Abstract
Objective(s):
To evaluated the effects of melatonin on early embryo competence and the expression rate of the primary implantation receptors (ErbB1 and ErbB4).
Materials and Methods:
Two-cell mouse embryos were cultured in 3 groups: simple media, melatonin-treated (10-9 M melatonin) and Luzindole-treated (10-9 M luzindole). Then, the rate of ErbB1 and ErbB4 gene and protein expression, the level of intracellular ROS, antioxidant capacity, and also the number of cells were evaluated and compared with the fourth group in vivo developed blastocysts (control group).
Results:
We concluded that melatonin significantly up-regulated the ErbB1 and ErbB4 gene and protein expression, decreased intracellular ROS, increased the total antioxidant capacity, and also elevated the cell numbers in the melatonin-treated group compared with the other groups (P≤ 0.05).
Conclusion:
The use of melatonin may be a helpful factor in improving the embryo quality and enhancing the expression of ErbB1 and ErbB4, two important implantation-related genes and proteins.
Keywords: Antioxidant, ErbB1, ErbB4, Melatonin, Pre-implantation embryo, Reactive oxygen species
Introduction
Infertility is a critical factor in reproductive health and has affected around 50 million couples worldwide (1).
In spite of many signs of progress in assisted reproductive technologies (ART), the proportion of well-developed embryos and successful implantations are still insufficient (2).
In ART, gametes and embryos are inadvertently exposed to various oxidative stress inducing environ-mental factors which could be due to a decrease of embryos’ defensive capacity against reactive oxygen species (ROS). Overproduction of ROS results in different types of cell injuries, adenosine triphosphate (ATP) depletion, mitochondrial dysfunction, DNA damage, apoptosis, and necrosis (3, 4). It was also reported that these negative consequences of oxidative stress were associated with suppression of genes expression involved in in vitro embryo development (4, 5). Successful implantation depends on the expression of ErbB receptors including ErbB1, ErbB2, ErbB3, and ErbB4 in the trophectoderm of the expanded blastocyst and their interaction with receptive uterus ligands. Among ErbB systems, ErbB1 and ErbB4 are the earliest expressed genes on pre-implantation embryos (6, 7).
So, introducing the best conditions for embryo culture media to neutralize the excess ROS has drawn increased attention improving the rate of pre-implantation embryo development and successful implantation.
Is a free radical scavenger and antioxidant which is one of the most effective substances in controlling ROS and reducing cellular oxidative damage (8). Furthermore, Luzindole, as a melatonin receptor antagonist, was used to inhibit the physiological action of melatonin receptors against probable endogenous secretion of melatonin.
Since, genes expression can alter in response to stress factors (9) so, the aim of this study was to investigate the potential effect of melatonin against environmental stress factors on pre-implantation embryo competence and clarify whether or not melatonin exerts its effects on the primary implantation receptors, ErbB1 and ErbB4, expression.
Materials and Methods
Animal preparation
NMRI mice were purchased from Pharmacy Faculty of Tehran University of Medical Sciences (Tehran, Iran). The local ethics committee of Tehran University of Medical Sciences approved the procedures for using and caring for animals in this project.
Six to 8-week-old female NMRI mice (28–30 g) were housed individually under controlled conditions of temperature (22–26 °C) and light (12 hr light: 12 hr dark cycle). Mice had access to commercial diet and water.
Embryos collection and in vitro culture of two-cell mouse embryos
Female mice were superovulated by an intra-peritoneal (IP) injection of 7.5 IU pregnant mare serum gonadotropin (PMSG, G 4877, Sigma-Aldrich, USA), followed by injection of 7.5 IU human chorionic gonadotropin (HCG, Karma, Germany) 48 hr later (10).
To ensure the reliability of gestation time, female mice were paired overnight with males of proven fertility (ratio = 1:1) and females with a vaginal plug were selected for the study.
At 42-48 hr after the HCG injection, the embryos were flushed into Ham’s F10 (Sigma, USA) medium under a stereomicroscope (Nikon SMZ-2T, Japan) (11).
