Abstract
Background
Gliomas are diverse neoplasms with multiple molecular subtypes. How tumor-initiating mutations relate to molecular subtypes as these tumors evolve during malignant progression remains unclear.
Methods
We used genetically engineered mouse models, histopathology, genetic lineage tracing, expression profiling, and copy number analyses to examine how genomic tumor diversity evolves during the course of malignant progression from low- to high-grade disease.
Results
Knockout of all 3 retinoblastoma (Rb) family proteins was required to initiate low-grade tumors in adult mouse astrocytes. Mutations activating mitogen-activated protein kinase signaling, specifically KrasG12D, potentiated Rb-mediated tumorigenesis. Low-grade tumors showed mutant Kras-specific transcriptome profiles but lacked copy number mutations. These tumors stochastically progressed to high-grade, in part through acquisition of copy number mutations. High-grade tumor transcriptomes were heterogeneous and consisted of 3 subtypes that mimicked human mesenchymal, proneural, and neural glioblastomas. Subtypes were confirmed in validation sets of high-grade mouse tumors initiated by different driver mutations as well as human patient-derived xenograft models and glioblastoma tumors.
Conclusion
These results suggest that oncogenic driver mutations influence the genomic profiles of low-grade tumors and that these, as well as progression-acquired mutations, contribute strongly to the genomic heterogeneity across high-grade tumors.
Keywords: genetically engineered mouse, glioblastoma, glioma, progression, transcriptome
Importance of the study
Gliomas are the most common and deadly primary brain cancer. Precision-medicine approaches require elucidating the causes of their extensive genomic heterogeneity. We used conditional, inducible mouse models and oncogenomics to show that driver mutations influence the natural history, transcriptomes, and copy number profiles of low-grade and high-grade tumors. Our results demonstrate that tumor-initiating mutations influence gliomagenesis and that genomic profiles evolve during malignant progression.
Diffuse gliomas are the most common primary brain cancers and are characterized by extensive morphological, molecular, genomic, and biological heterogeneity. Diagnostic classification has traditionally been based on tumor cell morphology and prognosis: astrocytomas resemble astrocytes and are biologically aggressive, while oligodendrogliomas resemble oligodendrocytes and are more indolent. Histology grade has been used to further predict prognosis and guide care.1,2 Low-grade gliomas (LGG; World Health Organization [WHO] grade II) have a 10- to 15-year survival and lack the proliferation, angiogenesis, and necrosis present in high-grade gliomas. These latter tumors include anaplastic gliomas (grade III) and glioblastoma (GBM, grade IV) and have prognoses of 3–5 years and 12–15 months, respectively.1,2 Recent genomics discoveries have provided an impetus to modify classification to include both morphological and genotypic features.2 Despite advances in classification, the failure of adjuvant therapies, inevitability of tumor recurrence, and dismal patient outcomes have continued to fuel research to define the sources of glioma heterogeneity.
Mutations are one major source of glioma heterogeneity. These have been extensively documented by The Cancer Genome Atlas (TCGA), which examined the genomic heterogeneity of primary GBM, LGG, and anaplastic gliomas.3–7 TCGA and other groups have defined 4 transcriptome subtypes of primary GBM by comprehensive molecular profiling and found that select mutations correlated with transcriptome subtype.3,5,8 The most frequently mutated genes functioned in 3 “core GBM pathways”—G1/S cell cycle, receptor tyrosine kinase (RTK), and TP53.3–5 TCGA and other groups have also defined 3–4 transcriptome subtypes of “lower-grade gliomas” (including grade II and grade III anaplastic gliomas) that were enriched for particular mutation profiles.7,9,10
Genetically engineered mouse (GEM) models are uniquely suited to address the genetic mechanisms of glioma pathogenesis because they allow temporal control of driver mutations at defined developmental time points. Despite the existence of numerous glioma GEM models, most studies have only examined the genomic profiles of tumors from terminally aged mice.11 The evolution of genomic profiles during the course of malignant progression has therefore yet to be examined. In this report, we expanded on our previous work with a series of conditional, inducible GEM models in which the mitogen-activated protein kinase (MAPK) and/or phosphatidylinositol-3-kinase (PI3K) effector arms of RTK signaling are mutationally activated in G1/S checkpoint-defective adult mouse astrocytes.12,13 We use this model system here to examine the genetic requirements for tumorigenesis within G1/S and MAPK pathways and the genomics of tumor evolution during progression from low- to high-grade disease.
