Abstract
Protein disulphide isomerase (PDI) is a highly unusual multifunctional polypeptide, identical to the beta-subunit of prolyl 4-hydroxylase. It has two -Cys-Gly-His-Cys- sequences which represent two independently acting catalytic sites of PDI activity. We report here on the expression in baculovirus vectors of various mutant PDI/beta-subunits together with a wild-type alpha-subunit of the human prolyl 4-hydroxylase alpha 2 beta 2 tetramer in Spodoptera frugiperda insect cells. When either one or both of the -Cys-Gly-His-Cys- sequences was converted to -Ser-Gly-His-Cys-, a tetramer was formed as with wild-type PDI/beta-subunit. This tetramer was fully active prolyl 4-hydroxylase. The data demonstrate that PDI activity of the PDI/beta-subunit is not required for tetramer assembly or for the prolyl 4-hydroxylase activity of the tetramer, and thus other sequences of the PDI/beta-subunit may be critical for keeping the alpha-subunits in a catalytically active, non-aggregated conformation. Measurements of the PDI activities of tetramers containing the various mutant PDI/beta-subunits demonstrated that the activity of the wild-type tetramer is almost exclusively due to the C-terminal PDI catalytic sites, which explains the finding that the PDI activity of the PDI/beta-subunit present in the tetramer is about half that in the free polypeptide.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
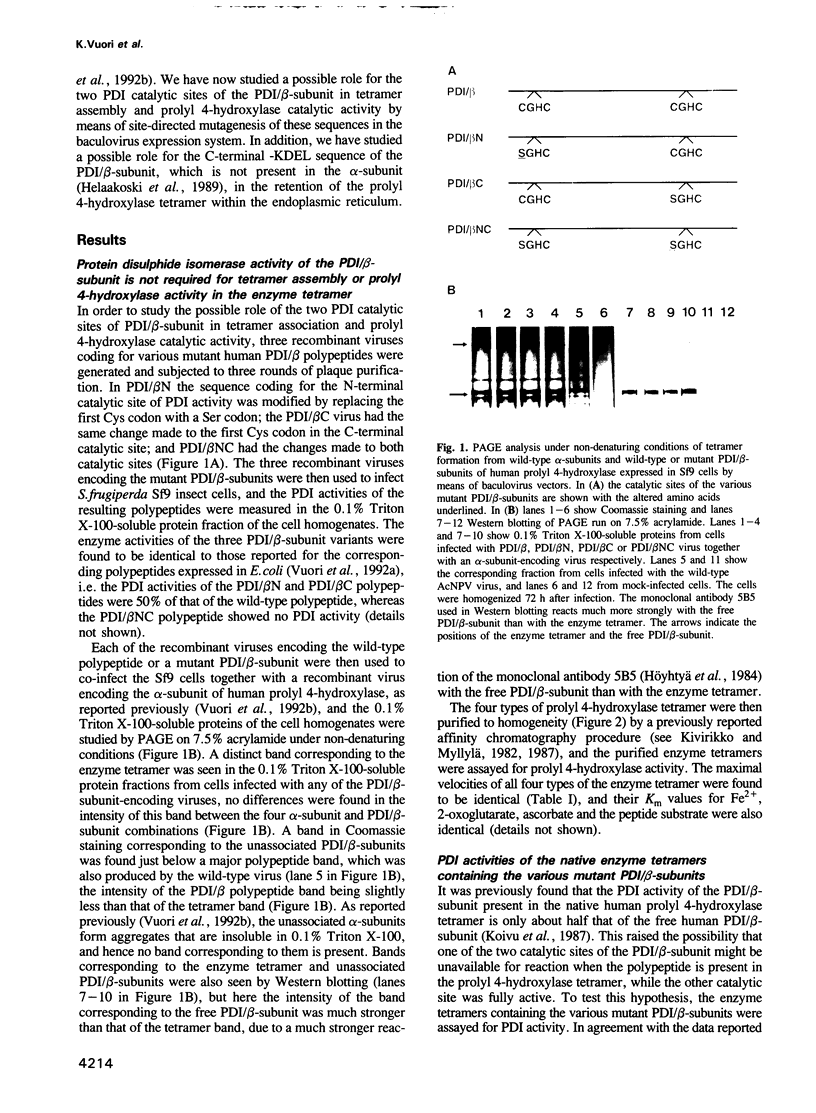



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassuk J. A., Kao W. W., Herzer P., Kedersha N. L., Seyer J., DeMartino J. A., Daugherty B. L., Mark G. E., 3rd, Berg R. A. Prolyl 4-hydroxylase: molecular cloning and the primary structure of the alpha subunit from chicken embryo. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7382–7386. doi: 10.1073/pnas.86.19.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. Y., Gong Q. H., Parkison C., Robinson E. A., Appella E., Merlino G. T., Pastan I. The nucleotide sequence of a human cellular thyroid hormone binding protein present in endoplasmic reticulum. J Biol Chem. 1987 Aug 15;262(23):11221–11227. [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Haugejorden S. M., Srinivasan M., Green M. Analysis of the retention signals of two resident luminal endoplasmic reticulum proteins by in vitro mutagenesis. J Biol Chem. 1991 Apr 5;266(10):6015–6018. [PubMed] [Google Scholar]
- Hawkins H. C., Freedman R. B. The reactivities and ionization properties of the active-site dithiol groups of mammalian protein disulphide-isomerase. Biochem J. 1991 Apr 15;275(Pt 2):335–339. doi: 10.1042/bj2750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaakoski T., Vuori K., Myllylä R., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höyhtyä M., Myllylä R., Piuva J., Kivirikko K. I., Tryggvason K. Monoclonal antibodies to human prolyl 4-hydroxylase. Eur J Biochem. 1984 Jun 15;141(3):472–482. doi: 10.1111/j.1432-1033.1984.tb08217.x. [DOI] [PubMed] [Google Scholar]