After three washes, embryos with normal develop-mental morphologies were randomly divided into 3 groups including:
α-MEM medium (Sigma, USA) containing 10% fetal bovine serum (FBS) and 10-9 M melatonin as the melatonin-treated group.
α-MEM medium with serum and without melatonin as the simple media group.
α-MEM medium containing serum and 10-9 M luzindole as the Luzindole-treated group.
Between 72–96 hr after initiation of embryo culture, the expanded blastocysts were randomly selected for further experiments and compared with the fourth group, in vivo developed blastocysts, as the control group.
Differential staining of blastocysts
Expanded blastocysts were randomly chosen for cell counting analysis as described elsewhere (11) with a few modifications. Briefly, 3–5 expanded blastocysts from each group (control, simple media, melatonin-treated, and Luzindole-treated) were picked up and placed in Ham’s F10 medium supplemented with 1% Triton X-100 and 10 μg/ml propidium iodide (PI) (P4170, sigma, Germany) at 37°C for approximately 30–50 sec. Then, the embryos were incubated in 25 μg/ml of bisbenzimide (Hoechst 33342, Sigma, Germany) in 500 μl absolute alcohol as a fixative, overnight at 4°C in a dark chamber. By using the Hoechst dye, the nucleus of the inner cell mass (ICM) was stained blue and with PI, trophectoderm (TE) nuclei appeared pink to red.
Then, embryos were mounted on microscope slides with glycerol and the numbers of ICM and TE nuclei were counted under a fluorescence microscope (Olympus BX51TRF, Japan).
Immunocytochemistry of ErbB1 and ErbB4 in mouse embryos
Three to five of expanded blastocysts from each group (control, simple media, melatonin-treated, and luzindole-treated) were randomly selected as described elsewhere (1, 12) with a few modifications. Briefly, selected embryos were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. Then blastocyst were washed three times with PBS. PBS containing 0.5% Triton X-100 was applied to permeabilize embryos for 5 min. Then, blastocysts were incubated in blocking solution (10% goat serum, Sigma, USA) for 10 min. Primary antibody, anti-EGFR antibody (ErbB1), anti-ErbB4 antibody (1:50, Abcam, USA) were added and incubated at 4 °C overnight. Immunodetection procedures were performed in duplicate for each ErbB antibody. After several washings in PBS, embryos were incubated with secondary antibody fluorescein isothiocyanate (FITC, Abcam, USA) conjugated IgG for 20 min at room temperature in the dark. After washing with PBS, blastocysts were mounted in 90% glycerol in PBS and then visualized by fluorescent microscope (Olympus BX51TRF, Japan).
Detection of ROS production with DCFH-DA
To determine the quantity of ROS levels, embryos from each group (n=3–5) were incubated for 30 min in HamsF10 containing 2’,7’-dichlorofluorescein diacetate (DCFH-DA; 2 μM, Sigma, USA) at 37°C in the dark. Incubated embryos with vehicle and with DCFH-DA plus H2O2 were designed as negative and positive controls, respectively.
Subsequently, specimens were washed three times in HamsF10 medium supplemented with FBS and then mounted on glass slides. DCFH fluorescence was measured using fluorescence microscopy (Olympus BX51, Tokyo, Japan) equipped with an E.30 digital camera (Olympus, Tokyo, Japan) with 450–490 nm (excitation) and 520 nm (emission) filters, under the lowest level of room light. The brightness of each blastocyst was analyzed using the Image Quant software (TotalLab Quant, UK) (2).
Quantitative real-time PCR
Twenty expanded blastocysts from each group were randomly chosen for total RNA extraction using Arcturus PicoPure RNA Isolation Kit. Embryos were washed with phosphate-buffered saline (PBS) and then added to the lysis buffer.
Then, samples were bonded to a silica-based filter where they were treated with RNase-free DNase I (Qiagen, Valencia, CA). After several washings, RNA was then eluted with 20 μl elution buffer and checked for quality. The 260: 280 nm ratios (range, 1.80 to 2) were determined.