Materials and Methods
See the Supplementary materials for further methodological details. Supplementary figures and tables can be found online.
Genetically Engineered Mice
Heterozygous TgGZT121 (T), KrasG12D (R), GFAP-CreER, PLP-CreER, NG2-CreER, and Rosa26-tdTomato mice and homozygous Pten (P), p53, Rb1, and Nf1 mice were maintained on C57/Bl6 background (>94%). PCR genotyping was performed as previously described.13–15 Recombination was induced with 1 mg of intraperitoneal 4-hydroxytamoxifen for 5 consecutive days. Animal studies were approved by the UNC Institutional Animal Care and Use Committee.
Histopathology, Immunohistochemistry, and Quantification of Tumor Burden
Histopathological grading was performed according to WHO 2007/2016 criteria for human astrocytomas and defined as low-grade (grade II) or high-grade tumors (grades III and IV, GBM). Chromogenic immunohistochemistry for SV40 large T antigen was performed as previously described on a Leica Bond automated stainer.13 Tumor burden was quantified 2 months after induction using hematoxylin and eosin (H&E) stained sections and digital image analysis.16 H&E stained slides were scanned on an Aperio ScanScope XT using a 20× objective. Brains were manually segmented with Aperio ImageScope. Aperio color deconvolution v9 algorithm was used to quantify the area occupied by hematoxylin-positive nuclei in each region on 1–3 serial sagittal brain sections per mouse using the Allen Brain Atlas as a reference.17
Genetic Lineage Tracing and Immunofluorescence Staining
Genetic lineage tracing and immunofluorescence staining were performed on mice sacrificed ~2 months after induction, as previously described.13 Immunofluorescence analysis was performed using primary antibodies against glial fibrillary acidic protein (GFAP), Ki-67, neuronal nuclei (NeuN), neuron specific enolase, and p16. Images were acquired using a Zeiss LSM 710 confocal microscope.
Microarrays
RNA from brains harvested 2 months after induction were hybridized to Agilent Whole Mouse Genome 4 × 44K microarrays (G4122F), while brains from terminally aged mice were hybridized to 4x44Kv2 (G4846A). Stratagene Universal Mouse Reference RNA (Agilent, #740100) was cohybridized to each array. DNA was hybridized to Agilent Mouse 244A microarrays (G4415A) using a pooled DNA reference from wild-type C57/Bl6 and phenotypically wild-type syngeneic littermates. All original raw microarray data are deposited in the UNC Microarray Database (http://genome.unc.edu) and the National Center for Biotechnology Information’s Gene Expression Omnibus (GSE49269).
Bioinformatics
Microarray data were normalized using Lowess. Further analyses, including CombatR removal of batch effects, consensus clustering with ConsensusClusterPlus, and principal components analysis (PCA), significance analysis of microarrays (SAM), SigClust, silhouette width, classification to nearest centroids (ClaNC), and single-sample gene set enrichment (ssGSEA) analyses were performed in R. SWITCHdna analyses were conducted in R using the current (GSE49269) and published datasets (GSE22927).18 Array comparative genomic hybridization (aCGH) data were summarized using nonoverlapping 1MB windows across the genome and intersecting them either with probe start coordinates or detected copy number abnormalities (CNA). Neural lineage-specific gene signatures were examined using GSE9566.19 Mouse genes were converted to human orthologs using the Jax MGI database. Human lower-grade astrocytoma signatures were examined using GSE35158.10 TCGA subtypes were predicted using murine orthologs of TCGA GBM ClaNC 840 classifier genes.12,25 High-grade tumor subtypes were validated using 3 similar datasets (GSE22927, GSE35917, and GSE29458).18,20,21 Data from 236 human GBMs (TCGA) with aCGH, sequencing, and mRNA and protein expression data were analyzed using the cBio Cancer Genomics Portal.22 ConsensusClusterPlus, gene set variation analysis, and ClaNC analyses were conducted on the current dataset (GSE49269), orthotopic patient-derived xenograft (PDX) models (GSE38814), and human GBMs (Level 3) from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/).
