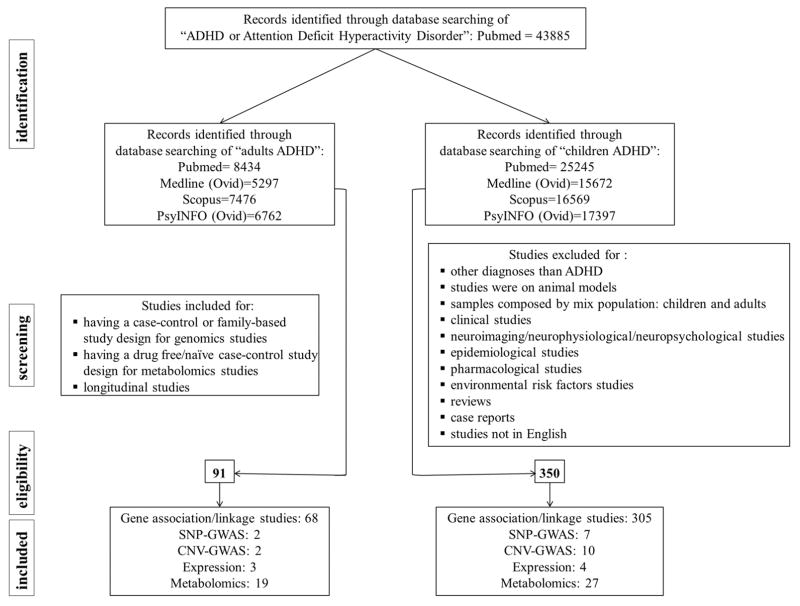
As indicated in the PRISMA flow-chart, a total of eligibility was 350 studies from genomics and metabolomics for cADHD and 91 for aADHD (Figure 1). Table 1 and Figure 2 reassumed the results obtained from genomic and metabolomic studies. Summary of positive/negative findings and statistical results for common and specific pathways in cADHD and aADHD are in Tables S1 and S2 respectively.
Figure 1.
PRISMA flow diagram for review and meta-analysis
Table 1.
Common and specific genes/proteins in children and adults with ADHD selected from genomics (candidate gene association, GWA, CNV, expression studies) to metabolomics.
| Genomics | Metabolomics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systems | Gene symbol | Location | Function | Linkage | CGAS | SNPs- GWA |
CNVs- GWA |
Expression | |
| Dopaminergic system | SLC6A3 | 5p15.3 | It is known to play a key role in the regulation of DA availability by removing DA from the synaptic cleft into the presynaptic neuron as well as releasing DA into the extracellular space. It is a target for methylphenidate and amphetamine, which are therapeutic for ADHD | * | c/a | c/a | |||
| DRD4 | 11p15.5 | It is a G protein-coupled receptor belonging to the DA D2-like receptor family, which act to inhibit adenyl cyclase. It is involved in ADHD aetiopathogenetic mechanisms because of its high expression in brain regions implicated in attention and inhibition such as anterior cingulate cortex. Moreover DRD4 was the first to be associated with personality trait common in ADHD (novelty-seeking). | c/a | c | |||||
| DRD5 | 4p16.1 | It is a G protein-coupled receptor that belongs to the D1 class of DA receptors and serves to stimulate adenyl cyclase activity. The expression of this gene is higher in hippocampus, a brain area involved in ADHD pathogenesis. Moreover DRD5 is implicated in synaptic strength in hippocampal memory formation. | c/a | c | c/a | ||||
| DRD3 | 3q13.3 | It codes a G protein-coupled receptor belonging to the D2 family of dopamine receptors, which act to inhibit adenylyl cyclase. It is primarily expressed in the nucleus accumbens and substantia nigra, and has been shown to play an important role in incentive-based learning. | c/a | ||||||
| DRD2 | 11q22–q23 | It codes a G protein-coupled receptor, which acts to inhibit adenyl cyclase. It is expressed in several brain regions thought to be relevant to ADHD such as the basal ganglia and prefrontal cortex. It plays a key role in regulating the mesolimbic “reward” pathways. | c/a | ||||||
| DRD1 | 5q34–q35 | DRD1 mutant mice exhibit locomotor hyperactivity and two animal models of ADHD show alterations in the expression and/or function of DRD1. | c/a | ||||||
| Serotoninergic system | SCL6A4 | 17q11.2 | It codes a soluble carrier protein responsible for the reuptake of 5-HT from the synaptic cleft back into the presynaptic neuron. It is a primary mechanism for the regulation of serotoninergic activity in the brain. 5-HT is expressed in brain regions implicated in attention, memory and motor activities. | c/a | |||||
| HTR1B | 6q13 | It is a G protein-coupled receptor that inhibits cyclic AMP formation. It is highly expressed in the dorsal raphe nucleus involved in the sleep/wake cycle. HTR1B “knockout”mice showed increased aggression and impulsive behavior, fail to show the normal hyperlocomotion associated with amphetamine administration, and display an increased response to novel stimuli. | c/a | a | |||||
| HTR2A | 13q14–q21 | It is highly expressed throughout the cortex, hippocampus, amygdala, nucleus accumbens. Inhibition of serotonin 2A receptors attenuates the increases in dopamine activity and hyperlocomotion caused by amphetamine administration and anti-psychotic medications such as clozapine. | c/a | ||||||
| Other receptors | HTR1A, HTR1D, HTR1E, HTR1F, HTR2B, HTR2C, HTR3A, HTR3B, HTR4, HTR5A, HTR6, HTR7 | c/a | |||||||
| Noradrenergic system | SLC6A2 | 16q12.2 | NET1 protein is responsible for the reuptake of norepinephrine from the synaptic cleft back into the presynaptic neuron. It is targeted by atomoxetine. It is most highly expressed in the frontal lobes where it plays a role in noradrenergic and dopaminergic reuptake. | c/a | |||||
| ADRA2A | 10q25.2 | In the prefrontal cortex, it influences executive functions impaired in ADHD. It is a target for two ADHD medications: guanfacine and clonidine. | c/a | ||||||
| ADRA2C | 4p16 | It plays a critical role in regulating neurotransmitter release from sympathetic nerves and from adrenergic neurons in the central nervous system. | c/a | ||||||
| Metabolic enzymes | COMT | 22q11.21 | It is an enzyme that catalyzes a major step in the degradation of DA, NE, and epinephrine; about 60% of the DA degradation in the prefrontal cortex, region implicated in ADHD, is performed by COMT. | c/a | a | ||||
| DBH | 9q34 | It catalyzes the primary enzyme responsible for conversion of DA to NE, and is found in sympathetic terminals, adrenal glands, and in the prefrontal cortex. DBH polymorphisms have been shown to strongly influence plasma levels of the protein and its low plasma levels have been associated with conduct disorder, which often co-occurs with ADHD. | c/a | ||||||
| MAOA | Xp11.4–p11.3 | It encodes a protein involved in the metabolism of DA, 5-HT and NE in the brain. MAOA knockout mouse, showing an aggressive behavior and higher monoaminergic neurotransmitter levels. | c/a | ||||||
| MAOB | Xp11.4–p11.3 | It preferentially metabolizes DA in the brain. Treatment studies suggested that monoamine oxidase inhibitors can reduce ADHD symptom levels. | c/a | ||||||
| TPH1 | 11p15.3–p14 | It catalyzes tryptophan to 5-hydroxytryptophan, which is subsequently decarboxylated to form the neurotransmitter 5-HT. Thus, tryptophan hydroxylase is the rate-limiting enzyme in the production of 5-HT. | c/a | c/a | |||||
| TPH2 | 12q21.1 | A second isoform coded by the gene TPH2, is responsible for tryptophan hydroxylase expression in the brain. | c/a | ||||||
| DDC | 7p12.1 | It catalyses the formation of functional DA through decarboxylation of a precursor tyrosine derivative. It participates in the synthesis of trace amine compounds that are believed to act as modulators of central neurotransmission. | c/a | ||||||
| TH | 11p15.5 | It catalyzes the conversion of the precursor of dopamine (dihydroxyphenylalanine, DOPA) and thus of the catecholamine. It is expressed both in the periphery neuroendocrine system and in the central nervous system. | c/a | ||||||
| BCHE | 3q26.1–q26.2 | It is an enzyme involved in the regulation of neuronal proliferation and differentiation. Variations in BCHE activity can influence the cholinergic system. | a | c | |||||
| SNARE system | SNAP25 | 20p12–p11.2 | It codes for a protein involved in axonal growth, synaptic plasticity, and in the docking and fusion of synaptic vesicles in presynaptic neurons, which is necessary for the regulation of neurotransmitter release. SNAP-25 is deleted in the Coloboma mouse, which shows hyperactivity that, in turn, decreases with amphetamine treatment. | c/a | a | ||||
| Neurodevelopmental network/neurite outgrowth | SYT1 SYT2 SYP STX1A VAMP2 VAMP1 SYNIII CPLX1 CPLX2 CPLX3 CPLX4 STXBP1 SNPH RAB3A NSF CACNA1A |
12q21.3 1q32.1 Xp11.23–p11.22 7q11.2 17p13.1 12p 22q12.3 4p16.3 5q35.2 15q24.1 18q21.32 9q34.1 20p13 19p13.2 17q21 19p13 |
SNARE system. It is involved in the neurotransmission release at the synapse. The SNARE complex is composed of three membrane-associated proteins: SNAP-25, Syntaxin 1A (STX1A) and the vesicular membrane-associated synaptobrevin (VAMP2), that form a bridge between the synaptic vesicle and the plasma membrane, driving the membrane fusion required for neurotransmitter release. In addition, the SNARE core complex interacts with other proteins that mediate the fusion process, such as synaptotagmins (SYT) and complexins (CPLX), or regulate the release of synaptic vesicles, such as synaptophysin (SYP). | c/a | c | ||||
| BDNF | 11p14.1 | It belongs to the family of neurotrophins, involved in promoting neurogenesis, neuronal survival, and synaptic plasticity. Animal models, pharmacological evidence, and molecular genetic studies suggest that it might be involved in the susceptibility to ADHD. | c/a | c/a | |||||
| CNTFR CNTF NTF3 NTRK2,1,3 NTF4/5 NGFR |
9p13 11q12 12p13 9q22.1 19q13.3 17q21–q22 |
They are neurotrophic factors, which participate in neuronal survival and synaptic efficiency and they are strong candidates to contribute to the neuroplasticity changes that take place in the human central nervous system during childhood, adolescence, and early adulthood. Two different groups of NTFs are distinguished according to their actions and signal transduction pathways: 1) NGF, BDNF, neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5), and whose effects are mediated through specific high-affinity neurotrophic tyrosine kinase receptors (NTRKs) 2) a heterogeneous group of molecules that belong to the cytokine family such as ciliary NTF (CNTF). Animal models, pharmacological evidence, and molecular genetic studies suggest that NTFs might be involved in the susceptibility to ADHD. | c/a | ||||||
| NGF | 1p13.1 | It is a neurotrophin that plays a critical role in the development and maintenance of cholinergic neurons implicated in the memory and learning processes. It participates in the regulation of brain plasticity. | c/a | c | |||||
| LPHN3 | 4q13.1 | It is a brain-specific member of the LPHN subfamily of G-protein-coupled receptors that is expressed in ADHD-related brain regions. Different lines of investigation suggest its involvement in synaptic neurotransmitter release as well as in neurodegeneration in response to ischemia and hypoxia. | * | c/a | |||||