Reverse transcription was performed to produce complementary DNA (cDNA) by using the High Capacity Reverse Transcription cDNA kit (Applied Biosystems, Foster City, CA) for real-time polymerase chain reaction (qPCR) (13). For reverse transcription 100 ng of total RNA was added to 0.5 mg oligo (dT) 12-18 primer (Invitrogen), 0.5 mM of each dNTP, 4 U Omniscript reverse transcriptase, and 1 U RT buffer (Omniscript Reverse Transcription kit; Qiagen GmbH, Hilden, Germany). The reaction was performed for 5 min at 25–30 ºC, 60 min at 60 ºC, and 5 min at 95 ° C.
Quantitative real-time PCR (qRT-PCR) was performed using the ABI 7300 Real-Time PCR System with the Power SYBR Green PCR Master Mix (Applied Biosystems) blindly on blastocyst samples. The following PCR protocol was used: denaturation step (95 °C for 10 min), amplification step for 40 cycles (95 °C for 15 sec, and 60 °C for 1 min), then dissociation step (95°C for 15 sec, 60 °C for 1 min, 95°C for 15 sec and 60 °C for 15 sec).
The quantification of 2 genes, ErbB1 and ErbB4 was calculated relative to the comparatively constant level of transcription in every sample of the housekeeping gene, GAPDH (13).
Finally, the 2-ΔΔCt technique was used for comparative quantification of data and further norma- lization to GAPDH and fold change comparison to control. The primers were designed with Gene runner (version 3) and primer expresses (version 3.05) software and the designed primers were blasted in http://www.ncbi.nlm.nih.gov/BLAST/.
Table 1 presents the nucleotide sequences of primers for the ErbB1, ErbB4m, and housekeeping genes.
Table 1.
Primer sequences for quantitative real-time PCR (qRT-PCR)
| Gene name | Forward and reverse primer sequence | Product size |
|---|---|---|
| ErbB1 | Forward:5’ TGGGTACGTTCAATGGCAGT 3’ | 318 bp |
| Reverse:5’ CCCTTGGGCTACTGAGAGGA 3’ | ||
| ErbB4 | Forward:5’ GGACGGGCCATTCCACTTTA 3’ | 172 bp |
| Reverse:5’ ACCAGCTCTGTCTCCAGGAA 3’ | ||
| GABDH | Forward:5’ AGCAACAGGGTGGTGGACCT 3’ | 133 bp |
| Reverse:5’ AGTGTGGCGGAGATGGGGCA 3’ |
Measurement of antioxidant capacity by radical cation (ABTS °+)
Trolox equivalent antioxidant capacity (TEAC) assay was first introduced by Rice–Evans & Miller. The TEAC assay is based on the inhibition by antioxidants of the absorbance of the radical cation of 2, 2’-azinobis (3-ethylbenzothiazoline 6-sulfonate) (ABTS).
ABTS was converted to radical cation ABTS (ABTS °+) by the addition of sodium persulfate. During this reaction, the bluish green ABTS radical cation was converted back to its colorless neutral form. Then, the reaction was monitored by measuring the absorbance of antioxidant – radical reaction mixture at 734 nm at a defined time with a spectrophotometer (4).
Statistical analysis
All experiments were repeated at least in triple and the data was expressed as mean ± SD. To evaluate the statistical significance between different groups, statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s and Tamhane’s post hoc tests using SPSS 16. A value of P≤ 0.05 was considered statistically significant.
Results
Differential blastocyst staining
In the present study, the expanded blastocysts were stained with PI/Hoechst dye then, the number of ICM and TE nuclei were calculated.
Under fluorescence microscopy, with excitation wavelength (355 nm) and barrier filter (465 nm), ICM nuclei appeared blue and TE nuclei with excitation wavelength (530 nm) and barrier filter (615 nm) were detected in red. We observed that the blastocysts in the melatonin-treated group had significantly (P<0.05) the highest number of ICM and TE nuclei compared with other groups. On the other hand, the lowest number of nuclei was shown among embryos in the Luzindole-treated group (Figure 1).
Figure 1.