Met Expression in Cultured T Astrocytes
Cultured T astrocytes were infected with recombinant murine stem cell virus retroviral particles encoding Met (Addgene #17493). Receptor expression and proliferation were analyzed by immunoblot and CellTiter AQ (Promega) proliferation assays as previously described.14,15
Magnetic Resonance Imaging
Pre- and post-gadolinium enhanced T1- and T2-weighted images of TRP+/− mouse brains were acquired at weekly intervals beginning 2 months after induction. Tumor volumes on T2 scans were calculated using MIPAV software.
Results
We previously used non-germline GEM cell culture and orthotopic allograft model systems to show that activating mutations in MAPK (KrasG12D, R) and PI3K (Pten deletion, P) pathways cooperate to induce GBM when the G1/S checkpoint is rendered defective in murine astrocytes via an N-terminal, 121 amino acid mutant SV40 large T antigen (T121, T) transgene expressed from the GFAP promoter.14,15,23 We developed germline GEM models with the same floxed oncogenic alleles to determine the effects of these mutations on adult astrocytes in situ. Mutations were induced via tamoxifen-induced, GFAP-CreER–mediated recombination.12 Lineage tracing showed that recombination occurred specifically in astrocytes and was extremely rare in microglia, oligodendrocyte precursor cells, and neurons.13 We used these GEM models here to examine the influence of driver mutations on gliomagenesis and how tumor genomic profiles evolve during progression.
Core GBM Pathway Mutations Influence Tumorigenesis in Adult Murine Astrocytes
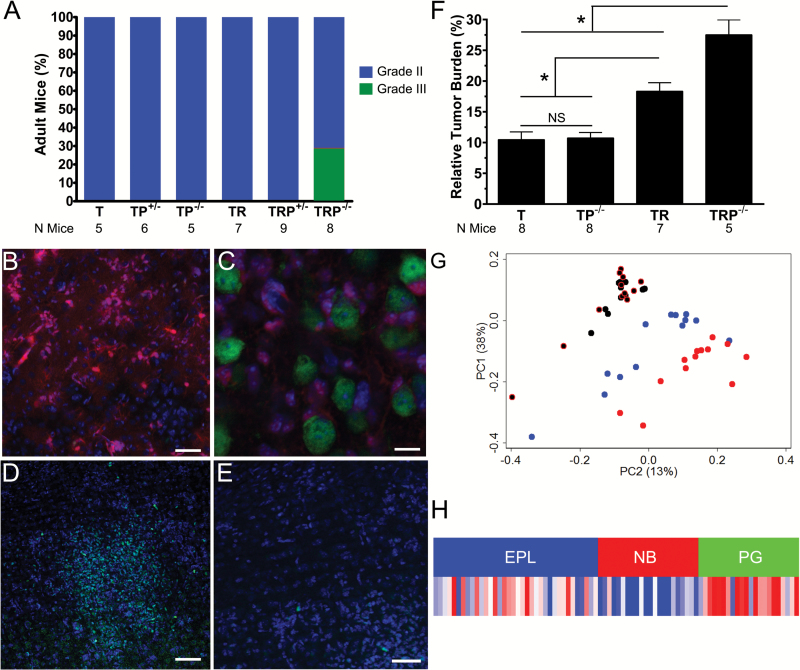
Histopathological evaluation of low-grade tumors at 2 months suggested that mutations in multiple pathways influenced tumor burden (Supplementary Figure S1, Supplementary Table S1).12 Low-grade tumors were evident in all mice containing T, and small tumors with elevated mitotic activity had only developed in a subset of TRP−/− mice at this time (Fig. 1A). We next used tdTomato lineage tracing to further explore whether progression had occurred in TRP+/− mice that had exclusively developed low-grade tumors by 2 months.13 TRP+/− mutations induced increased tdTomato cells (Fig. 1B) and produced classic glioma features, including perineuronal satellitosis (Fig. 1C). Ki-67 staining revealed hypercellular foci with increased proliferation (Fig. 1D) relative to surrounding diffuse tumor (Fig. 1E), suggesting that TRP+/− mutations induce focal progression even at this early time point in tumor evolution.
