Abstract
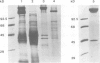
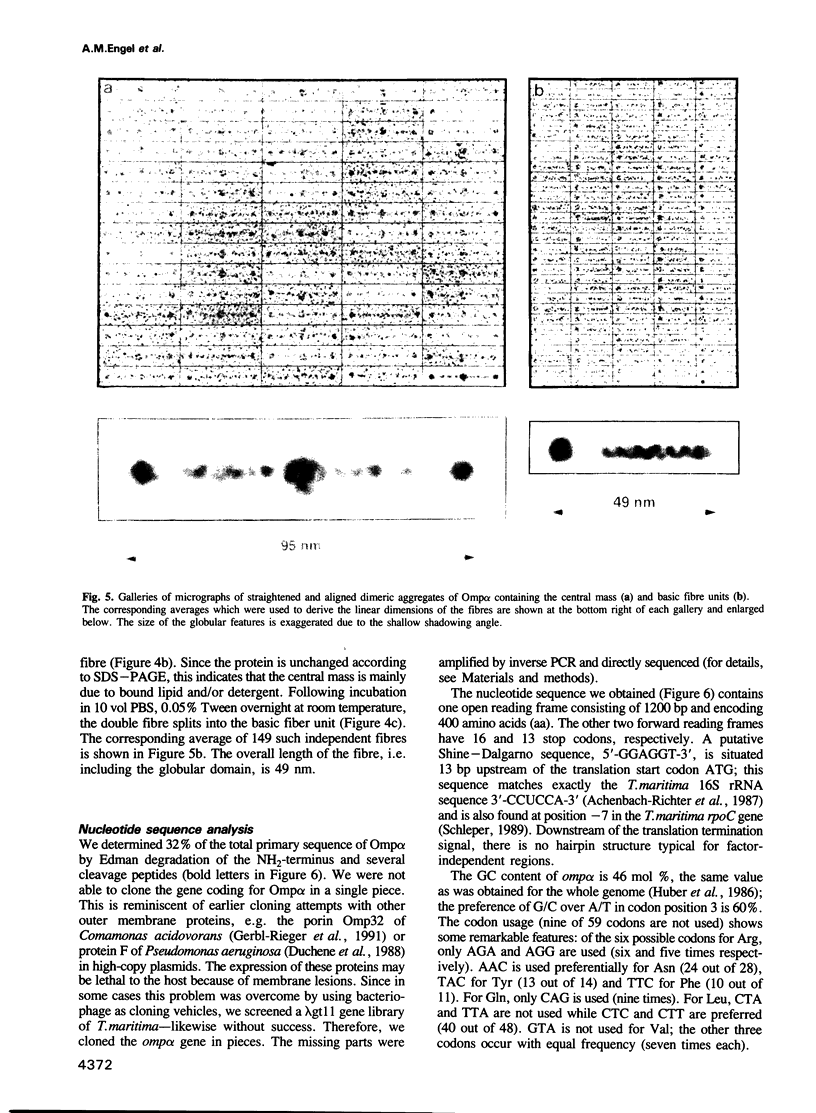
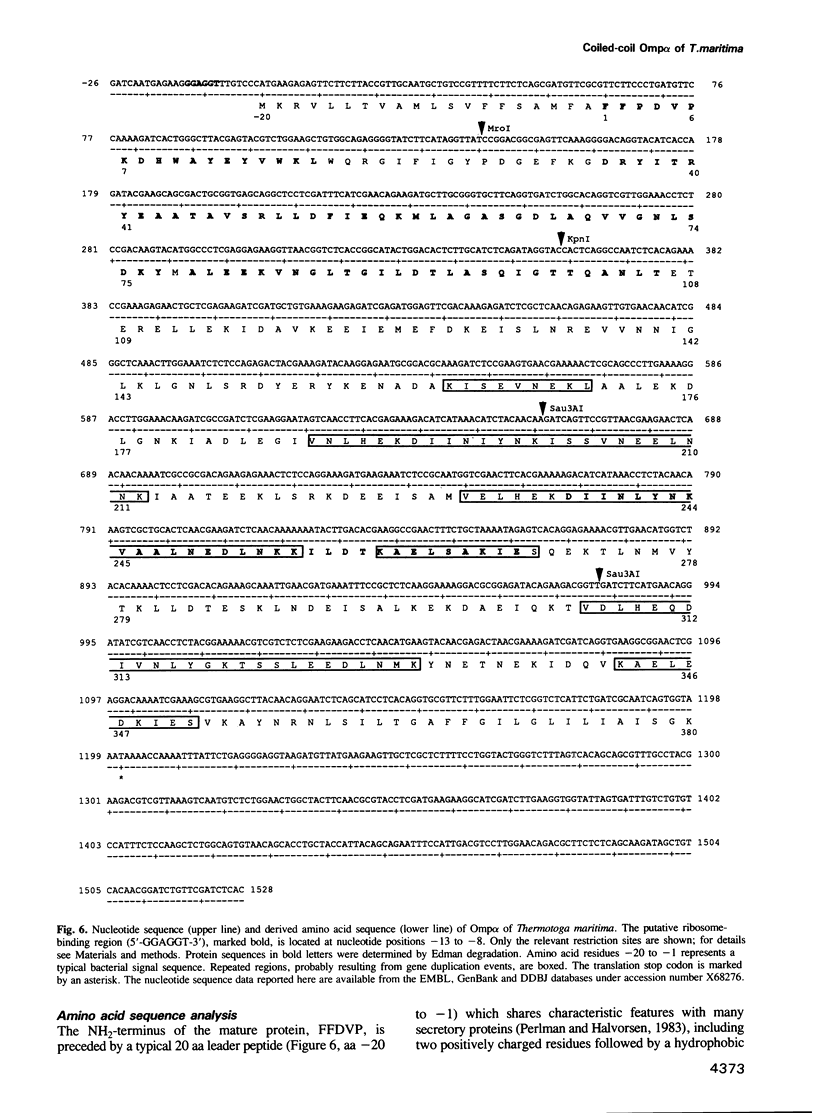
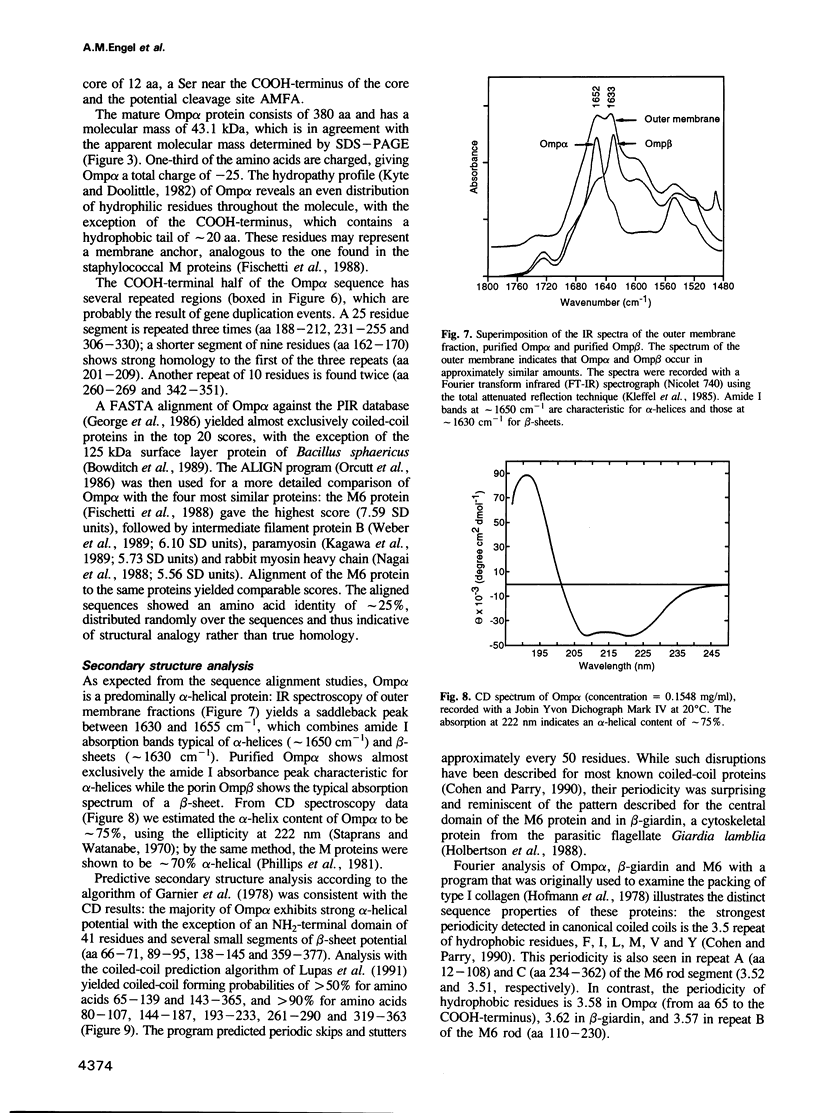
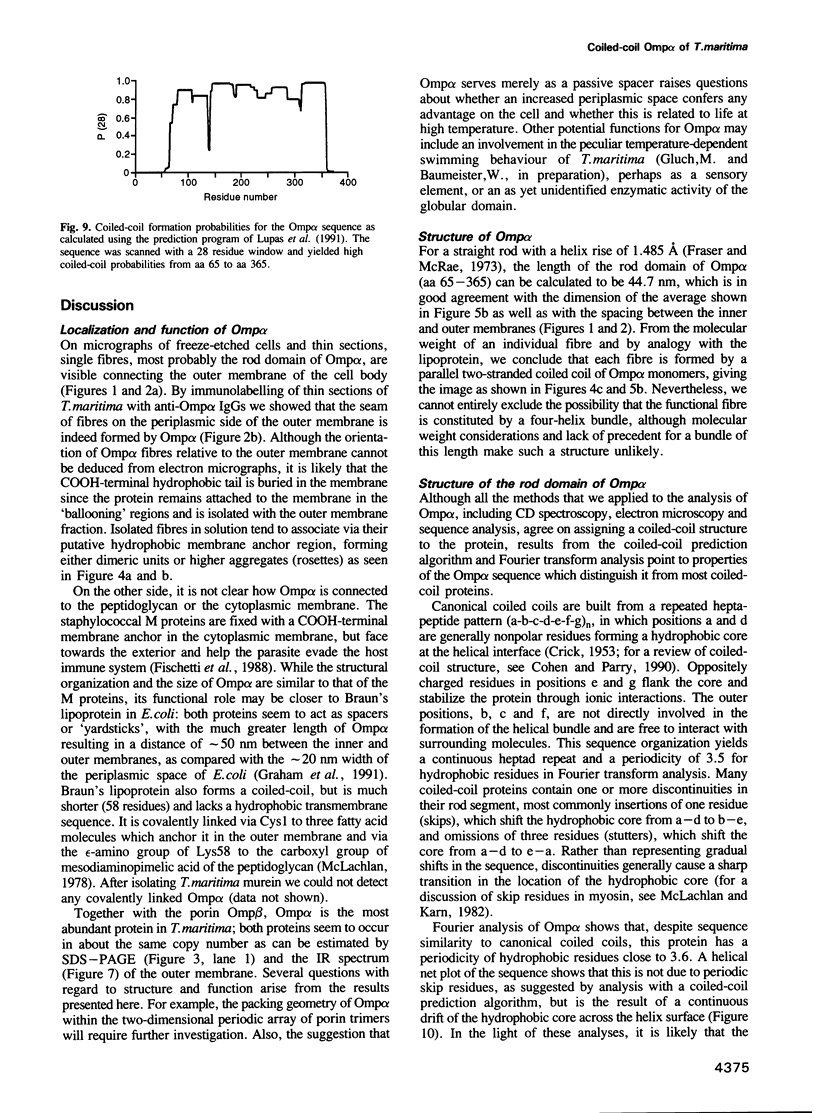
We have discovered a new oligomeric protein component associated with the outer membrane of the ancestral eubacterium Thermotoga maritima. In electron micrographs, the protein, Omp alpha, appears as a rod-shaped spacer that spans the periplasm, connecting the outer membrane to the inner cell body. Purification, biochemical characterization and sequencing of Omp alpha suggest that it is a homodimer composed of two subunits of 380 amino acids with a calculated M(r) of 43,000 and a pI of 4.54. The sequence of the omp alpha gene indicates a tripartite organization of the protein with a globular NH2-terminal domain of 64 residues followed by a putative coiled-coil segment of 300 residues and a COOH-terminal, membrane-spanning segment. The predicted length of the coiled-coil segment (45 nm) correlates closely with the spacing between the inner and outer membranes. Despite sequence similarity to a large number of coiled-coil proteins and high scores in a coiled-coil prediction algorithm, the sequence of the central rod-shaped domain of Omp alpha does not have the typical 3.5 periodicity of coiled-coil proteins but rather has a periodicity of 3.58 residues. Such a periodicity was also found in the central domain of staphylococcal M protein and beta-giardin and might be indicative of a subclass of fibrous proteins with packing interactions that are distinct from the ones seen in other two-stranded coiled-coils.