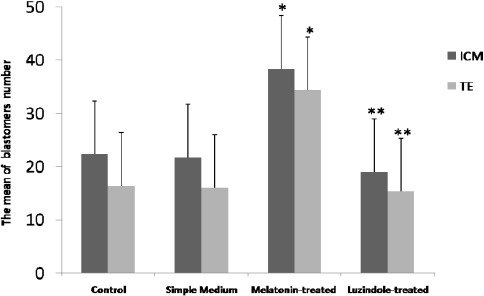
Melatonin increases the number of ICM and TE nuclei in the expanded blastocysts. Bars show the mean of blastomers number ± SD in all groups.* P<0.05 vs. other groups; ** P<0.05 vs. the melatonin-treated group (one-way ANOVA)
As Figure 1 shows treating with melatonin can significantly (P<0.05) enhance the blastomere numbers in cultured embryos compared with the control group.
Analysis of ErbB1 and ErbB4 genes expression
The results of qRT-PCR showed that treatment blastocysts with melatonin at the concentration of 10-9 M can up-regulate ErbB1 and ErbB4 genes expression compared with the other groups (P<0.05) (Figures 2a, b).
Figure 2.
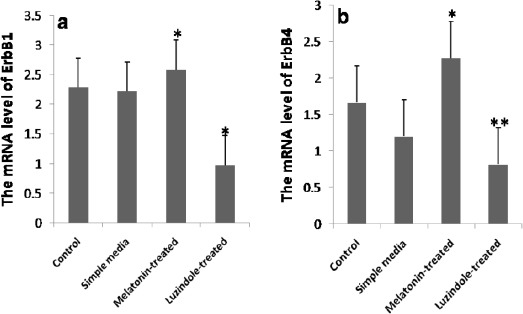
Up-regulation of ErbB1 and ErbB4 genes expression following treating the expanded blastocysts with melatonin. a Quantitative real-time PCR (qRT-PCR) analysis of ErbB1 mRNA. * P<0.05 vs. all groups. b Quantitative real-time PCR (qRT-PCR) analysis of ErbB4 mRNA.* P<0.05 vs. all groups; ** P<0.05 vs. the control and the melatonin-treated groups
The expanded blastocysts were labeled with anti-ErbB1 then the quantitation was measured using Image Quant software (TotalLab Quant). Bars are representative of mean fluorescence intensity ± SD. * P<0.05 vs. the simple media and the Luzindole-treated groups; ** P=0.003 vs. the melatonin-treated group
On the other hand, statistical analysis revealed that supplemented culture media with luzindole can significantly (P<0.05) decrease the mRNA level of ErbB1 in comparison to the other groups (Figure 2a) and can significantly (P<0.05) decrease the mRNA level of ErbB4 compared with the control and the melatonin-treated groups (Figure 2b). In the level of ErbB4 expression, no significant difference was detected between the Luzindole-treated and the simple media groups.
Immunocytochemistry of mouse blastocysts
The obtained results from ICC showed that the expanded blastocysts in all groups were uniformly labeled with anti-ErbB1 antibody and anti-ErbB4. To evaluate the likely changes of expression in cultured embryos, the quantitation of ErbB1 and ErbB4 was performed using the ImageQuant software (TotalLab Quant, UK).
The significant overexpression of ErbB1 and ErbB4 proteins were detected in developed embryos in the melatonin-treated group not those in the simple media and the Luzindole-treated groups. When we compared the quantity of ErbB1 and ErbB4 protein expression in the melatonin-treated group and the control group, no significant difference was shown. As the data shows, the lowest ErbB1 and ErbB4 protein expression belonged to the luzindole-treated group (Figures 3 and 4).
Figure 3.
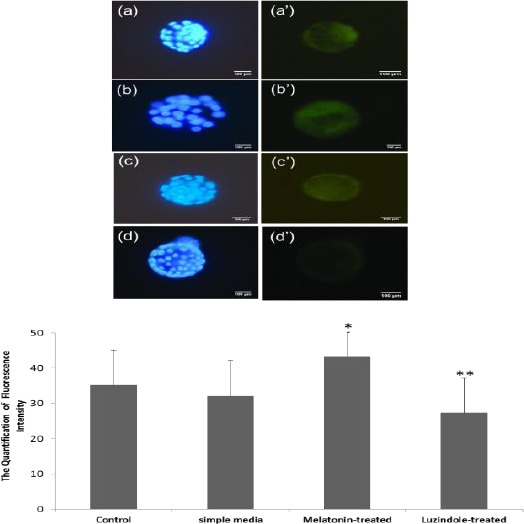
Increased proteins expression of ErbB1 following treatment with melatonin. a, b, c, and d show the stained embryos with Hoechst dye (blue) and a’, b’, c’, and d’ present the fluorescence intensity (green) in the control, the melatonin-treated, the simple media, and the Luzindole-treated groups, respectively.