| BAIAP2 | 17q25 | It has been identified as a gene differentially expressed between hemispheres. There is evidence suggesting that abnormal left-right brain asymmetries in ADHD patients may be involved in a variety of ADHD-related cognitive processes, including sustained attention, working memory, response inhibition and planning. Although mechanisms underlying cerebral lateralization are unknown, left-right cortical asymmetry has been associated with transcriptional asymmetry at embryonic stages. Other brain lateralization genes include DAPPER1, LMO4, NEUROD6, ATP2B3, ID2 |
c/a | ||||||
| DIRAS2 | 9q22.32 | Brain-expressed DIRAS2 is thought to regulate neurogenesis. It is expressed in structures involved in working memory, error detection and executive control, all of which are impaired in ADHD. | * | c/a | |||||
| CDH13 | 16q23.3 | It codes for T-cadherin that is an atypical member of the cadherin family of cell adhesion molecules. Unlike classical cadherins, it lacks a transmembrane domain, is attached to the cell membrane via a glycosylphosphatidylinositol anchor and has low adhesive properties. Its functions could be potentially related to neurite outgrowth, dendrite arborizations, synapse development, and maintenance of synaptic contacts. T-cadherin is also a receptor for adiponectin and low-density lipoprotein. | * | c/a | c/a | c | |||
| CTNNA2 | 2p12–p11.1 | It encodes the aN-catenin protein that binds typical cadherins with the actin cytoskeleton and it maintains the stability of dendritic spines and synaptic contact. The aN-catenin is highly expressed in the central nervous system, particularly in hypothalamus, amygdala, cingulate cortex, temporal lobe, and PFC. A N-catenins seems to stabilize synapse formation mediated by typical cadherins during the development of nervous system. | c/a | a | c | ||||
| KCNIP4 | 4p15.31 | KCNIP proteins are known as calcium binding proteins that are interaction partners of the voltage-gated potassium channel subunit Kv4 family. This family of subunits is believed to be responsible for the A type potassium current in neurons which is defined as a low-threshold, rapidly activating current that inactivates very fast. This current regulates the firing rate of neurons and the sensitivity to synaptic inputs at the soma and the dendrites. Its interaction with presenilin as thus with Wnt/b-catenin pathway, it can be assumed that KCNIP4 is also involved in developmental processes in the brain. | c/a | c | |||||
| SPOCK3 | 4q32.3 | It is coding for putative Ca(2)-binding extracellular heparan/chondroitin sulfate proteoglycan. Proteoglycanes are constituents of the extracellular matrix and most abundant in the adult brain. In vitro studies showed that proteoglycanes play essential roles in development and interconnection of the neuronal system and in synaptic plasticity. | a | c | |||||
| NOS1/NO | 12q24.22 | Nitric oxide (NO) is a gaseous transmitter produced by nitric oxide synthases (NOSs). The neuronal isoform (NOS-I, encoded by NOS1) is the main source of NO in the CNS. Animal studies suggest that nitrinergic dysregulation may lead to behavioral abnormalities and could be a risk factors for ADHD. | c/a | c | c/a | ||||
| MAP1B | 5q13 | It encodes the microtubule-associated protein 1B that could act in several different categories of cellular functions, such as expression of glutamatergic, systems, neuronal migration, myelination, axon guidance and corpus callosum formation. | a/c | a | |||||
| PRKG1 | 10q11.2 | The cyclic guanosine monophosphate (cGMP) dependent Protein Kinase (PKG) is a transduction pathway enzyme. PKG is operative in a variety of cell responses that are also typical of signal transduction components. It is involved in neuron migration. | a | c | |||||
| ITGAE/ITGA11 | 17p13 | It encodes adhesion molecules and it is involved in neuron projection morphogenesis. | c | a/c | |||||
| DISC1 | 1q42 | It has the highest expression of brain tissue in the hippocampus and the cerebral cortex and have been implicated to be involved in neuronal migration, neurite outgrowth and axon targeting during brain development. | a/c | a | |||||
| Circadian rythms | CLOCK PER2 Melatonin Cortisol |
4q12 2q37.3 |
They are clock genes. The circadian clock is responsible for the generation of rhythms of behaviour and physiology on a near 24 period base, and has a key role in determining the rhythm of the sleep/wake cycle. The molecular basis of such circadian rhythm generation consists of positive and negative transcriptional/translational feedback loops of ‘clock’ genes and their protein products. Some evidence supported that this circadian clock may be compromised in ADHD. | c/a | a | c/a | |||
| Other Systems | OPRM1 | 6q24–q25 | It codes for the υ-opioid receptor, which is the molecular target of endogenous opioid peptides as well as for morphine and other opioids. | c/a | |||||
| ZNF804A | 2q32.1 | It encodes the transcription factor zinc-finger protein 804A. A polymorphism rs1344706 is predicted to enhance the maintenance of binding sites for brain-expressed transcription factors. Another study suggests that rs1344706 is associated with increased response latency on a task assessing the executive control network of attention in healthy volunteers. | c/a | ||||||
| Genes from SNP/CNV/GWAs | GRM | 11q14.3 | It codes for glutamate metabotropic receptors that play a role in neuronal communication, synaptogenesis, cognitive processes. GRM5 is critical for inhibitory learning mechanisms because impaired receptor function results in inappropriate retention of aversive memories. GRM7 is expressed in brain areas related to ADHD such as the cerebral cortex, hippocampus, cerebellum. Glutamate is the major excitatory neurotransmitter in the brain and is involved in a number of processes relevant for ADHD: brain development, modulation of neuronal activity, bidirectional regulation of DA signaling, synaptic plasticity, memory formation and learning. | a/c | c | c/a | |||
| ASTN2 | 9q33.1 | It is a second member of the astrotactin protein family. It has recently been found to interact with ASTN1 in the neuronal membrane and regulate its expression on the neuronal surface, thus mediating the formation and release of neuronal-glial adhesions during migration. | * | a | c | ||||
| GPC5/6 | 13q32 | They are glypican 5/6, belonging to the glypican family. Glypicans comprise a family of glycosyl-phosphatidylinositol-anchored heparin sulfate proteoglycans. Control of cell growth and division seems to be influenced by glypicans. | c/a | ||||||
| Genes from expression studies | microRNA | They are 18–25 nucleotides single-stranded non-coding RNAs that play an important role in the regulation of gene expression at the post-transcriptional level. Mature miRNAs bind to target mRNAs through a 3′UTR interaction region of about 6–8 nucleotides. Once the interaction is established, and depending on the degree of complementarity between the two strands, mRNA translation is repressed either by interference with ribosome binding or by destabilization or degradation of the mRNA molecule. Through this mechanism, miRNAs regulate approximately 60% of human protein-coding gene expression at the posttranscriptional level. Computational estimations indicate that there are more than 800 miRNAs in the human genome and the study of their expression profiles has contributed to the understanding of the role of these non-coding RNA molecules in different biological processes found altered in complex diseases, such as cell development, differentiation or oncogenesis. In the central nervous system, miRNAs are abundant and participate in neuronal survival, development, differentiation and neuronal function. | c/a | ||||||
| Metabolomics | Polyunsaturated fatty acids (PUFAs) | A role for polyunsaturated fatty acids (PUFAs) in ADHD was originally proposed following the observation that hyperactive children had physical signs of fatty acid deficiency, including polydispia, polyuria, dry hair, and skin and follicular keratosis. These signs have been found to be at least 30% more frequent in children with ADHD than in controls. | c/a | ||||||
| Oxidative stress proteins | Oxidative stress parameters. PON: Paraoxonase, TAS: total antioxidant status; TOS: total oxidant status. The term “oxidative stress” describes the biological damage that arises from the imbalance of oxidants and antioxidants. Oxidants damage the protein structure of cell membranes. It is thought that oxidants inhibit uptake of enzymes and/or neurotransmitters involved in the physiological functioning of cells, which may be a predisposing factor for disease. This mechanism could be altered in ADHD. - | c/a | |||||||
| Kynurenines | The kynurenine pathway constitutes the major route for catabolism of the essential amino acid tryptophan. Tryptophan metabolites can modulate several neurotransmitter systems, including dopaminergic and serotoninergic transmissions, systems altered in ADHD. | c/a | |||||||
| Adiponectin | Recent studies have shown co-occurrence of obesity and ADHD in children and adults. Thus, these comorbidities may reflect a common etiology or the involvement of common pathways, as well as a cross-talk between adipose tissue and the central nervous system. Adiponectin is an adipokine hormone that has insulin-sensitizing and anti-inflammatory effects, stimulates fatty acid oxidation and its expression is regulated by insulin, testosterone and glucocorticoids. It thus influence appetite and weight gain. | c/a | |||||||
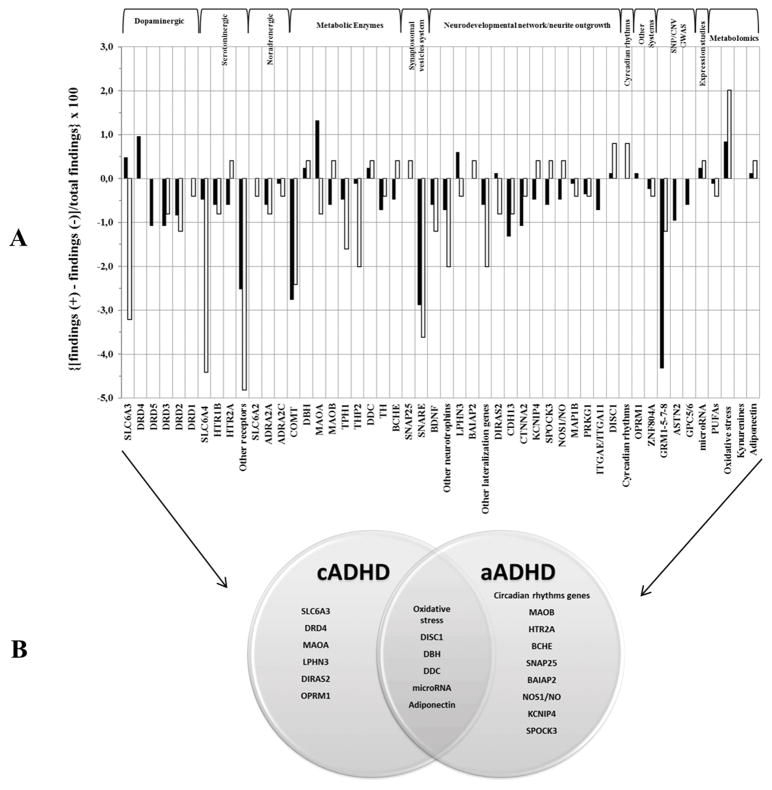
Figure 2.
A. The histogram showed the ratio for each gene/polymorphism/protein from genomic and metabolomic studies of children with ADHD (cADHD) and adults with ADHD (aADHD). The columns in black refer to cADHD, whereas those in white refer to aADHD. Details in calculations are reported in supplementary material. B. The Venn diagram where common and specific genes for cADHD and aADHD are reported.
