Abstract
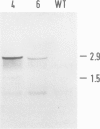
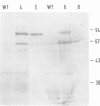
Upon transfer of a genetically engineered Escherichia coli gene for glycerol-3-phosphate acyltransferase (plsB) to Arabidopsis thaliana (L.) Heynh., the gene is transcribed and translated into an enzymatically active polypeptide. This leads to an alteration in fatty acid composition of membrane lipids. From these alterations it is evident that the enzyme is located mainly inside the plastids. The amount of saturated fatty acids in plastidial membrane lipids increased. In particular, the fraction of high-temperature melting species of phosphatidylglycerol is elevated. These molecules are thought to play a crucial role in determining chilling sensitivity of plants. An increase in sensitivity could be observed in the transgenic plants during recultivation after chilling treatment. Implications for the hypothesis of phosphatidylglycerol-determined chilling sensitivity are discussed.
Full text
PDF

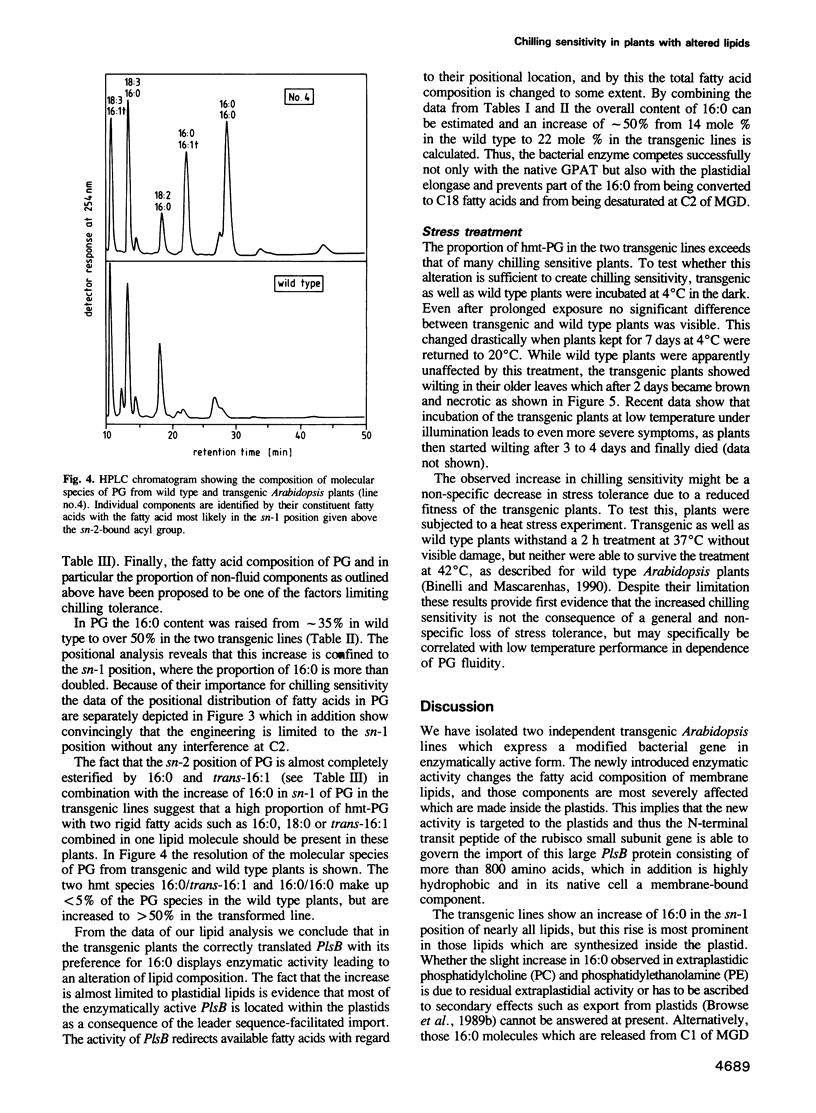



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrams M., Heinz E. Positional Specificity and Fatty Acid Selectivity of Purified sn-Glycerol 3-Phosphate Acyltransferases from Chloroplasts. Plant Physiol. 1981 Sep;68(3):653–657. doi: 10.1104/pp.68.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J., Kunst L., Anderson S., Hugly S., Somerville C. A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol. 1989 Jun;90(2):522–529. doi: 10.1104/pp.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J., McCourt P., Somerville C. A mutant of Arabidopsis deficient in c(18:3) and c(16:3) leaf lipids. Plant Physiol. 1986 Jul;81(3):859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C. R., Slack C. R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the '16:3' plant Arabidopsis thaliana. Biochem J. 1986 Apr 1;235(1):25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Roughan P. G. Fatty Acid Specificity and Selectivity of the Chloroplast sn-Glycerol 3-Phosphate Acyltransferase of the Chilling Sensitive Plant, Amaranthus lividus. Plant Physiol. 1987 Mar;83(3):676–680. doi: 10.1104/pp.83.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Green P. R., Merrill A. H., Jr, Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Purification, reconstitution, and characterization of sn-glycerol-3-phosphate acyltransferase. J Biol Chem. 1981 Nov 10;256(21):11151–11159. [PubMed] [Google Scholar]