Figure 4.
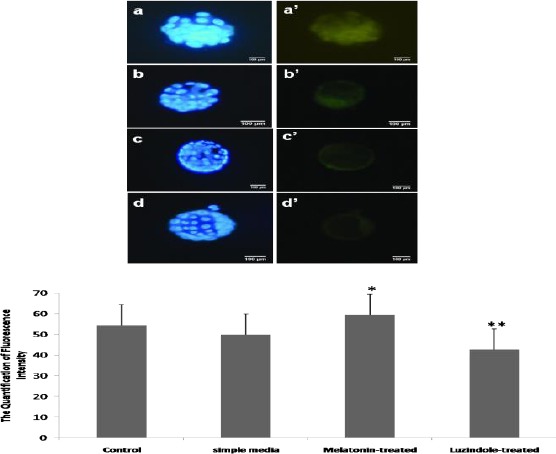
Increased proteins expression of ErbB4 following treatment with melatonin. a, b, c, and d show the stained embryos with Hoechst (blue) and a’, b’, c’, and d’ present the fluorescence intensity (green) in the control, the melatonin-treated, the simple media, and the Luzindole-treated groups, respectively.
The expanded blastocysts were labeled with anti-ErbB4 then the quantitation was measured by Image Quant software (TotalLab Quant). Bars are representative of mean fluorescence intensity ± SD.* P<0.05 vs. the simple media and the Luzindole-treated groups; ** P=0.003 vs. the melatonin-treated group
Measurement of reactive oxygen species (ROS)
To evaluate oxidative stress in blastocysts, the level of intracellular ROS was measured. The quantification analysis showed that supplemented culture medium with melatonin can significantly (P<0.05) decrease the mean of intracellular ROS in the expanded blastocysts in comparison with the other groups (37.01 ± 2.94 in the control, 38.14 ± 4.57 in the simple media, 21.66 ± 2.88 in the melatonin–treated, and 40.94 ± 4.95 in the Luzindole-treated).
When we measured the DCFH-DA fluorescence intensity in the Luzindole-treated group it was markedly (P=0.001) higher than in the melatonin-treated group and insignificantly higher than in the control and the simple media groups (Figure 5).
Figure 5.
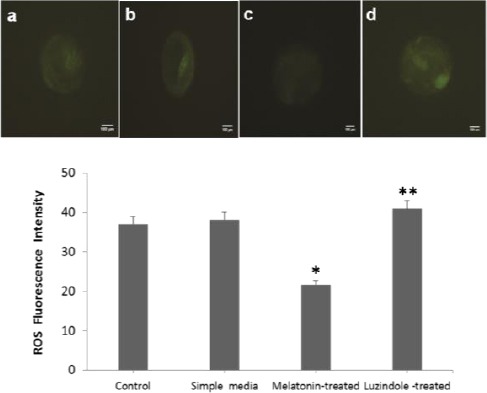
Melatonin decreases environmental stress factors-induced intracellular ROS level in mouse expanded blastocysts. The rate of intracellular ROS shown by the DCFH-DA fluorescence intensity (green) in all studied groups. a, b, c and d are representative of the control, the simple media, the melatonin-treated and the Luzindole-treated groups, respectively.
Bars show the quantification of fluorescence intensity of ROS in all groups. Data are presented as the mean ± SD from each group. * P<0.05 vs. all groups; ** P=0.001 vs. the melatonin-treated group (one-way ANOVA)
Measurement of total antioxidant capacity (TAC)
Since environmental stress factors may lead to a decrease of antioxidant capability in cultured preimplantation embryos, we measured the TAC in the studied blastocysts after 72 to 96 hr from initiation of in vitro culture.