Captions: SLC6A3=dopamine transporter; DRD4=dopamine receptor D4; DRD5=dopamine receptor D5; DRD3=dopamine receptor D3; DRD2=dopamine receptor D2; DRD1=dopamine receptor D1; SLC6A4=serotonin transporter; HTR1B=serotonin receptor 1B; HTR2A=serotonin receptor 2A; other serotonin receptors=HTR1A, HTR1D, HTR1E, HTR1F, HTR2B, HTR2C, HTR3A, HTR3B, HTR4, HTR5A, HTR6, HTR7; SLC6A2=norepinephrine transporter; ADRA2A=alpha-2A-adrenergic receptor; ADRA2C=alpha-2C-adrenergic receptor; COMT =catechol-O-methyltransferase; DBH=dopamine beta-hydroxylase; MAOA=monoamine oxidase A; MAOB=monoamine oxidase B; TPH1=tryptophan hydroxylase 1; THP2=tryptophan hydroxylase 2; DDC=dopamine decarboxylase; TH=tyrosine hydroxilase; BCHE=acetylcholine metabolizing butyrylcholinesterase; SNAP25=synaptosomal-Associated Protein 25; SYT1,2/SYP/STX1A/VAMP1,2/CPLX1,2,3,4/STXBP1/SNPH/RAB3A/NSF/CACNA1A/SYNIII=SNARE system; BDNF=brain derived neurotrophic factor; CNTF/CNTFR/NGF/NTF3,4,5/NTRK1,2,3/NGFR=neurotrophins; LPHN3=latrophin-3; BAIAP2=BAI1-associated protein 2; other brain lateralization genes=DAPPER1, LMO4, NEUROD6, ATP2B3, ID2; DIRAS2=GTP-binding RAS-like 2; CDH13=T-cadherin 3; CTNNA2=catenin (cadherin-associated protein); KCNIP4=kv channel interacting protein 4; SPOCK3=Sparc/Osteonectin, Cwcv And Kazal-Like Domains Proteoglycan (Testican) 3; NOS1=nitric Oxide Synthase-1, NO (Nitric Oxide metabolite); MAP1B=Microtubule-Associated Protein 1B; PRKG1=Protein kinase G gene; ITGAE/ITAGA11=Integrin, Alpha E (Antigen CD103, Human Mucosal Lymphocyte Antigen 1; Alpha Polypeptide); DISC1=Disrupted In Schizophrenia 1; CLOCK/PER2/MELATONIN/Cortisol=circadian rhythms genes; OPRM1=Opioid Receptor, Mu 1; ZNF804A=Zinc Finger Protein 804A; GRM1,5,7,8=gluatamate receptor, metabotropic; ASTN2=Astrotactin 2; GPC5/6=Glypican 5/6; microRNA; Polyunsaturated Fatty Acids (PUFAs)=arachidonic acid (AA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA); Oxidative stress proteins=MAD (Malondialdehyde), SOD (Superoxide Dismutase), Paraoxonase (PON1), Arylesterase (ARES), total antioxidant status (TAS), total oxidant status (TOS) oxidative stress index (OSI) ; Kynurenine pathway=tryptophan, kynurenine, kynurenic acid, 3-hydroxykynurenine
Genomics
Linkage studies in ADHD children and adults
Since 2002, there have been 14 linkage studies performed for cADHD, including 11 GW-linkage analyses (Li et al. 2014). However, few loci were implicated and the only regions showing a meta-analytic significance were 16q23.3 (Zhou et al. 2008) (CDH13, T-cadherin 3) and 5p13 (Ogdie et al. 2006) (SLC6A3, dopamine transporter). One study reported associations to three SNPs in LPHN3 (latrophin-3) locus, the 4q13.1 (Li et al. 2014). Moreover 9q22 (DIRAS2, GTP-binding RAS-like 2) and 9q33 (ASTN2, astrotactin 2) were associated to cADHD (Li et al. 2014).
In adults, two studies were conducted on pure aADHD, one of them pointed to 16q23.3 as a disease gene harboring region (Lesch et al. 2008).
Candidate gene association studies in cADHD and aADHD
Dopaminergic system
SLC6A3. A frequently studied variant is a 40bp variable number of tandem repeats (VNTR) polymorphism located in the 3′-untranslated region (3′-UTR). Gizer et al.’s meta-analysis reported an association between the 10R allele and cADHD (Gizer, Ficks, Waldman 2009). Another VNTR in intron 8 widely investigated is the 30-bp repeat sequence. The 6R allele was associated with cADHD by meta-analysis (Gizer, Ficks, Waldman 2009). Some studies indicated associations of the haplotypes formed by the 10R allele of the 3′UTR and the 3R allele of the intron 8 or the 10-6 with increased risk for ADHD (Gao et al. 2014; Li et al. 2014). Other single polymorphisms have been investigated: rs6347 (exon 8) and rs40184 (intron 13) were not associated, whereas rs27072 (3′UTR) was associated to cADHD (Gizer, Ficks, Waldman 2009).
The association of the 9-6 haplotype and the 9-9 genotype with aADHD was confirmed by a meta-analysis of 1,440 cases and 1,769 controls, and in single successive studies on 9R risk allele (Franke et al. 2012). A new allele 9.5R was detected and found associated to aADHD (Hasler et al. 2015). Moreover de Azeredo et al. (2014) reported that rs2652511 in the promoter region was a risk factor for aADHD. Notwithstanding these positive results, others supported negative findings (Table S1).
Dopamine receptor DRD4. Most association studies examined a highly polymorphic VNTR in the third exon, which presents 2 to 11 copies of a 48-bp repeat sequence. The 7R allele has been consistently associated with cADHD (Gizer, Ficks, Waldman 2009; Smith 2010; Wu et al. 2012). Recently, rare variants and non-synonymous mutations in the VNTR region of the 7R allele have been identified in ADHD subjects (Grady et al. 2003), suggesting presence of allelic heterogeneity. Meta-analyses of other polymorphisms in/del (Gizer, Ficks, Waldman 2009; Wu et al. 2012) and rs747302 (Wu et al. 2012) showed negative results. Contrasting results were obtained by two meta-analyses for the rs1800955 (Gizer, Ficks, Waldman 2009; Wu et al. 2012). A small number of association studies focused on other SNPs in the promoter region of DRD4, such as −615A/G and −376C/T showed negative results (Wu et al. 2012). Different haplotypes showed prevalently associations with cADHD (Gao et al. 2014; Li et al. 2014).
Contrasting results were obtained for the association of the VNTR and aADHD (Franke et al. 2012). A large meta-analysis in 1608 aADHD patients and 2358 controls was negative for this polymorphism as well as for the promoter 120-bp insertion/deletion, however evidence for association of a haplotype formed by the 4R allele and the long (L) allele was observed (Sanchez-Mora et al. 2011). A different distribution of the 6R allele was found in aADHD (Hasler et al. 2015). Negative results were also obtained (Table S1).
Dopamine receptor DRD5. A highly polymorphic dinucleotide repeat (CA)n, located in 18.5 kb at the end of the 5′ flanking region, has been the most studied DNA variant. The 148-bp allele was associated with ADHD according to meta-analyses (Gizer, Ficks, Waldman 2009; Wu et al. 2012). However, considering other studies, no associations were found in cADHD but also in aADHD (Table S1).
Dopamine receptor DRD3. Two meta-analyses (Gizer, Ficks, Waldman 2009; Wu et al. 2012) of the functional polymorphism in exon 1 Ser9Gly (rs6280) were negative. Additional studies on other polymorphisms/haplotypes showed exclusively negative findings (Table S1).
Similar conclusions were obtained in aADHD (Franke et al. 2012; Table 1S).
Dopamine receptor DRD2. The results from the first meta-analysis (Gizer, Ficks, Waldman 2009) were negative for TaqIA restriction site (rs1800497) polymorphism. Successive meta-analyses showed positive results (Wu et al. 2012; Pan et al. 2015). Further evidences consisted in negative studies for other polymorphisms/haplotypes (Table S1).
Similar conclusions were obtained in aADHD (Franke et al. 2012; Table S1).
Dopamine Receptor DRD1. A single and multiple marker analysis in 322 cADHD, 533 controls and in 196 nuclear ADHD families provided evidence for the contribution of DRD1 to cADHD (Ribases et al. 2012). This association was confirmed in a replication sample. A meta-analysis on rs4532 showed no association (Wu et al. 2012). Studies on other polymorphisms/haplotypes reported additional associations with this gene (Table S1).
In 211 aADHD and 533 controls, DRD1 was not found associated to aADHD (Ribases et al. 2012).
Serotoninergic system
Serotonin transporter SLC6A4. Results of a meta-analysis (Gizer, Ficks, Waldman 2009) indicated an association between ADHD and the functional 5HTTLPR 44-bp insertion/deletion “long” allele. In addition to the 5HTTLPR, a 17-bp repeat in intron 2 (STin2) and a SNP in the 3′ UTR (rs3813034) of SLC6A4 have been studied in relation to cADHD with no associations (Gizer, Ficks, Waldman 2009). Additional studies on other polymorphisms/haplotypes reported positive results (Table S1).
In adults, a meta-analysis of 5HTTLPR did not support a major role (Landaas et al. 2010). Subsequent studies were negative (Franke et al. 2012; Table S1).
Serotonin receptors. The HTR1B’s meta-analysis of the G861C (rs6296) indicated an association with G allele (Gizer, Ficks, Waldman 2009). However the gene as whole resulted not associated to cADHD (Table S1). Concerning the Serotonin 2A receptor (HTR2A), a meta-analysis (Gizer, Ficks, Waldman 2009) indicated no association for three polymorphisms (rs6314, rs6313, rs6311). More recently other SNPs and haplotypes were associated to cADHD (Table S1).
In adults, positive and negative results were found for HTR2A and HTR1B genes respectively (Franke et al. 2012; Table S1).
Other serotonin receptor genes HTR1A, HTR1D, HTR1E, HTR1F, HTR2B, HTR2C, HTR3A, HTR3B, HTR4, HTR5A, HTR6, HTR7 showed prevalently negative results in cADHD and aADHD.
Noradrenergic system
Norepinephrine Transporter SLC6A2. A frequently studied SNP (rs5569 or G1287A) located in exon 9 was not associated with cADHD in a meta-analysis (Gizer, Ficks, Waldman 2009). No association was also observed between cADHD and rs2242447 (Gizer, Ficks, Waldman 2009). More recently associations were reported with specific haplotypes also in relation to sex and subtypes (Table S1).
Alpha-2A-adrenergic receptor, ADRA2A. Two meta-analyses confirmed no association with ADHD and the A-1291 C to G variant creating an MspI site in the promoter region (Gizer, Ficks, Waldman 2009; Shiffrin et al. 2013). Similarly rs553668 and rs1800545 were not associated with cADHD (Gizer, Ficks, Waldman 2009). Associations were observed with some haplotypes (Table S1). ADRA2C. For this gene a lacking of association was found (Hawi et al. 2013). Further studies confirmed this finding (Table S1).
There has been no evidence of association for these three genes with aADHD ( Table S1).
Metabolic enzymes
Catechol-O-methyltransferase, COMT. Meta-analyses indicated no association between ADHD and the most popular marker Val156Met (Gizer, Ficks, Waldman 2009; Sun et al. 2014; Lee and Song 2015). Further studies on other polymorphisms/haplotypes indicated prevalently negative results.