Fig. 1.
Initiating mutations influence low-grade tumor burden and transcriptomes. All T mice, with and without RP mutations, developed low-grade tumors (blue, grade II). Tumors in a subset (25%) of TRP−/− mice had progressed to high-grade (green, grade III) (A). Lineage tracing in TRP+/− mice showed tdTomato (red) tumor cells (B) that developed perineuronal satellitoses around NeuN+ (green) neurons (C). Hypercellular, hyperproliferative (Ki-67+ [green]) tumor foci were also evident (D) adjacent to areas of less proliferative tumor (E, DAPI [blue]). Morphometric analyses showed that initiating mutations affected tumor burden (one-way ANOVA P < 0.0001; *indicates t-test P < 0.05; NS, not significant) (F). PCA showed that tumor transcriptomes with (red) and without (blue) KrasG12D were distinct, while transcriptomes of histologically normal brain with (black with red outlines) and without (black) KrasG12D were indistinguishable (G). A KrasG12D-related gene signature derived from low-grade tumors was enriched in pre-glioblastoma (PG), but not neuroblastic (NB) or early progenitor-like (EPL) subtypes of human lower-grade astrocytomas (H). Scale bars 50 (B), 10 (C), 100 µm (D), and 50 µm (E).
We next examined whether initiating genotype influenced tumor burden using a newly developed histological and digital image analysis method.16 The use of H&E stained sections was more straightforward and avoided complex breeding and confocal imaging required with genetic lineage tracing. We validated this method by quantifying cellularity of histologically normal brains and controlling for regional variability along the sagittal sectioning axis (Supplementary Figure S2A). We found that the cellularity of mouse brains with R, P, or RP mutations was not significantly different than wild-type C57Bl/6 mice, a quantitative result consistent with their lack of tumor (Supplementary Figure S2BC, Supplementary Table S1). In contrast, TR±P mutations significantly affected tumor burden (Fig. 1F). These results suggest that MAPK and/or PI3K pathway genotype influences tumor development in G1/S-defective adult astrocytes.
KrasG12D Significantly Influences Low-Grade Tumor Transcriptomes
We next examined the transcriptomes of low-grade tumors harvested 2 months after induction. Unsupervised PCA showed separation of normal brain and low-grade tumors. Moreover, low-grade tumors with and without KrasG12D grouped separately (Fig. 1G). Consensus clustering confirmed the effects of KrasG12D status on tumor transcriptomes (Supplementary Figure S3). These findings demonstrate that low-grade tumors have KrasG12D mutation-specific transcriptome signatures.
SAM was conducted to identify genes differentially expressed between low-grade tumors and normal brain (Supplementary Table S2A), as well as low-grade tumors with and without KrasG12D (Supplementary Table S2B).24 Gene ontology (GO) analyses of genes upregulated in tumor versus normal brains showed enrichment of proliferation, cell cycle, and mitosis-related processes (Supplementary Table S3A). In contrast, genes upregulated in tumors with KrasG12D versus those without showed enrichment of immune and cell stress responses (Supplementary Table S3B). To determine whether KrasG12D signature genes (Supplementary Table S3C) were differentially expressed among human gliomas, we assessed their enrichment in lower-grade (non-GBM) astrocytomas.10 The KrasG12D signature was manifest in the “pre-glioblastoma” (PG) subtype (Fig. 1H). These patients had the shortest survival and their tumors consisted of more anaplastic (grade III) than diffuse (grade II) astrocytomas. Moreover, PG gliomas had genomic landscapes similar to GBM, including frequent EGFR amplification and CDKN2A and PTEN deletions.7,10 Taken together, these data suggest that the molecular phenotype of rapidly progressing KrasG12D-driven low-grade murine tumors is similar to aggressive lower-grade human astrocytomas.12
GO analysis showed that low-grade tumors harbored dysregulated G1/S and G2/M cell cycle checkpoints (Supplementary Figure S4, Supplementary Table S3A). We therefore tested for aberrant G1/S signaling by p16 immunofluorescence. Unlike adult, age-matched, wild-type mice that lack p16 expression in astrocytes (Supplementary Figure S4BC), TRP+/− mutations induced p16 expression in transformed astrocytes (Supplementary Figure S4D).25 These findings are consistent with compensatory upregulation of G1/S checkpoint genes in TRP-induced low-grade tumors.