As the data shows, supplemented embryo culture media with melatonin could significantly (P<0.05) increase TAC and supplemented media with luzindole markedly (P<0.001) decreased total antioxidant capacity in comparison with the other groups (Figure 6).
Figure 6.
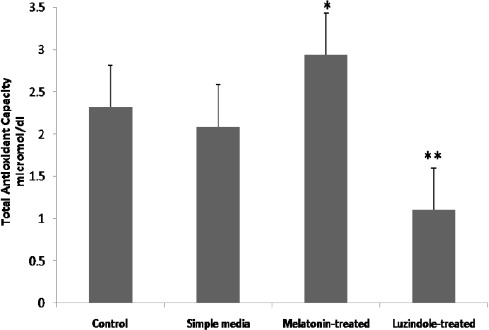
Melatonin protects mouse expanded blastocysts from oxidative stress by increasing the total antioxidant capability. Bars are representative of total antioxidant capacity in the samples.* P<0.05 and ** P<0.001 vs. all groups
Discussion
Blastocyst implantation is a critical step in mammalian reproduction involving a complicated sequence of genetic and cellular interactions so that all of them must be executed in a defined time (4).
The signaling pathway is initiated by HB-EGF, an early molecular marker of embryo–uterine crosstalk, produced in the uterus and adhering to blastocysts that display cell-surface ErbB4 and ErbB1 (6, 14).
So disturbances in this bidirectional cross talk could cause implantation failure. The imperfection of ErbB4 and ErbB1 expressions in in vitro cultured embryos will result in poor embryo development and implantation failure (15).
Although the environment in in vitro embryo development mimics the in vivo environment but this simulation is not exact and in vitro produced embryos are inevitably exposed to environmental stress factors (16).
The dissimilarity between environmental condi- tions such as the composition of culture media (17), pH fluctuations (18) etc., can induce the production of ROS and negatively impact the early embryonic development (11, 19) and also may alter the gene expressions in embryos (20).
Since embryo defense mechanisms are insufficient to protect their cellular structures, it seems likely that the presence of antioxidants in embryo culture media is undoubtedly essential (1).
Melatonin as a direct free radical scavenger and universal antioxidant (21) is widely used in the protection of in vitro cultured embryos. It has been reported that melatonin supplementation in a concentration-dependent manner significantly improved the development of pre-implantation embryos in different species (22).
For example, the addition of 10−13 to 10−5 M melatonin dramatically enhanced the rates of blastocyst formation, expanded blastocysts and cell numbers in the mouse blastocysts. In this range, a remarkable positive effect belongs to melatonin at a concentration of 10-9 M (4, 23). It is also observed that at an extremely high-dose (10-3 M), melatonin retards embryo development (22).
Since many studies declared that embryo culture medium supplemented with melatonin in a dose-dependent manner can up-regulate the expression of some genes during pre-implantation embryo development (24), the current study was designed to investigate the effect of melatonin on the probable changes of ErbB1 and ErbB4 expression, two crucial implantation related genes and proteins in cultured embryos.
Our results showed that the expression of both ErbB1 and ErbB4 mRNAs and proteins were significantly up-regulated in the cultivation drops with melatonin supplementation.
According to the broad search on the web, there were no findings that declared the effect of melatonin on the expression rate of the primary implantation receptors, ErbB1, and ErbB4, in in vitro cultured embryos and our reported results may be the first. Although the results of one similar study show that melatonin can significantly increase the expression of ErbB1 in blastocysts but the effect of melatonin was investigated in in vivo developed embryos and following intraperitoneal injection (25).
In the current study melatonin antagonist (Luzindole) was used to confirm the role of melatonin in increasing the level of ErbB mRNAs and proteins. Our results show that melatonin antagonist could significantly decrease the expression of ErbB1 and ErbB4 genes and proteins.
In addition to the positive effects of melatonin at the molecular level, it works as a direct scavenger of existing ROS and has the ability to reduce the oxidative damage (17). Several reports have stated that oxidative stress leads to nuclear and mitochondrial DNA damage which is expected to cause cell death (21, 26, 27).
Melatonin also stimulates the activity of other antioxidant enzymes (4). Our results also agree with recent results that melatonin reduced ROS accumulation (28) and increased total antioxidants enzymes (4).