Dopamine beta-hydroxylase, DBH. Many studies focused on a TaqI restriction polymorphism (rs2519152) and the results from meta-analysis showed no association between the A2 allele and cADHD (Gizer, Ficks, Waldman 2009). Meta-analyses of rs1611115 and rs1108580 did not detect associations (Gizer, Ficks, Waldman 2009). Further studies on other SNPs/haplotypes found positive and negative findings (Table S1).
In aADHD, contrasting results with a tendency toward negative results were obtained for COMT and positive for DBH (Franke et al. 2012; Table S1).
Monoamine oxidase, MAO. Negative results were obtained according to meta-analysis (Gizer, Ficks, Waldman 2009) but considering more recent studies, the data were prevalently positive for MAOA gene (Table S1). Fewer studies were conducted for MAOB, but they did not support its involvement in cADHD (Table S1).
Instead, in 188 aADHD patients and 400 controls, MAOB was associated with aADHD (Ribases et al. 2009a).
Tryptophan hydroxylase, TPH. For TPH1, a meta-analysis of the silent A to C substitution in intron 7 (rs1800532) indicated no association to cADHD (Gizer, Ficks, Waldman 2009). For TPH2, no meta-analytic associations were found for rs1843809 and rs1386493. Further studies for both genes showed prevalently negative results (Table S1).
In adults, a recent meta-analysis of 1,636 cases and 1,923 controls reported no associations for rs17794760 in TPH1 and for rs7305115, rs7963717, rs4760818, rs1386496, rs4760820 in TPH2 (Franke et al. 2012; Table S1).
Dopamine decarboxylase, DDC. More papers reported associations of this gene with cADHD (Table S1).
In 188 patients and 400 controls, Ribases et al. 2009a found a strong association with aADHD.
Tyrosine hydroxylase, TH. Ribases et al. 2012 reported an association of rs2070762 with cADHD. In contrast, other studies showed negative findings (Table S1).
In aADHD, the same authors showed no association.
Butyrylcholinesterase, BCHE. A study showed an association with aADHD (Jacob et al. 2013).
Synaptosomal vesicles system
Synaptosome Associated Protein 25kDa, SNAP-25. rs3746544 was found associated to cADHD according to meta-analysis (Gizer, Ficks, Waldman 2009). Meta-analyses of rs362987 (intron 4), rs363006 (intron 6) and rs1051312 (3′UTR) were negative (Gizer, Ficks, Waldman 2009). In addition associations were identified between other polymorphisms or different combinations of haplotypes and cADHD. In Asian population, an association was found between 1065G>T and cADHD (Gao et al. 2014) (Table S1).
Herken et al. 2014 detected a significant association of the MnlI polymorphism with aADHD and with symptom severity in the Turkish population. This result was not confirmed in Olgiati et al. 2014. Other studies showed contrasting results (Table S1).
Other SNARE genes. Guan et al. (2009) assessed SYP (Synaptophysin), STX1A (Syntaxin 1A), SYT1 (Synaptotagmin 1) and VAMP2 (Vesicle-associated membrane protein 2) and found that SYP was associated with cADHD in a Chinese population. Liu et al. (2013a) replicated this result in a larger sample of Han Chinese subjects. Other studies showed prevalently negative findings. The gene CPLX2 (complexin 2) was investigated in Sanchez-Mora et al. (2013a) with a significant association (Table S1).
In adults, STX1A was associated in two studies (Sanchez-Mora et al. 2013a; Kenar et al. 2014) and VAMP2 and SYT2 according to Kenar et al. (2014) and Sanchez-Mora et al. (2013a) respectively. The complex as whole showed no association with aADHD (Table S1).
Neurodevelopmental network/neurite outgrowth
Brain derived neurotrophic factor, BDNF. Two meta-analyses found no association of the functional Val66Met (rs6265) with cADHD (Gizer, Ficks, Waldman 2009; Lee and Song 2015). Specific haplotypes or other polymorhisms were associated with cADHD (Table S1).
Three studies investigated the association with aADHD, including a meta-analysis of 1,445 cases and 2,247 controls. The results showed just one positive finding (Franke et al. 2012; Table S1).
Other neurotrophins. Studies of other genes belonging to this family showed an association between CNTFR (ciliary neurotrophic factor receptor), NTF3 (neurotrophin 3) and NTRK2 (neurotrophic tyrosine kinase receptor) in cADHD (Ribases et al. 2008). Other studies showed negative results for CNTF (ciliary neurotrophic factor), CNTFR and NTF3. Two negative findings were observed for NGF (Nerve Growth Factor) (Table S1).
In aADHD, one study reported an association with CNTFR but not with NTF3 (Ribases et al. 2008). The same study found association with NGF.
LPHN3. The first association was observed in a genetic Paisa isolate and was confirmed in a 2,627 cases, 2,531 controls and 1,202 relatives from five different populations. Additional positive associations were found, even more recently (Hwang et al. 2015) (Table S1).
One case-control studies on large samples reported a significant association of several polymorphisms (43 SNPs) and aADHD (Ribases et al. 2011). Other studies did not support this finding (Arcos-Burgos et al. 2010; Sanchez-Mora et al. 2015a).
BAI1-associated protein 2, BAIAP2 and other cerebral lateralization genes. A study showed that BAIAP2 was associated with cADHD in Han Chinese descents (Liu et al. 2013b). These results were not confirmed in a Caucasian cADHD population (Ribases et al. 2009b).
In aADHD, BAIAP2 was found to be associated in two Caucasian populations (Ribases et al. 2009b).
DIRAS2. Reif et al. (2011) conducted an association study in 166 families with a ADHD child and 420 controls. The results showed an association of a promoter specific haplotype block.
In adults, a meta-analysis of rs7854469, rs16906711, rs1331503, rs1412005 and of the risk haplotype ACGCTT performed in four populations showed associations for rs1412005 and for the risk haplotype only in German population (Reif et al. 2011).
CDH13. An association study indicated positive findings of rs11150556 to cADHD (Salatino-Oliveira et al. 2015), no confirmed in Gomez-Sanchez et al. (2016).
Mavroconstanti et al. (2013) identified seven coding variants none of them showed associations with aADHD. Further negative results were obtained (Salatino-Oliveira et al. 2015).
Catenin (Cadherin-Associated Protein), Alpha 2, CTNNA2. No associations were found for the rs1339502 and cADHD and aADHD (Salatino-Oliveira et al. 2015).
Kv Channel Interacting Protein 4, KCNIP4. Two SNPs showed association with cADHD in 171 families, whereas six SNPs and haplotypes showed association with aADHD in 594 patients and 974 controls from Germany (Weiflog et al. 2013).
Sparc/Osteonectin, Cwcv And Kazal-Like Domains Proteoglycan (Testican) 3, SPOCK3. Associations of rs7689440 and rs897511 was observed in 624 aADHD and 536 controls (Weber et al. 2014).
Nitric Oxide Synthase-1, NOS-1, Nitric Oxide (NO). The rs478597 was not associated to cADHD (Salatino-Oliveira et al. 2016).
Two variants of the NOS1 (exon 1c, 1f VNTR) were genotyped in aADHD reporting no association (Kittel-Schneider et al. 2015). In other studies, the exon 1f was found associated to aADHD (Franke et al. 2012; Table S1).
Microtubule-Associated Protein 1B, MAP1B. The rs2199161 was not associated to cADHD and in aADHD (Salatino-Oliveira et al. 2016).
Protein kinase G, PRKG1. In 125 nuclear families with aADHD patients, the C2276T (3′UTR) was not associated (De Luca et al. 2002).
Integrin, Alpha E (Antigen CD103, Human Mucosal Lymphocyte Antigen 1; Alpha Polypeptide), ITGAE/ITGA11. No association of this gene was found with cADHD (Laurin et al. 2008).
Disrupted In Schizophrenia 1, DISC1. rs11122330, rs6675281, rs11122319 were associated to cADHD (Kayyal et al. 2015).
In adults, one positive study was performed in Spain and Norwegian populations (Jacobsen et al. 2013).
Circadian rhythms
CLOCK, PER2, Melatonin, Cortisol. An over transmission of the T allele (rs1801260) of the CLOCK (Circadian locomotor output cycles kaput) gene was observed in both Taiwanese and British cADHD (Xu et al. 2010). Negative results were obeserved for PER2 (Period Circadian Clock 2) (Table S1).
An association of rs1801260 was found in Caucasian aADHD (Kissling et al. 2008).
Other systems
Opioid Receptor, Mu 1, OPRM1. An association was observed in cADHD (Drtilkova et al. 2008).
Contrasting results were obtained in aADHD (Table S1).
Zinc Finger Protein 804A, ZNF804A. Xu et al. 2013 reported no association of rs1344706 with cADHD in UK and Taiwanese samples.
Similar results in aADHD (Landaas et al. 2011).
Glutamate Receptor, Metabotropic 7, GRM7. Contrasting results were found with cADHD (Table S1).
In aADHD, one negative finding was available for GRM7 (Akutagava-Martins et al. 2014a).
Genome wide Association studies (GWAs)
Common Variants: SNPs-GWAs
In cADHD, seven GWAs and one meta-analysis were performed (Hawi et al. 2015). In aADHD, two were conducted (Lesch et al. 2008; Sanchez-Mora et al. 2015b).
CDH13. SNPs at this gene ranked among the top results in two SNPs-GWAs in cADHD (p=10−5) (Hawi et al. 2015).
In 343 aADHD and 304 controls, the findings provide significant results (p=10−6) (Lesch et al. 2008).
NOS1. One study showed an association (p=10−6) in cADHD (Lasky-Su et al. 2008).
GRM5. For this gene an association (p=10−6) was observed for cADHD (Hinney et al. 2011).
KCNIP4 (Neale et al. 2008) (p=10−5), SPOCK3 (Neale et al. 2008) (p=10−5), PRKG1 (Neale et al. 2010; Yang et al. 2013) (p=10−7), Glypican 5/6 (GPC5/6) (Hinney et al. 2011) (p=10−5), ITGAE/ITGA11 (Yang et al. 2013) (p=10−5). Significant associations were reported.
ASTN2 (p=10−7), CTNNA2 (p=10−6), GPC5/6 (p=10−8), MAPB1 (p=10−6), ITGAE/ITGAE11 (p=10−7) showed associations to aADHD (Lesch et al. 2008).
Rare Variants: CNVs-GWAs(Hinney et al. 2011)
To date ten CNVs-GWAs have been published for cADHD (Hawi et al. 2015). In aADHD, two studies were available (Akutagava-Martins et al. 2014b; Ramos-Quiroga et al. 2014b).
GRM5. It has been demontrated that GRM5 and GRM7 were among the genes affected by CNVs. The same authors detected again an overrepresentation of CNVs affecting GRM5 and GRM1, GRM7, GRM8 in cADHD (Hawi et al. 2015; Table S1). Akutagava-Martins et al. 2014b did not confirm this association.
In adults, a higher CNVs frequency was detected in aADHD for GRM1,5,8 (Akutagava-Martins et al. 2014b).