- Ibbetson A. L., Freedman R. B. Thiol-protein disulphide oxidoreductases. Assay of microsomal membrane-bound glutathione-insulin transhydrogenase and comparison with protein disulphide-isomerase. Biochem J. 1976 Nov;159(2):377–384. doi: 10.1042/bj1590377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Helaakoski T., Tasanen K., Vuori K., Myllylä R., Parkkonen T., Pihlajaniemi T. Molecular biology of prolyl 4-hydroxylase. Ann N Y Acad Sci. 1990;580:132–142. doi: 10.1111/j.1749-6632.1990.tb17925.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989 Mar;3(5):1609–1617. [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Lewis M. J., Sweet D. J., Pelham H. R. The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell. 1990 Jun 29;61(7):1359–1363. doi: 10.1016/0092-8674(90)90699-f. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Mazzarella R. A., Srinivasan M., Haugejorden S. M., Green M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990 Jan 15;265(2):1094–1101. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Kaska D. D., Kivirikko K. I. The catalytic mechanism of the hydroxylation reaction of peptidyl proline and lysine does not require protein disulphide-isomerase activity. Biochem J. 1989 Oct 15;263(2):609–611. doi: 10.1042/bj2630609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Tuderman L., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977 Nov 1;80(2):349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- Noiva R., Kimura H., Roos J., Lennarz W. J. Peptide binding by protein disulfide isomerase, a resident protein of the endoplasmic reticulum lumen. J Biol Chem. 1991 Oct 15;266(29):19645–19649. [PubMed] [Google Scholar]
- Noiva R., Lennarz W. J. Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992 Feb 25;267(6):3553–3556. [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990 Dec;15(12):483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza J. C., Hardwick K. G., Dean N., Pelham H. R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990 Jun 29;61(7):1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Sissom J., Ellis L. Secretion of the extracellular domain of the human insulin receptor from insect cells by use of a baculovirus vector. Biochem J. 1989 Jul 1;261(1):119–126. doi: 10.1042/bj2610119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Vernet T., Tessier D. C., Richardson C., Laliberté F., Khouri H. E., Bell A. W., Storer A. C., Thomas D. Y. Secretion of functional papain precursor from insect cells. Requirement for N-glycosylation of the pro-region. J Biol Chem. 1990 Sep 25;265(27):16661–16666. [PubMed] [Google Scholar]
- Vuori K., Myllylä R., Pihlajaniemi T., Kivirikko K. I. Expression and site-directed mutagenesis of human protein disulfide isomerase in Escherichia coli. This multifunctional polypeptide has two independently acting catalytic sites for the isomerase activity. J Biol Chem. 1992 Apr 15;267(11):7211–7214. [PubMed] [Google Scholar]
- Vuori K., Pihlajaniemi T., Marttila M., Kivirikko K. I. Characterization of the human prolyl 4-hydroxylase tetramer and its multifunctional protein disulfide-isomerase subunit synthesized in a baculovirus expression system. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7467–7470. doi: 10.1073/pnas.89.16.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., McLean L. R., Spinner S. N., Aggerbeck L. P. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991 Oct 8;30(40):9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., Spinner S. N., Joiner B. J. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990 Jun 15;265(17):9800–9807. [PubMed] [Google Scholar]
- Yamauchi K., Yamamoto T., Hayashi H., Koya S., Takikawa H., Toyoshima K., Horiuchi R. Sequence of membrane-associated thyroid hormone binding protein from bovine liver: its identity with protein disulphide isomerase. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1485–1492. doi: 10.1016/0006-291x(87)90817-5. [DOI] [PubMed] [Google Scholar]