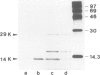
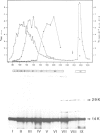
Abstract
RNA-1 molecules from tobacco rattle virus (TRV) and pea early-browning virus (PEBV), two members of the tobravirus group, have recently been shown to contain internal, in-frame UGA termination codons which are suppressed in vitro. Our results suggest that a UGA stop codon also exists in RNA-1 of pepper ringspot virus (PRV), another tobravirus. UGA suppression may therefore be a universal feature of the expression of tobravirus genomes. We have isolated two natural suppressor tRNAs from uninfected tobacco plants on the basis of their ability to promote readthrough over the leaky UGA codon of TRV RNA-1 in a wheat germ extract depleted of endogenous mRNAs and tRNAs. Their amino acid acceptance and nucleotide sequences identify the two UGA-suppressor tRNAs as chloroplast (chl) and cytoplasmic (cyt) tryptophan-specific tRNAs with the anticodon CmCA. These are the first UGA suppressor tRNAs to be identified in plants. They have several interesting features. (i) Chl tRNA(Trp) suppresses the UGA stop codon more efficiently than cyt tRNA(Trp). (ii) Chl tRNA(Trp) contains an A24:U11 pair in the D-stem as does the mutated Escherichia coli UGA-suppressor tRNA(Trp) which is a more active suppressor than wild-type tRNA(Trp). (iii) The suppressor activity of chl tRNA(Trp) is dependent on the nucleotides surrounding the stop codon because it recognizes UGA in the TRV context but not the UGA in the beta-globin context.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Sickinger H. D. The molecular basis for the differential translation of TMV RNA in tobacco protoplasts and wheat germ extracts. EMBO J. 1984 May;3(5):1091–1096. doi: 10.1002/j.1460-2075.1984.tb01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Mundry K. W., Issinger O. G. In vivo and in vitro translation of the RNAs of four tobamoviruses. Intervirology. 1980;14(5-6):292–299. doi: 10.1159/000149199. [DOI] [PubMed] [Google Scholar]
- Bossi L. Context effects: translation of UAG codon by suppressor tRNA is affected by the sequence following UAG in the message. J Mol Biol. 1983 Feb 15;164(1):73–87. doi: 10.1016/0022-2836(83)90088-8. [DOI] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Cordell B., DeNoto F. M., Atkins J. F., Gesteland R. F., Bishop J. M., Goodman H. M. The forms of tRNATrp found in avian sarcoma virus and uninfected chicken cells have structural identity but functional distinctions. J Biol Chem. 1980 Oct 10;255(19):9358–9368. [PubMed] [Google Scholar]
- Daniels C. J., Gupta R., Doolittle W. F. Transcription and excision of a large intron in the tRNATrp gene of an archaebacterium, Halobacterium volcanii. J Biol Chem. 1985 Mar 10;260(5):3132–3134. [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H. UGA suppression by normal tRNA Trp in Escherichia coli: codon context effects. Nucleic Acids Res. 1981 Feb 25;9(4):983–991. doi: 10.1093/nar/9.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J. U., Björk G. R. tRNA anticodons with the modified nucleoside 2-methylthio-N6-(4-hydroxyisopentenyl)adenosine distinguish between bases 3' of the codon. J Mol Biol. 1991 Apr 5;218(3):509–516. doi: 10.1016/0022-2836(91)90697-5. [DOI] [PubMed] [Google Scholar]
- Fritsch C., Mayo M. A., Hirth L. Further studies on the translation products of tobacco rattle virus RNA in vitro. Virology. 1977 Apr;77(2):722–732. doi: 10.1016/0042-6822(77)90494-9. [DOI] [PubMed] [Google Scholar]
- Fritsch C., Mayo M. A., Hirth L. Proceedings: In vitro translation of tobacco rattle virus RNA. Ann Microbiol (Paris) 1976 Jan;127A(1):93–95. [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P. Structure and function of tryptophan tRNA from wheat germ. Nucleic Acids Res. 1984 Jun 25;12(12):4997–5003. doi: 10.1093/nar/12.12.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Chantrenne H. On codon- anticodon interactions. Mol Biol Biochem Biophys. 1980;32:347–367. doi: 10.1007/978-3-642-81503-4_27. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Dietrich A., Maréchal L., Weil J. H. Plant mitochondria and chloroplast tRNAsTrp do not suppress the UGA stop codon. Nucleic Acids Res. 1986 Aug 26;14(16):6775–6775. doi: 10.1093/nar/14.16.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Boccara M., Robinson D. J., Baulcombe D. C. The complete nucleotide sequence of tobacco rattle virus RNA-1. J Gen Virol. 