DRD5. Lionel et al. (2011) found an overlapping with DRD5 locus and cADHD probands which was absent in controls.
BCHE. Elia et al. (2010); Lesch et al. (2011); Lionel et al. (2011) gave evidence for a 3q26.1 deletion and among the involved genes, BCHE showed the stronger association in cADHD.
CDH13. One study reported a deletion in one patient with cADHD, affecting part of this gene (Lionel et al. 2011).
CPLX2 (SNARE), ASTN2 were found associated to cADHD (Lionel et al. 2011).
CTNNA2. A deletion was reported in cADHD (Elia et al. 2010).
HTR1B, COMT, DISC1. CNVs were involved in these genes in aADHD (Ramos-Quiroga et al. 2014b).
Expression studies in peripheral systems in cADHD and aADHD
DRD4. Taurines et al. (2011) reported that the concentrations of blood DRD4-mRNA and not of DRD5 and TPH1 were lower in cADHD compared to controls. In another study the expression levels of SLC6A3, DRD4 and DRD5 were different between patients and controls (Xu et al. 2015).
SLC6A3, DRD5, TPH1, SNAP25. In a pilot study in aADHD, some authors demonstrated that combining the gene expression levels of these genes as predictors resulted in sensitivity and specificity of over 80% (Grunblatt et al. 2012).
Circadian rhythms (CLOCK, PER2, Melatonin, Cortisol). Alterations in circadian rhythms were observed in aADHD, with loss of PER2 and melatonin expression in patients (Baird et al. 2012).
microRNAs (miRNAs). Kandemir et al. (2014) reported, in 52 cADHD patients, that miRNA 18a-5p, 22-3p, 24-3p, 106b-5p and 107 levels were decreased whereas miRNA 155a-5p were increased in patients. Sanchez-Mora et al. (2013) evaluated the contribution of one SNP in the miR-96 target site at HTR1B and eight tagSNPs within the region containing this miRNA in 695 aADHD and 485 controls from Spain. Associations were observed between rs2402959 and rs6965643 of miR-96.
Metabolomics
Peripheral protein levels in cADHD and aADHD
BDNF. No difference with controls was found in serum levels in an Italian cADHD patients (Scassellati et al. 2014) whereas higher plasma levels were associated in Asian population (Shim et al., 2008).
Corominas-Roso et al. (2013) found serum levels lower in aADHD compared to controls.
NGF. Guney et al. (2014) reported that the serum NGF levels of the cADHD patients were higher than those of the controls.
NOS1, NO. NO activity for cADHD patients was different than those of the controls (Ceylan et al. 2010; Ceylan et al. 2012). Contrasting results were obtained in aADHD (Table 1S).
Circadian rhythms (CLOCK, PER2, Melatonin, Cortisol). Cortisol rhythms significantly phase delayed in the aADHD group (Baird et al. 2012). Contrasting results were observed for cortisol and for melatonin (Table S1).
Reduced salivary levels of cortisol were observed also in cADHD (Scassellati et al. 2012).
Polyunsaturated Fatty Acids (PUFAs). More negative results were observed for the serum arachidonic acid (AA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) levels in cADHD as compared to controls (Scassellati et al., 2012).
Similarly, in adults studies confirmed no alterations in AA, DHA, EPA (Table S1).
Oxidative stress proteins (MAD, SOD, PON1, ARES, TAS, TOS, OSI). Prevalently positive results were observed in ADHD adults and children for molecules belonging to the oxidative stress system (MAD Malondialdehyde; SOD Superoxide Dismutase; Paraoxonase PON1; Arylesterase ARES) and the total antioxidant status (TAS), total oxidant status (TOS) and oxidative stress index (OSI) (Table S1).
Kynurenine Pathway (Tryptophan, kynurenine, Kynurenic acid, 3-hydroxykynurenine). Same positive and negative results were obtained for this pathway both in cADHD and aADHD (Table S1).
Adiponectin. Two positive studies were available in aADHD (Mavroconstanti, Halmoy, Haavik 2014) and cADHD (Ozcan et al. 2015).
Discussion
Consistently with a developmental twin study where different sources of additive genetic variance accounted for the expression of ADHD in childhood and adulthood (Chang et al. 2013), our review found some divergence in findings between cADHD and aADHD, which suggests differential etiological pathways for different age ranges.
Through a convergent functional genomic approach and statistical analyses, the results (Figure 2, Table 1) indicate that SLC6A3, DRD4, MAOA, LPHN3, DIRAS2 and OPRM1 are specific for cADHD (p=0.016; OR=2.27 95%CI:1.15–4.47, Table S2) whereas circadian rhythms genes, MAOB, HTR2A, BCHE, SNAP25, BAIAP2, NOS1, KCNIP4, SPOCK3 are specific for aADHD (p=0.007; OR=3.30 95%CI:1.33–8.29, Table S2). Moreover we detected some genes in common: MAD, SOD, PON1, ARES, TOS, TAS OSI, followed by DISC1, DBH, DDC, microRNA and adiponectin (Table S2).
Specific genes
Longitudinal designs suggest that hyperactive/impulsive ADHD symptoms decrease with age (Biederman, Mick, Faraone 2000; Larsson, Lichtenstein, Larsson 2006). Given that expression levels of genes can differ across different stages of development (Elia and Devoto 2007; Langley et al. 2009), the contribution of some risk alleles/proteins to ADHD may not be constant across the life course. Such a pattern would be consistent with the developmental twin study of Chang et al. 2013.
Our results indicate that three dopaminergic genes, SLC6A3, DRD4 and MAOA, are specifically associated to cADHD. The list of ten candidate genes showing replicated evidence of association with cADHD (Hawi et al. 2015) includes SLC6A3 and DRD4. These data add further weight to the dopamine hypothesis where a dysregulation of dopaminergic neurotansmission is due to dopamine deficit in ADHD (Genro et al. 2010). Recently a hypothetical signature of genetic and biochemical markers that, someday, might be useful for diagnosing ADHD in childhood has been suggested (Faraone, Bonvicini, Scassellati 2014; Scassellati and Bonvicini 2015). This signature includes SLC6A3, DRD4 and MAOA, which is consistent with our conclusions. In aADHD MAOB, which encodes the enzyme that metabolizes dopamine, was more strongly associated. This could suggest a differential effect of this pathway with SLC6A3, DRD4, MAOA being more implicated in cADHD and MAOB in aADHD.
The differential effect of these genes has some precedent in the literature. A well-known example are two meta-analytic findings. One associates, even though not significant after Bonferroni correction, the 9R allele of the 40bp SLC6A3 VNTR with aADHD (Bonvicini, Faraone, Scassellati 2016), another associates the 10R to cADHD (Gizer, Ficks, Waldman 2009). One hypothesis about this difference is that, because DAT1 density decreases during life and ADHD symptoms are known to change during adolescence, the differential association of DAT1 with ADHD might reflect changing requirements on the dopaminergic system during life, probably influenced also by the functional effect of this polymorphism, with the 9R allele associated to increased in vivo DAT activity (Faraone et al. 2014).
Moreover, the DRD4 7R allele is associated with cortical thickness trajectories in early development but this association was no longer found around 16–18 years of age (Shaw et al. 2007; Shaw et al. 2013).
Finally, MAOA and MAOB were associated differentially to cADHD and aADHD respectively (Ribases et al. 2009a), confirming our results.
Further evidence of specific associations with cADHD comes from some genes linked to neurodevelopmental network such as LPHN3 and DIRAS2. In particular they are involved in synaptic plasticity, neurite outgrowth (LPHN3), and are expressed in those structure involved in working memory, error detection and executive control (DIRAS2), impaired in ADHD. LPHN3 is in the list of ten candidate genes showing replicated evidence of association with cADHD (Hawi et al. 2015).
In aADHD, other systems have been associated to this adult form; in particular circadian rhythms genes, HTR2A, and a more generic neurodevelopmental/neurite outgrowth represented by BCHE, SNAP25, BAIAP2, NOS1, KCNIP4 and SPOCK3 genes. These specific associations could reflect a certain versatility of these genes throughout the lifespan, first with a less significant role on early brain development and a larger role later in the lifecycle.
A recent review (Coogan et al. 2016) reported several studies supporting that circadian timekeeping appears to be altered in ADHD. One interesting facet of such alterations is the relatively strong concordance between different studies indicating phase delays associated with exclusively aADHD as assessed by endocrine, molecular, activity, sleep, and psychometric parameters. Although the significance of the observed phase alterations and desynchronisation of rhythms observed in ADHD is not fully understood, these may provide novel important therapeutic targets.
BCHE, SNAP25, BAIAP2, NOS1, KCNIP4 and SPOCK3 are as whole implicated in the neurite outgrowth, synaptic plasticity and neuronal proliferation/differentiation systems that were already demonstrated to be involved in ADHD by the bioinformatic analyses of Poelmans et al. 2011 and Hawi et al. 2015. These mechanisms, which are believed to underpin processes relevant for ADHD such as cognitive performance, attention, learning and memory formation, could be linked to a potential differential effect in the developing (LPHN3 and DIRA2) and adult (BCHE, SNAP25, BAIAP2, NOS1, KCNIP4, SPOCK3) brain. BAIAP2 was meta-analitic associated to aADHD (Bonvicini, Faraone, Scassellati 2016). The list of ten candidate genes showing replicated evidence of association with ADHD included SNAP25 and NOS1 (Hawi et al. 2015). SNAP25 plays an important role in axonal growth, synaptic plasticity and neurotransmitter release, acting in essential steps for wiring the nervous system. NOS1 influences cadherin systems and plays a direct role in neurite outgrowth. As reported in Poelmans et al. (2011) several of these proteins appear to be under control of the stimulants methylphenidate and amphetamine that are used to treat ADHD symptoms. Both stimulants have been shown to stimulate neurite outgrowth and directly or indirectly regulate the expression and/or funtion of several genes/proteins implicated in this network.
In summary, these data suggest that specific genetic and biological mechanisms can differ with age and suggest differential effects for dopaminergic and neurodevelopmental/neurite outgrowth systems in cADHD (SLC6A3, DRD4, MAOA, LPHN3, DIRAS2) and in aADHD (MAOB, BCHE, SNAP25, BAIAP2, NOS1, KCNIP4, SPOCK3). Fewer studies were performed for KCNIP4, SPOCK3, and DIRAS2 genes. Given their important roles in brain (Table 1), further investigations are needed.
Common genes
The involvement of the oxidative stress system as major actor followed by some dopamine metabolism enzymes (DBH, DDC), DISC1, microRNA and adiponectin in cADHD and in aADHD strengthens the hypothesis that dysregulation of oxidative stress mechanisms as well as of dopamine metabolism are implicated in ADHD independently of age.
Research suggests that oxidative stress predispones to a diverse range of psychiatric conditions, including ADHD (Joseph et al. 2015). A meta-analysis concluded that measures of oxidative stress were elevated among ADHD patients both children and adults (Joseph et al. 2015). Our results confirm the involvement of this system throught lifespan. Validating the oxidative stress as a component of ADHD’s pathophysiology in children and adults with further studies, could be mandatory mainly because antioxidants might be useful for the treatment of ADHD.
More findings should be carried out on DISC1, a gene implicated in neuronal migration, neurite outgrowth and axon targeting during brain development.