As regards mechanism, it can be concluded from our data that melatonin by removing extra ROS from embryo culture media had an important role in protecting nuclear DNA from induced damage and resulted in up-regulation of implantation-related genes.
Despite findings on the melatonin effects on the morphological variations such as total cell numbers in cultured embryos (4), the current results confirmed again that supplemented culture media with 10-9 M melatonin increased the number of ICM and TE nuclei in in vitro developed embryos. Our results are in line with previous studies in which 10-10M melatonin provided the highest increase in total cell number of blastocysts and significantly promoted cleavage and blastocyst formation rates (24, 29). Since the blastocyst cell numbers reflect the incidence of cell division blastomeres (1), our evidence can prove that application of melatonin increases the blastomere numbers and provides the developmental competence of pre-implantation embryos.
Conclusion
The obtained data from the present study show that treating embryos with melatonin enhances the pre-implantation mouse embryo quality and increases the expression of ErbB1 and ErbB4, important genes relating in implantation. Based on the results of this study, melatonin enhanced the level of ErbB1 and ErbB4 mRNAs probably via its role as a radical scavenger. Following reducing intracellular ROS and because of the direct and indirect antioxidant action of melatonin , the protection of embryo nuclei gets raised. Moreover, melatonin could improve the cell division of embryos and increase the blastomere numbers. So, the use of melatonin could be a helpful tool for solving embryo development impairment and also implantation failure.
Acknowledgments
The results presented in this paper were part of a student thesis and it was financially supported by grant number: 93-02-30-25193 from Tehran University of Medical Sciences, Tehran, Iran. Authors would like to thank Miss Fatemeh Hassani and Mr Asgari (researcher in Embryology Department and Stem Cells Department, respectively, Royan Institute, Tehran, Iran, for useful comments and suggestions for conducting this project.
References
- 1.Peters VM, Spray DC, Mendez-Otero R. Effect of mesenchymal stem cells and mouse embryonic fibroblasts on the development of preimplantation mouse embryos. In Vitro Cell Dev Bidl Anim. 2016;52:497–506. doi: 10.1007/s11626-015-9997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu S, Long H, Lyu Qf, Zhang Qh, Yan Z-g, Liang Hx, et al. Protective effect of quercetin on the development of preimplantation mouse embryos against hydrogen peroxide-induced oxidative injury. PLoS One. 2014;9:e89520. doi: 10.1371/journal.pone.0089520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Mehaisen GM, Saeed AM, Gad A, Abass AO, Arafa M, El-Sayed A. Antioxidant capacity of melatonin on preimplantation development of fresh and vitrified rabbit embryos: morphological and molecular aspects. PLoS One. 2015;10:e0139814. doi: 10.1371/journal.pone.0139814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrenzycki C, Herrmann D, Keskintepe L, Martins A Jr, Sirisathien S, Brackett B, et al. Effects of culture system and protein supplementation on mRNA expression in pre-implantation bovine embryos. Hum Reprod. 2001;16:893–901. doi: 10.1093/humrep/16.5.893. [DOI] [PubMed] [Google Scholar]
- 6.Jeong W, Jung S, Bazer FW, Song G, Kim J. Epidermal growth factor: Porcine uterine luminal epithelial cell migratory signal during the peri-implantation period of pregnancy. Mol Cell Endocrinol. 2016;420:66–74. doi: 10.1016/j.mce.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Lim HJ, Dey S. HB-EGF: a unique mediator of embryo-uterine interactions during implantation. Exp Cell Res. 2009;315:619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao C, Han HB, Tian XZ, Tan DX, Wang L, Zhou GB, et al. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res. 2012;52:305–311. doi: 10.1111/j.1600-079X.2011.00944.x. [DOI] [PubMed] [Google Scholar]
- 9.Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging. 2012;4:768–789. doi: 10.18632/aging.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Movaghar B, Askarian S. Expression of e-cadherin, leukemia inhibitory factor and progesterone receptor in mouse blastocysts after ovarian stimulation. Cell J. 2012;14:225–230. [PMC free article] [PubMed] [Google Scholar]