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch R. D., Baumann P., Yousten A. A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989 Aug;171(8):4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Duchêne M., Schweizer A., Lottspeich F., Krauss G., Marget M., Vogel K., von Specht B. U., Domdey H. Sequence and transcriptional start site of the Pseudomonas aeruginosa outer membrane porin protein F gene. J Bacteriol. 1988 Jan;170(1):155–162. doi: 10.1128/jb.170.1.155-162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Parry D. A., Trus B. L., Hollingshead S. K., Scott J. R., Manjula B. N. Conformational characteristics of the complete sequence of group A streptococcal M6 protein. Proteins. 1988;3(1):60–69. doi: 10.1002/prot.340030106. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbl-Rieger S., Peters J., Kellermann J., Lottspeich F., Baumeister W. Nucleotide and derived amino acid sequences of the major porin of Comamonas acidovorans and comparison of porin primary structures. J Bacteriol. 1991 Apr;173(7):2196–2205. doi: 10.1128/jb.173.7.2196-2205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J., Nanninga N. Periplasmic space and the concept of the periplasm. Trends Biochem Sci. 1991 Sep;16(9):328–329. doi: 10.1016/0968-0004(91)90135-i. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Fietzek P. P., Kühn K. The role of polar and hydrophobic interactions for the molecular packing of type I collagen: a three-dimensional evaluation of the amino acid sequence. J Mol Biol. 1978 Oct 25;125(2):137–165. doi: 10.1016/0022-2836(78)90342-x. [DOI] [PubMed] [Google Scholar]