This review has several limitations/comments: -to give the same weight to the different findings (single study and meta-analysis), we divided each meta-analysis available for a certain gene or protein, into single studies. Moreover, as the SNPs-CNVs-GWAS studies available for a gene showed mostly negative results for children, more genes resulted in associations with aADHD, -some genes implicated by our review should be replicated to yield definite conclusions: OPRM1, adiponectin, KCNIP4, SPOCK3, microRNA, DIRAS2, DISC1, ZNF804A, ASTN2, GPC5/6, kynurenine pathway, CTNNA2, MAP1B, ITGAE/ITGA11, -all genetic studies performed in childhood and/or adulthood ADHD so far are characterized by a lack of power and their results have not provided genome-wide association hit or robust candidate gene, -considering the decrease in hyperactive/impulsive symptoms over the life cycle, future analyses of this symptom domain with the different genes at different age ranges could to be useful (Bralten et al. 2013).
To the best of our knowledge, this is the first review that with a convergent functional genomics approach and statistical analyses contribute to clarify which genetic/biological mechanisms associated with ADHD differ by age. There are some genes whose effects do not differ throughout lifetime, whereas others are linked to age specific stages. Additional research and further studies are needed to generate firmer conclusions that might someday be useful for predicting the remission and persistence of the disorder. Although the limitations and the needing to confirm these results, some of these genes/proteins could be potential useful biomarkers to discriminate cADHD versus aADHD.
Supplementary Material
Numbers of positive and negative findings from linkage studies, genomics (candidate gene association, GWA, CNVs, expression studies) and metabolomics conducted in children and adults with ADHD
2-way Contingency Table Analyses for pathways in children and adults with ADHD
Acknowledgments
This research was supported by grants from the Italian Ministry of Health [Ricerca Corrente]. Professor Faraone is supported by the K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 602805 and NIMH grant R01MH094469.
Abbreviations
- SLC6A3
dopamine transporter
- DRD4
dopamine receptor D4
- MAOA
monoamine oxidase A
- LPHN3
latrophin-3
- DIRAS2
GTP-binding RAS-like 2
- OPRM1
Opioid Receptor, Mu 1
- HTR2A
serotonin receptor 2A
- MAOB
monoamine oxidase B
- BCHE
acetylcholine metabolizing butyrylcholinesterase
- SNAP25
synaptosomal-Associated Protein 25
- BAIAP2
BAI1-associated protein 2
- NOS1/NO
nitric Oxide Synthase-1, NO, Nitric Oxide metabolite
- KCNIP4
kv channel interacting protein 4
- SPOCK3
Sparc/Osteonectin, Cwcv And Kazal-Like Domains Proteoglycan (Testican) 3
- MAD
Malondialdehyde
- SOD
Superoxide Dismutase
- PON1
Paraoxonase
- ARES
Arylesterase
- TAS
total antioxidant status
- TOS
total oxidant status
- OSI
oxidative stress index
- DISC1
Disrupted In Schizophrenia 1
- DBH
dopamine beta hydroxylase
- DDC
dopamine decarboxylase
Footnotes
Authors’ contributions
Writing group: CS, FSV
Analytic group: CB, CS, FSV
Study design: CS, CB
Data preparation: CB, CS
Conflict of interest
The authors CB, CS declare that they have no conflict of interest and have no financial interests of any kind in publishing the results of this study.
In the past year, Dr. Faraone received income, potential income, travel expenses and/or research support from Arbor, Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Neurovance, Impax, NeuroLifeSciences. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD.
References
- Akutagava-Martins GC, Salatino-Oliveira A, Bruxel EM, Genro JP, Mota NR, Polanczyk GV, Zeni CP, Grevet EH, Bau CH, Rohde LA, et al. Lack of association between the GRM7 gene and attention deficit hyperactivity disorder. Psychiatr Genet. 2014a;24(6):281–2. doi: 10.1097/YPG.0000000000000059. [DOI] [PubMed] [Google Scholar]
- Akutagava-Martins GC, Salatino-Oliveira A, Genro JP, Contini V, Polanczyk G, Zeni C, Chazan R, Kieling C, Anselmi L, Menezes AM, et al. Glutamatergic copy number variants and their role in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2014b;165B(6):502–9. doi: 10.1002/ajmg.b.32253. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Jain M, Acosta MT, Shively S, Stanescu H, Wallis D, Domené S, Vélez JI, Karkera JD, Balog J, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010;15(11):1053–66. doi: 10.1038/mp.2010.6. [DOI] [PubMed] [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17(10):988–95. doi: 10.1038/mp.2011.149. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Kiely K, Guite J, Ablon JS, Reed E, Warburton R. High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: A pilot study. Am J Psychiatry. 1995;152(3):431–5. doi: 10.1176/ajp.152.3.431. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, Ouellette C, Moore P, Spencer T. Predictors of persistence and remission of ADHD into adolescence: Results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35(3):343–51. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816–8. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Bonvicini C, Faraone SV, Scassellati C. Attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol Psychiatry. 2016;21(7):872–84. doi: 10.1038/mp.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, Saviouk V, Hottenga JJ, Distel MA, de Moor MH, Vink JM, Geels LM, van Beek JH, Bartels M, de Geus EJ, et al. Genetic epidemiology of attention deficit hyperactivity disorder (ADHD index) in adults. PLoS One. 2010;5(5):e10621. doi: 10.1371/journal.pone.0010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bralten J, Franke B, Waldman I, Rommelse N, Hartman C, Asherson P, Banaschewski T, Ebstein RP, Gill M, Miranda A, et al. Candidate genetic pathways for attention-deficit/hyperactivity disorder (ADHD) show association to hyperactive/impulsive symptoms in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2013;52(11):1204, 1212.e1. doi: 10.1016/j.jaac.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Brikell I, Kuja-Halkola R, Larsson H. Heritability of attention-deficit hyperactivity disorder in adults. Am J Med Genet B Neuropsychiatr Genet. 2015:30. doi: 10.1002/ajmg.b.32335. [DOI] [PubMed] [Google Scholar]
- Ceylan M, Sener S, Bayraktar AC, Kavutcu M. Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1491–4. doi: 10.1016/j.pnpbp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Ceylan MF, Sener S, Bayraktar AC, Kavutcu M. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2012;66(3):220–6. doi: 10.1111/j.1440-1819.2012.02330.x. [DOI] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: High heritabilities throughout development. JAMA Psychiatry. 2013;70(3):311–8. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- Coogan AN, Baird AL, Popa-Wagner A, Thome J. Circadian rhythms and attention deficit hyperactivity disorder: The what, the when and the why. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:74–81. doi: 10.1016/j.pnpbp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Corominas-Roso M, Ramos-Quiroga JA, Ribases M, Sanchez-Mora C, Palomar G, Valero S, Bosch R, Casas M. Decreased serum levels of brain-derived neurotrophic factor in adults with attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2013;30:1–9. doi: 10.1017/S1461145712001629. [DOI] [PubMed] [Google Scholar]
- de Azeredo LA, Rovaris DL, Mota NR, Polina ER, Marques FZ, Contini V, Vitola ES, Belmonte-de-Abreu P, Rohde LA, Grevet EH, et al. Further evidence for the association between a polymorphism in the promoter region of SLC6A3/DAT1 and ADHD: Findings from a sample of adults. Eur Arch Psychiatry Clin Neurosci. 2014;264(5):401–8. doi: 10.1007/s00406-014-0486-8. [DOI] [PubMed] [Google Scholar]
- De Luca V, Muglia P, Jain U, Basile VS, Sokolowski MB, Kennedy JL. A drosophila model for attention deficit hyperactivity disorder (ADHD): No evidence of association with PRKG1 gene. Neuromolecular Med. 2002;2(3):281–7. doi: 10.1385/NMM:2:3:281. [DOI] [PubMed] [Google Scholar]
- Drtilkova I, Sery O, Theiner P, Uhrova A, Zackova M, Balastikova B, Znojil V. Clinical and molecular-genetic markers of ADHD in children. Neuro Endocrinol Lett. 2008;29(3):320–7. [PubMed] [Google Scholar]
- Elia J, Devoto M. ADHD genetics: 2007 update. Curr Psychiatry Rep. 2007;9(5):434–9. doi: 10.1007/s11920-007-0057-z. [DOI] [PubMed] [Google Scholar]
- Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, D’arcy M, deBerardinis R, Frackelton E, Kim C, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2010;15(6):637–46. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux MC. Toward guidelines for pedigree selection in genetic studies of attention deficit hyperactivity disorder. Genet Epidemiol. 2000;18(1):1–16. doi: 10.1002/(SICI)1098-2272(200001)18:1<1::AID-GEPI1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Feighner JA, Monuteaux MC. Assessing symptoms of attention deficit hyperactivity disorder in children and adults: Which is more valid? J Consult Clin Psychol. 2000;68(5):830–42. [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–65. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33(1):159–80. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Bonvicini C, Scassellati C. Biomarkers in the diagnosis of ADHD--promising directions. Curr Psychiatry Rep. 2014;16(11):497. doi: 10.1007/s11920-014-0497-1. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J. Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: A meta-analysis. Mol Psychiatry. 2014;19(8):880–9. doi: 10.1038/mp.2013.126. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJS, Tannock R, Franke B. Attention deficit hyperactivity disorder. Nature Reviews Disease Primers. 2015:1. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CH, Ramos-Quiroga JA, Mick E, Grevet EH, Johansson S, Haavik J, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry. 2012;17(10):960–87. doi: 10.1038/mp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Liu L, Qian Q, Wang Y. Advances in molecular genetic studies of attention deficit hyperactivity disorder in china. Shanghai Arch Psychiatry. 2014;26(4):194–206. doi: 10.3969/j.issn.1002-0829.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genro JP, Kieling C, Rohde LA, Hutz MH. Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev Neurother. 2010;10(4):587–601. doi: 10.1586/ern.10.17. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Hum Genet. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CI, Riveiro-Alvarez R, Soto-Insuga V, Rodrigo M, Tirado-Requero P, Mahillo-Fernandez I, Abad-Santos F, Carballo JJ, Dal-Re R, Ayuso C. Attention deficit hyperactivity disorder: Genetic association study in a cohort of spanish children. Behav Brain Funct. 2016;12(1) doi: 10.1186/s12993-015-0084-6. 2,015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunblatt E, Geissler J, Jacob CP, Renner T, Muller M, Bartl J, Gross-Lesch S, Riederer P, Lesch KP, Walitza S, et al. Pilot study: Potential transcription markers for adult attention-deficit hyperactivity disorder in whole blood. Atten Defic Hyperact Disord. 2012;4(2):77–84. doi: 10.1007/s12402-012-0074-6. [DOI] [PubMed] [Google Scholar]