- 11.Moshkdanian G, Nematollahi-mahani SN, Pouya F, Nematollahi-mahani A. Antioxidants rescue stressed embryos at a rate comparable with co-culturing of embryos with human umbilical cord mesenchymal cells. J Assist Reprod Genet. 2011;28:343–349. doi: 10.1007/s10815-010-9529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown N, Deb K, Paria BC, Das SK, Reese J. Embryo-uterine interactions via the neuregulin family of growth factors during implantation in the mouse. Biol Reprod. 2004;71:2003–2011. doi: 10.1095/biolreprod.104.031864. [DOI] [PubMed] [Google Scholar]
- 13.Parks JC, McCallie BR, Janesch AM, Schoolcraft WB, Katz-Jaffe MG. Blastocyst gene expression correlates with implantation potential. Fertil Steril. 2011;95:1367–1372. doi: 10.1016/j.fertnstert.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 15.Altmäe S, Reimand J, Hovatta O, Zhang P, Kere J, Laisk T, et al. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol. 2011;26:203–217. doi: 10.1210/me.2011-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Malhotra N, Sharma D, Chandra A, Ashok A. Oxidative stress and its role in female infertility and assisted reproduction: clinical implications. Int J Fertil Steril. 2009;2:147–164. [Google Scholar]
- 17.Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol. 2014;12:112. doi: 10.1186/1477-7827-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nematollahi-mahani SN, Nematollahi-mahani A, Moshkdanian G, Shahidzadehyazdi Z, Labibi F. The role of co-culture systems on developmental competence of preimplantation mouse embryos against pH fluctuations. J Assist Reprod Genet. 2009;26:597–604. doi: 10.1007/s10815-009-9363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asgari Z, Ghasemian F, Ramezani M, Bahadori MH. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012;14:203–208. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Tian X, Zhou Y, Tan D, Zhu S, Dai Y, et al. Melatonin improves the quality of in vitro produced (IVP) bovine embryos: implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS One. 2014;9:e93641. doi: 10.1371/journal.pone.0093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:1. doi: 10.1186/1757-2215-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Tian X, Zhang L, Gao C, He C, Fu Y, et al. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J Pineal Res. 2014;56:333–342. doi: 10.1111/jpi.12126. [DOI] [PubMed] [Google Scholar]
- 23.Tian XZ, Wen Q, Shi JM, Liang W, Zeng SM, Tian JH, et al. Effects of melatonin on in vitro development of mouse two-cell embryos cultured in HTF medium. Endocr Res. 2010;35:17–23. doi: 10.3109/07435800903539607. [DOI] [PubMed] [Google Scholar]
- 24.Choi J, Park SM, Lee E, Kim JH, Jeong YI, Lee JY, et al. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol Reprod Dev. 2008;75:1127–1135. doi: 10.1002/mrd.20861. [DOI] [PubMed] [Google Scholar]
- 25.He C, Wang J, Li Y, Zhu K, Xu Z, Song Y, et al. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J Pineal Res. 2015;58:300–309. doi: 10.1111/jpi.12216. [DOI] [PubMed] [Google Scholar]
- 26.Chen YC, Sheen JM, Tiao MM, Tain YL, Huang LT. Roles of melatonin in fetal programming in compromised pregnancies. Int J Mol Sci. 2013;14:5380–5401. doi: 10.3390/ijms14035380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih YF, Lee TH, Liu CH, Tsao HM, Huang CC, Lee MS. Effects of reactive oxygen species levels in prepared culture media on embryo development: a comparison of two media. Taiwan J Obstet Gynecol. 2014;53:504–508. doi: 10.1016/j.tjog.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan D-X, Manchester LC, Paredes SD, Mayo JC, Sainz RM. Melatonin and reproduction revisited. Biol Reprod. 2009;81:445–456. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 29.Zhao LH, Cui XZ, Yuan HJ, Liang B, Zheng LL, Liu YX, et al. Restraint stress inhibits mouse implantation: temporal window and the involvement of HB-EGF, estrogen and progesterone. PLoS One. 2013;8:e80472. doi: 10.1371/journal.pone.0080472. [DOI] [PMC free article] [PubMed] [Google Scholar]