- Holberton D., Baker D. A., Marshall J. Segmented alpha-helical coiled-coil structure of the protein giardin from the Giardia cytoskeleton. J Mol Biol. 1988 Dec 5;204(3):789–795. doi: 10.1016/0022-2836(88)90370-1. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Walian P. J. Biophysics of the structure and function of porins. Q Rev Biophys. 1990 Nov;23(4):367–403. doi: 10.1017/s003358350000559x. [DOI] [PubMed] [Google Scholar]
- Kagawa H., Gengyo K., McLachlan A. D., Brenner S., Karn J. Paramyosin gene (unc-15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence and models for thick filament structure. J Mol Biol. 1989 May 20;207(2):311–333. doi: 10.1016/0022-2836(89)90257-x. [DOI] [PubMed] [Google Scholar]
- Kleffel B., Garavito R. M., Baumeister W., Rosenbusch J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985 Jun;4(6):1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei P., Altamura S., Huber R., Stetter K. O., Cammarano P. Ribosomes of the extremely thermophilic eubacterium Thermotoga maritima are uniquely insensitive to the miscoding-inducing action of aminoglycoside antibiotics. J Bacteriol. 1988 Sep;170(9):4353–4360. doi: 10.1128/jb.170.9.4353-4360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W., Weizenegger M., Betzl D., Leidel E., Lenz T., Ludvigsen A., Möllenhoff D., Wenzig P., Schleifer K. H. Complete nucleotide sequences of seven eubacterial genes coding for the elongation factor Tu: functional, structural and phylogenetic evaluations. Arch Microbiol. 