- Grady DL, Chi HC, Ding YC, Smith M, Wang E, Schuck S, Flodman P, Spence MA, Swanson JM, Moyzis RK. High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Mol Psychiatry. 2003;8(5):536–45. doi: 10.1038/sj.mp.4001350. [DOI] [PubMed] [Google Scholar]
- Guan L, Wang B, Chen Y, Yang L, Li J, Qian Q, Wang Z, Faraone SV, Wang Y. A high-density single-nucleotide polymorphism screen of 23 candidate genes in attention deficit hyperactivity disorder: Suggesting multiple susceptibility genes among chinese han population. Mol Psychiatry. 2009;14(5):546–54. doi: 10.1038/sj.mp.4002139. [DOI] [PubMed] [Google Scholar]
- Guney E, Ceylan MF, Kara M, Tekin N, Goker Z, Senses Dinc G, Ozturk O, Eker S, Kizilgun M. Serum nerve growth factor (NGF) levels in children with attention deficit/hyperactivity disorder (ADHD) Neurosci Lett. 2014;560:107–11. doi: 10.1016/j.neulet.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Hasler R, Salzmann A, Bolzan T, Zimmermann J, Baud P, Giannakopoulos P, Perroud N. DAT1 and DRD4 genes involved in key dimensions of adult ADHD. Neurol Sci. 2015;36(6):861–9. doi: 10.1007/s10072-014-2051-7. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Cummins TD, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 2015;20(3):289–97. doi: 10.1038/mp.2014.183. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Matthews N, Barry E, Kirley A, Wagner J, Wallace RH, Heussler HS, Vance A, Gill M, Bellgrove MA. A high density linkage disequilibrium mapping in 14 noradrenergic genes: Evidence of association between SLC6A2, ADRA1B and ADHD. Psychopharmacology (Berl ) 2013;225(4):895–902. doi: 10.1007/s00213-012-2875-x. [DOI] [PubMed] [Google Scholar]
- Herken H, Erdal ME, Kenar AN, Unal GA, Cakaloz B, Ay ME, Yücel E, Edgünlü T, Sengül C. Association of SNAP-25 gene ddel and mnll polymorphisms with adult attention deficit hyperactivity disorder. Psychiatry Investig. 2014;11(4):476–80. doi: 10.4306/pi.2014.11.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney A, Scherag A, Jarick I, Albayrak Ö, Pütter C, Pechlivanis S, Dauvermann MR, Beck S, Weber H, Scherag S, et al. Genome-wide association study in German patients with attention deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(8):888–97. doi: 10.1002/ajmg.b.31246. [DOI] [PubMed] [Google Scholar]
- Hwang IW, Lim MH, Kwon HJ, Jin HJ. Association of LPHN3 rs6551665 A/G polymorphism with attention deficit and hyperactivity disorder in korean children. Gene. 2015;566(1):68–73. doi: 10.1016/j.gene.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Weber H, Retz W, Kittel-Schneider S, Heupel J, Renner T, Lesch KP, Reif A. Acetylcholine-metabolizing butyrylcholinesterase (BCHE) copy number and single nucleotide polymorphisms and their role in attention-deficit/hyperactivity syndrome. J Psychiatr Res. 2013;47(12):1902–8. doi: 10.1016/j.jpsychires.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Jacobsen KK, Halmoy A, Sanchez-Mora C, Ramos-Quiroga JA, Cormand B, Haavik J, Johansson S. DISC1 in adult ADHD patients: An association study in two european samples. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(3):227–34. doi: 10.1002/ajmg.b.32136. [DOI] [PubMed] [Google Scholar]
- Joseph N, Zhang-James Y, Perl A, Faraone SV. Oxidative stress and ADHD: A meta-analysis. J Atten Disord. 2015;19(11):915–24. doi: 10.1177/1087054713510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan KJ, Dolan CV, Nivard MG, Middeldorp CM, van Beijsterveldt CE, Willemsen G, Boomsma DI. Genetic and environmental stability in attention problems across the lifespan: Evidence from the netherlands twin register. J Am Acad Child Adolesc Psychiatry. 2013;52(1):12–25. doi: 10.1016/j.jaac.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Kandemir H, Erdal ME, Selek S, Ay Öİ, Karababa IF, Kandemir SB, Ay ME, Yılmaz ŞG, Bayazıt H, Taşdelen B. Evaluation of several micro RNA (miRNA) levels in children and adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2014;580:158–62. doi: 10.1016/j.neulet.2014.07.060. [DOI] [PubMed] [Google Scholar]
- Kayyal M, Movafagh A, Hashemi M, Sayad A, Emamalizadeh B, PourIran K, Kayyal M, Amirabadi MR, Zamani M, Darvish H. Association analysis of DISC1 gene polymorphisms with attention-deficit hyperactivity disorder in iranian population. Pak J Med Sci. 2015;31(5):1162–6. doi: 10.12669/pjms.315.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenar AN, Ay O, Herken H, Erdal ME. Association of VAMP-2 and syntaxin 1A genes with adult attention deficit hyperactivity disorder. Psychiatry Investig. 2014;11(1):76–83. doi: 10.4306/pi.2014.11.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling C, Retz W, Wiemann S, Coogan AN, Clement RM, Hünnerkopf R, Conner AC, Freitag CM, Rösler M, Thome J. A polymorphism at the 3′-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):333–8. doi: 10.1002/ajmg.b.30602. [DOI] [PubMed] [Google Scholar]
- Kittel-Schneider S, Reuß M, Meyer A, Weber H, Gessner A, Leistner C, Kopf J, Schmidt B, Hempel S, Volkert J, et al. Multi-level biomarker analysis of nitric oxide synthase isoforms in bipolar disorder and adult ADHD. J Psychopharmacol. 2015;29(1):31–8. doi: 10.1177/0269881114555251. [DOI] [PubMed] [Google Scholar]
- Landaas ET, Johansson S, Halmoy A, Oedegaard KJ, Fasmer OB, Haavik J. Bipolar disorder risk alleles in adult ADHD patients. Genes Brain Behav. 2011;10(4):418–23. doi: 10.1111/j.1601-183X.2011.00680.x. [DOI] [PubMed] [Google Scholar]
- Landaas ET, Johansson S, Jacobsen KK, Ribasés M, Bosch R, Sánchez-Mora C, Jacob CP, Boreatti-Hümmer A, Kreiker S, Lesch KP, et al. An international multicenter association study of the serotonin transporter gene in persistent ADHD. Genes Brain Behav. 2010;9(5):449–58. doi: 10.1111/j.1601-183X.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- Langley K, Fowler TA, Grady DL, Moyzis RK, Holmans PA, van den Bree MB, Owen MJ, O’Donovan MC, Thapar A. Molecular genetic contribution to the developmental course of attention-deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18(1):26–32. doi: 10.1007/s00787-008-0698-4. [DOI] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2006;45(8):973–81. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Larsson H, Asherson P, Chang Z, Ljung T, Friedrichs B, Larsson JO, Lichtenstein P. Genetic and environmental influences on adult attention deficit hyperactivity disorder symptoms: A large swedish population-based study of twins. Psychol Med. 2013;43(1):197–207. doi: 10.1017/S0033291712001067. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1345–54. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- Laurin N, Lee J, Ickowicz A, Pathare T, Malone M, Tannock R, Kennedy JL, Schachar RJ, Barr CL. Association study for genes at chromosome 5p13-q11 in attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(5):600–5. doi: 10.1002/ajmg.b.30654. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Song GG. J Atten Disord. 2015. BDNF 196 G/A and COMT Val158Met polymorphisms and susceptibility to ADHD: A meta-analysis; p. 17. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Röser C, Nguyen TT, Craig DW, Romanos J, Heine M, Meyer J, et al. Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm (Vienna) 2008;115(11):1573–85. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Selch S, Renner TJ, Jacob C, Nguyen TT, Hahn T, Romanos M, Walitza S, Shoichet S, Dempfle A, et al. Genome-wide copy number variation analysis in attention-deficit/hyperactivity disorder: Association with neuropeptide Y gene dosage in an extended pedigree. Mol Psychiatry. 2011;16(5):491–503. doi: 10.1038/mp.2010.29. [DOI] [PubMed] [Google Scholar]
- Li Z, Chang SH, Zhang LY, Gao L, Wang J. Molecular genetic studies of ADHD and its candidate genes: A review. Psychiatry Res. 2014;219(1):10–24. doi: 10.1016/j.psychres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, Gazzellone M, Carson AR, Howe JL, Wang Z, et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med. 2011;3(95):95ra75. doi: 10.1126/scitranslmed.3002464. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen Y, Li H, Qian Q, Yang L, Glatt SJ, Faraone SV, Wang Y. Association between SYP with attention-deficit/hyperactivity disorder in chinese han subjects: Differences among subtypes and genders. Psychiatry Res. 2013a;210(1):308–14. doi: 10.1016/j.psychres.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Liu L, Sun L, Li ZH, Li HM, Wei LP, Wang YF, Qian QJ. BAIAP2 exhibits association to childhood ADHD especially predominantly inattentive subtype in chinese han subjects. Behav Brain Funct. 2013b;9:48. doi: 10.1186/1744-9081-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, O’Donovan MC, Thapar A, Langley K, Williams N. The relative contribution of common and rare genetic variants to ADHD. Transl Psychiatry. 2015;5:e506. doi: 10.1038/tp.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroconstanti T, Johansson S, Winge I, Knappskog PM, Haavik J. Functional properties of rare missense variants of human CDH13 found in adult attention deficit/hyperactivity disorder (ADHD) patients. PLoS One. 2013;8(8):e71445. doi: 10.1371/journal.pone.0071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroconstanti T, Halmoy A, Haavik J. Decreased serum levels of adiponectin in adult attention deficit hyperactivity disorder. Psychiatry Res. 2014;216(1):123–30. doi: 10.1016/j.psychres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Neale BM, Lasky-Su J, Anney R, Franke B, Zhou K, Maller JB, Vasquez AA, Asherson P, Chen W, Banaschewski T, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1337–44. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Medland S, Ripke S, Anney RJ, Asherson P, Buitelaar J, Franke B, Gill M, Kent L, Holmans P, et al. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):906–20. doi: 10.1016/j.jaac.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogdie MN, Bakker SC, Fisher SE, Francks C, Yang MH, Cantor RM, Loo SK, van der Meulen E, Pearson P, Buitelaar J, et al. Pooled genome-wide linkage data on 424 ADHD ASPs suggests genetic heterogeneity and a common risk locus at 5p13. Mol Psychiatry. 2006;11(1):5–8. doi: 10.1038/sj.mp.4001760. [DOI] [PubMed] [Google Scholar]
- Olgiati P, Mandelli L, Alberti S, Lia L, Serretti A, Tiwari AK, Jain U, Kennedy JL, Muller DJ. Role of synaptosome-related (SNARE) genes in adults with attention deficit hyperactivity disorder. Psychiatry Res. 2014;215(3):799–800. doi: 10.1016/j.psychres.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Ozcan O, Arslan M, Gungor S, Yuksel T, Selimoglu MA. Plasma leptin, adiponectin, neuropeptide Y levels in drug naive children with ADHD. J Atten Disord. 2015:15. doi: 10.1177/1087054715587095. [DOI] [PubMed] [Google Scholar]
- Pan YQ, Qiao L, Xue XD, Fu JH. Association between ANKK1 (rs1800497) polymorphism of DRD2 gene and attention deficit hyperactivity disorder: A meta-analysis. Neurosci Lett. 2015;590:101–5. doi: 10.1016/j.neulet.2015.01.076. [DOI] [PubMed] [Google Scholar]