1987 Oct;68(Pt 10):2563–2575. doi: 10.1099/0022-1317-68-10-2563. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hofstetter H., Monstein H. J., Weissmann C. The readthrough protein A1 is essential for the formation of viable Q beta particles. Biochim Biophys Acta. 1974 Dec 6;374(2):238–251. doi: 10.1016/0005-2787(74)90366-9. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Tashiro F., Nishimura S. Tetrahymena thermophila glutamine tRNA and its gene that corresponds to UAA termination codon. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4758–4762. doi: 10.1073/pnas.82.14.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzin M. I. Khirurgicheskaia pomosch' v Sovetskom Soize (k 50-letiiu obrazoaniia SSSR. Klin Med (Mosk) 1972 Dec;50(12):7–10. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Li G. P., Rice C. M. Mutagenesis of the in-frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J Virol. 1989 Mar;63(3):1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Y., March R., Scanlon S. R., Folk W. R. Isolation and transcriptional competence of three tRNA(Trp) genes from Arabidopsis thaliana L. Plant Mol Biol. 1992 Jan;18(1):159–160. doi: 10.1007/BF00018472. [DOI] [PubMed] [Google Scholar]
- MacFarlane S. A., Taylor S. C., King D. I., Hughes G., Davies J. W. Pea early browning virus RNA1 encodes four polypeptides including a putative zinc-finger protein. Nucleic Acids Res. 1989 Mar 25;17(6):2245–2260. doi: 10.1093/nar/17.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal L., Guillemaut P., Grienenberger J. M., Jeannin G., Weil J. H. Sequence and codon recognition of bean mitochondria and chloroplast tRNAsTrp: evidence for a high degree of homology. Nucleic Acids Res. 1985 Jun 25;13(12):4411–4416. doi: 10.1093/nar/13.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M. A. Polypeptides induced by tobacco rattle virus during multiplication in tobacco protoplasts. Intervirology. 1982;17(4):240–246. doi: 10.1159/000149294. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Hashimoto A. Purification and properties of suppressor seryl-tRNA: ATP phosphotransferase from bovine liver. FEBS Lett. 1984 Apr 24;169(2):319–322. doi: 10.1016/0014-5793(84)80342-7. [DOI] [PubMed] [Google Scholar]
- Ohme M., Kamogashira T., Shinozoki K., Sugiura M. Locations and sequences of tobacco chloroplast genes for tRNAPro(UGG), tRNATrp, tRNAfMet and tRNAGly(GCC): the tRNAGly contains only two base-pairs in the D stem. Nucleic Acids Res. 1984 Sep 11;12(17):6741–6749. doi: 10.1093/nar/12.17.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R., Knight C. A. Protein synthesis in tobacco protoplasts infected with tobacco mosaic virus. Virology. 1975 Mar;64(1):10–22. doi: 10.1016/0042-6822(75)90074-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Pintor-Toro J. A., Langridge P., Feix G. Isolation and characterization of maize genes coding for zein proteins of the 21000 dalton size class. Nucleic Acids Res. 1982 Jul 10;10(13):3845–3860. doi: 10.1093/nar/10.13.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S. J., Stewart T. S. The characterization of phosphoseryl tRNA from lactating bovine mammary gland. Nucleic Acids Res. 1977 Jul;4(7):2123–2136. doi: 10.1093/nar/4.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A., Hari V., Kolacz K. The effect of tobacco mosaic virus infection on host and virus-specific protein synthesis in protoplasts. Virology. 1978 Apr;85(2):494–503. doi: 10.1016/0042-6822(78)90456-7. [DOI] [PubMed] [Google Scholar]
- Skuzeski J. M., Nichols L. M., Gesteland R. F., Atkins J. F. The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J Mol Biol. 1991 Mar 20;218(2):365–373. doi: 10.1016/0022-2836(91)90718-l. [DOI] [PubMed] [Google Scholar]
- Smith D., Yarus M. Transfer RNA structure and coding specificity. I. Evidence that a D-arm mutation reduces tRNA dissociation from the ribosome. J Mol Biol. 1989 Apr 5;206(3):489–501. doi: 10.1016/0022-2836(89)90496-8. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Dank N., Nock S., Schön A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2127–2171. doi: 10.1093/nar/19.suppl.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Wandelt C., Feix G. Sequence of a 21 kd zein gene from maize containing an in-frame stop codon. Nucleic Acids Res. 1989 Mar 25;17(6):2354–2354. doi: 10.1093/nar/17.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]