1990;153(3):241–247. doi: 10.1007/BF00249075. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. The double helix coiled coil structure of murein lipoprotein from Escherichia coli. J Mol Biol. 1978 Jun 5;121(4):493–506. doi: 10.1016/0022-2836(78)90396-0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nagai R., Larson D. M., Periasamy M. Characterization of a mammalian smooth muscle myosin heavy chain cDNA clone and its expression in various smooth muscle types. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1047–1051. doi: 10.1073/pnas.85.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Staprans I., Watanabe S. Optical properties of troponin, tropomyosin, and relaxing protein of rabbit skeletal muscle. J Biol Chem. 1970 Nov 25;245(22):5962–5966. [PubMed] [Google Scholar]
- Tiboni O., Cantoni R., Creti R., Cammarano P., Sanangelantoni A. M. Phylogenetic depth of Thermotoga maritima inferred from analysis of the fus gene: amino acid sequence of elongation factor G and organization of the Thermotoga str operon. J Mol Evol. 1991 Aug;33(2):142–151. doi: 10.1007/BF02193628. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J. 1989 Nov;8(11):3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wrba A., Schweiger A., Schultes V., Jaenicke R., Závodszky P. Extremely thermostable D-glyceraldehyde-3-phosphate dehydrogenase from the eubacterium Thermotoga maritima. Biochemistry. 1990 Aug 21;29(33):7584–7592. doi: 10.1021/bi00485a007. [DOI] [PubMed] [Google Scholar]