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated genome-wide association study findings: Identification of a neurodevelopmental network for attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168(4):365–77. doi: 10.1176/appi.ajp.2010.10070948. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Quiroga JA, Nasillo V, Fernandez-Aranda F, Fernandez-Arana F, Casas M. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014a;14(5):553–67. doi: 10.1586/14737175.2014.908708. [DOI] [PubMed] [Google Scholar]
- Ramos-Quiroga JA, Sánchez-Mora C, Casas M, Garcia-Martínez I, Bosch R, Nogueira M, Corrales M, Palomar G, Vidal R, Coll-Tané M, et al. Genome-wide copy number variation analysis in adult attention-deficit and hyperactivity disorder. J Psychiatr Res. 2014b;49:60–7. doi: 10.1016/j.jpsychires.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Reif A, Nguyen TT, Weissflog L, Jacob CP, Romanos M, Renner TJ, Buttenschon HN, Kittel-Schneider S, Gessner A, Weber H, et al. DIRAS2 is associated with adult ADHD, related traits, and co-morbid disorders. Neuropsychopharmacology. 2011;36(11):2318–27. doi: 10.1038/npp.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribasés M, Hervás A, Ramos-Quiroga JA, Bosch R, Bielsa A, Gastaminza X, Fernández-Anguiano M, Nogueira M, Gómez-Barros N, Valero S, et al. Association study of 10 genes encoding neurotrophic factors and their receptors in adult and child attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(10):935–45. doi: 10.1016/j.biopsych.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Ribasés M, Ramos-Quiroga JA, Hervás A, Bosch R, Bielsa A, Gastaminza X, Artigas J, Rodriguez-Ben S, Estivill X, Casas M, et al. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol Psychiatry. 2009a;14(1):71–85. doi: 10.1038/sj.mp.4002100. [DOI] [PubMed] [Google Scholar]
- Ribases M, Bosch R, Hervás A, Ramos-Quiroga JA, Sanchez-Mora C, Bielsa A, Gastaminza X, Guijarro-Domingo S, Nogueira M, Gomez-Barros N, et al. Case-control study of six genes asymmetrically expressed in the two cerebral hemispheres: Association of BAIAP2 with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009b;66(10):926–34. doi: 10.1016/j.biopsych.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Ribases M, Ramos-Quiroga JA, Sanchez-Mora C, Bosch R, Richarte V, Palomar G, Gastaminza X, Bielsa A, Arcos-Burgos M, Muenke M, et al. Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: A replication study. Genes Brain Behav. 2011;10(2):149–57. doi: 10.1111/j.1601-183X.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- Ribases M, Ramos-Quiroga JA, Hervás A, Sanchez-Mora C, Bosch R, Bielsa A, Gastaminza X, Lesch KP, Reif A, Renner TJ, et al. Candidate system analysis in ADHD: Evaluation of nine genes involved in dopaminergic neurotransmission identifies association with DRD1. World J Biol Psychiatry. 2012;13(4):281–92. doi: 10.3109/15622975.2011.584905. [DOI] [PubMed] [Google Scholar]
- Sánchez-Mora C1, Ribasés M, Casas M, Bayés M, Bosch R, Fernàndez-Castillo N, Brunso L, Jacobsen KK, Landaas ET, Lundervold AJ, et al. Exploring DRD4 and its interaction with SLC6A3 as possible risk factors for adult ADHD: A meta-analysis in four european populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(5):600–12. doi: 10.1002/ajmg.b.31202. [DOI] [PubMed] [Google Scholar]
- Sánchez-Mora C, Cormand B, Ramos-Quiroga JA, Hervás A, Bosch R, Palomar G, Nogueira M, Gómez-Barros N, Richarte V, Corrales M, et al. Evaluation of common variants in 16 genes involved in the regulation of neurotransmitter release in ADHD. Eur Neuropsychopharmacol. 2013a;23(6):426–35. doi: 10.1016/j.euroneuro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Sánchez-Mora C, Ramos-Quiroga JA, Garcia-Martínez I, Fernàndez-Castillo N, Bosch R, Richarte V, Palomar G, Nogueira M, Corrales M, Daigre C, et al. Evaluation of single nucleotide polymorphisms in the miR-183-96-182 cluster in adulthood attention-deficit and hyperactivity disorder (ADHD) and substance use disorders (SUDs) Eur Neuropsychopharmacol. 2013b;23(11):1463–73. doi: 10.1016/j.euroneuro.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Sánchez-Mora C, Richarte V, Garcia-Martínez I, Pagerols M, Corrales M, Bosch R, Vidal R, Viladevall L, Casas M, Cormand B, et al. Dopamine receptor DRD4 gene and stressful life events in persistent attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2015a:14. doi: 10.1002/ajmg.b.32340. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mora C, Ramos-Quiroga JA, Bosch R, Corrales M, Garcia-Martinez I, Nogueira M, Pagerols M, Palomar G, Richarte V, Vidal R, et al. Case-control genome-wide association study of persistent attention-deficit hyperactivity disorder identifies FBXO33 as a novel susceptibility gene for the disorder. Neuropsychopharmacology. 2015b;40(4):915–26. doi: 10.1038/npp.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salatino-Oliveira A, Genro JP, Polanczyk G, Zeni C, Schmitz M, Kieling C, Anselmi L, Menezes AM, Barros FC, Polina ER, et al. Cadherin-13 gene is associated with hyperactive/impulsive symptoms in attention/deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(3):162–9. doi: 10.1002/ajmg.b.32293. [DOI] [PubMed] [Google Scholar]
- Salatino-Oliveira A, Akutagava-Martins GC, Bruxel EM, Genro JP, Polanczyk GV, Zeni C, Kieling C, Karam RG, Rovaris DL, Contini V, et al. NOS1 and SNAP25 polymorphisms are associated with attention-deficit/hyperactivity disorder symptoms in adults but not in children. J Psychiatr Res. 2016;75:75–81. doi: 10.1016/j.jpsychires.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1003, 1019.e20. doi: 10.1016/j.jaac.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Scassellati C, Zanardini R, Tiberti A, Pezzani M, Valenti V, Effedri P, Filippini E, Conte S, Ottolini A, Gennarelli M, et al. Serum brain-derived neurotrophic factor (BDNF) levels in attention deficit-hyperactivity disorder (ADHD) Eur Child Adolesc Psychiatry. 2014;23(3):173–7. doi: 10.1007/s00787-013-0447-1. [DOI] [PubMed] [Google Scholar]
- Scassellati C, Bonvicini C. Role of dopaminergic and noradrenergic systems as potential biomarkers in ADHD diagnosis and treatment. In: Norvilitis Jill M., Dr, editor. ADHD - new directions in diagnosis and treatment. InTech; 2015. [Google Scholar]
- Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, Sharp W, Evans A, Giedd JN, Castellanos FX, et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):921–31. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin ND, Gruber J, Glatt SJ, Faraone SV. No association between MspI allele of the ADRA2A polymorphism and ADHD: Meta-analysis of family-based studies. Psychiatr Genet. 2013;23(4):174–5. doi: 10.1097/YPG.0b013e3283631509. [DOI] [PubMed] [Google Scholar]
- Shim SH, Hwangbo Y, Kwon YJ, Jeong HY, Lee BH, Lee HJ, Kim YK. Increased levels of plasma brain-derived neurotrophic factor (BDNF) in children with attention deficit-hyperactivity disorder (ADHD) Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1824–8. doi: 10.1016/j.pnpbp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br J Psychiatry. 2009;194(3):204–11. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Smith TF. Meta-analysis of the heterogeneity in association of DRD4 7-repeat allele and AD/HD: Stronger association with AD/HD combined type. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(6):1189–99. doi: 10.1002/ajmg.b.31090. [DOI] [PubMed] [Google Scholar]
- Sun H, Yuan F, Shen X, Xiong G, Wu J. Role of COMT in ADHD: A systematic meta-analysis. Mol Neurobiol. 2014;49(1):251–61. doi: 10.1007/s12035-013-8516-5. [DOI] [PubMed] [Google Scholar]
- Taurines R, Segura M, Schecklmann M, Albantakis L, Grünblatt E, Walitza S, Jans T, Lyttwin B, Haberhausen M, Theisen FM, Martin B, Briegel W, Thome J, et al. Altered mRNA expression of monoaminergic candidate genes in the blood of children with attention deficit hyperactivity disorder and autism spectrum disorder. World J Biol Psychiatry. 2011;12(Suppl 1):104–8. doi: 10.3109/15622975.2011.600297. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Clinical practice: Adult attention deficit-hyperactivity disorder. N Engl J Med. 2013;369(20):1935–44. doi: 10.1056/NEJMcp1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Scholz CJ, Jacob CP, Heupel J, Kittel-Schneider S, Erhardt A, Hempel S, Schmidt B, Kiel T, Gessner A, et al. SPOCK3, a risk gene for adult ADHD and personality disorders. Eur Arch Psychiatry Clin Neurosci. 2014;264(5):409–21. doi: 10.1007/s00406-013-0476-2. [DOI] [PubMed] [Google Scholar]
- Weißflog L1, Scholz CJ, Jacob CP, Nguyen TT, Zamzow K, Groß-Lesch S, Renner TJ, Romanos M, Rujescu D, Walitza S, et al. KCNIP4 as a candidate gene for personality disorders and adult ADHD. Eur Neuropsychopharmacol. 2013;23(6):436–47. doi: 10.1016/j.euroneuro.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics. 2012;9(3):490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Franke B, Mick E, Anney RJ, Freitag CM, Gill M, Thapar A, O’Donovan MC, Owen MJ, Holmans P, et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: The role of rare variants and duplications at 15q13.3. Am J Psychiatry. 2012;169(2):195–204. doi: 10.1176/appi.ajp.2011.11060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xiao H, Sun H, Zou L, Zhu LQ. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol Neurobiol. 2012;45(3):605–20. doi: 10.1007/s12035-012-8278-5. [DOI] [PubMed] [Google Scholar]
- Xu X, Breen G, Chen CK, Huang YS, Wu YY, Asherson P. Association study between a polymorphism at the 3′-untranslated region of CLOCK gene and attention deficit hyperactivity disorder. Behav Brain Funct. 2010;6:48. doi: 10.1186/1744-9081-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chen XT, Luo M, Tang Y, Zhang G, Wu D, Yang B, Ruan DY, Wang HL. Multiple epigenetic factors predict the attention deficit/hyperactivity disorder among the chinese han children. J Psychiatr Res. 2015;64:40–50. doi: 10.1016/j.jpsychires.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Yang L, Neale BM, Liu L, Lee SH, Wray NR, Ji N, Li H, Qian Q, Wang D, Li J, et al. Polygenic transmission and complex neuro developmental network for attention deficit hyperactivity disorder: Genome-wide association study of both common and rare variants. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(5):419–30. doi: 10.1002/ajmg.b.32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Dempfle A, Arcos-Burgos M, Bakker SC, Banaschewski T, Biederman J, Buitelaar J, Castellanos FX, Doyle A, Ebstein RP, et al. Meta-analysis of genome-wide linkage scans of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1392–8. doi: 10.1002/ajmg.b.30878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Numbers of positive and negative findings from linkage studies, genomics (candidate gene association, GWA, CNVs, expression studies) and metabolomics conducted in children and adults with ADHD
2-way Contingency Table Analyses for pathways in children and adults with ADHD