- Heemskerk J. W., Schmidt H., Hammer U., Heinz E. Biosynthesis and desaturation of prokaryotic galactolipids in leaves and isolated chloroplasts from spinach. Plant Physiol. 1991 May;96(1):144–152. doi: 10.1104/pp.96.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S., Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 1992 May;99(1):197–202. doi: 10.1104/pp.99.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Olsson O., Langridge W. H., Schell J., Szalay A. A. Expression and assembly of functional bacterial luciferase in plants. Proc Natl Acad Sci U S A. 1987 Jan;84(1):131–135. doi: 10.1073/pnas.84.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L., Browse J., Somerville C. A mutant of Arabidopsis deficient in desaturation of palmitic Acid in leaf lipids. Plant Physiol. 1989 Jul;90(3):943–947. doi: 10.1104/pp.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L., Browse J., Somerville C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner V. A., Bell R. M., Modrich P. The DNA sequences encoding plsB and dgk loci of Escherichia coli. J Biol Chem. 1983 Sep 25;258(18):10856–10861. [PubMed] [Google Scholar]
- Murata N., Yamaya J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984 Apr;74(4):1016–1024. doi: 10.1104/pp.74.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman H. A., John J. B. Metabolism of Unsaturated Monogalactosyldiacylglycerol Molecular Species in Arabidopsis thaliana Reveals Different Sites and Substrates for Linolenic Acid Synthesis. Plant Physiol. 1986 Jul;81(3):731–736. doi: 10.1104/pp.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. O., Goelz S. E., Cronan J. E., Jr Phospholipid synthesis in Escherichia coli. Characteristics of fatty acid transfer from acyl-acyl carrier protein to sn-glycerol 3-phosphate. J Biol Chem. 1981 Jan 25;256(2):736–742. [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol. 1985 Mar;77(3):740–746. doi: 10.1104/pp.77.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Thompson G. A., Jr, Cho S. H. Metabolism of exogenous long-chain fatty acids by spinach leaves. Arch Biochem Biophys. 1987 Dec;259(2):481–496. doi: 10.1016/0003-9861(87)90515-7. [DOI] [PubMed] [Google Scholar]
- Takamura H., Narita H., Urade R., Kito M. Quantitative analysis of polyenoic phospholipid molecular species by high performance liquid chromatography. Lipids. 1986 May;21(5):356–361. doi: 10.1007/BF02535701. [DOI] [PubMed] [Google Scholar]
- Weber S., Wolter F. P., Buck F., Frentzen M., Heinz E. Purification and cDNA sequencing of an oleate-selective acyl-ACP:sn-glycerol-3-phosphate acyltransferase from pea chloroplasts. Plant Mol Biol. 1991 Nov;17(5):1067–1076. doi: 10.1007/BF00037145. [DOI] [PubMed] [Google Scholar]
- Wolter F. P., Fritz C. C., Willmitzer L., Schell J., Schreier P. H. rbcS genes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci U S A. 1988 Feb;85(3):846–850. doi: 10.1073/pnas.85.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]