Astrocyte Tumorigenesis and Progression Require Rb Family Ablation and KrasG12D
To determine if mutations within the retinoblastoma (Rb) or MAPK pathways could substitute for one another at tumor initiation, we next compared Rb1 loss with T121 and KrasG12D with Nf1 loss (Supplementary Figure S5). TRP mice were examined in all genotypic combinations and floxed Rb1 and Nf1 were substituted for T and R, respectively. Rb1 deletion, Nf1 deletion, and KrasG12D alone failed to initiate tumorigenesis in astrocytes, in both the presence and the absence of Pten deletion (Supplementary Figure S2BC, Supplementary Table S4AB).18 These results contrast with those of Marumoto et al, showing that activated Hras and Akt mutations transform mouse astrocytes with and without Trp53 deletion.26 Neither KrasG12D nor Nf1 loss induced tumors in Rb1;Pten mice. RP mutations were also insufficient for oligodendrocyte tumorigenesis in PLP-CreER mice (Supplementary Table S4B).
In contrast, all GFAP-CreER mice harboring astrocyte-targeted T±P mutations developed tumors.12 Previous work showed that Kras, but not other Ras isoforms, was activated upon Nf1 deletion and that KrasG12D phenocopied Nf1 deletion in neonatal murine astrocytes in vitro and in vivo.27 However, only KrasG12D, but not Nf1 deletion, increased progression to high-grade tumors (Supplementary Figure S5).
KrasG12D-Driven Tumors Stochastically Progress and Acquire CNA
We next analyzed the genomics of malignant progression in a cohort of mice with all possible TRP genotypes (Supplementary Table S4B). Within each genotype, mice had similar survival (Supplementary Figure S6A), tumor grades (Supplementary Figure S6B), and histopathological features (Supplementary Figure S6C–R) as in our previous report.12 The vast majority of T and TP mice developed low-grade tumors (WHO grade II) but remained asymptomatic 9–18 months after induction. In contrast, TR and TRP mice developed tumors that frequently progressed to lethal high-grade disease.
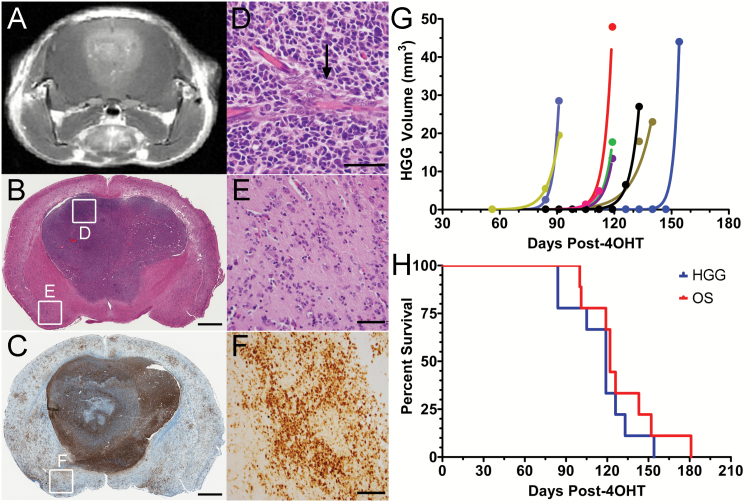
We next monitored progression in TRP+/− mice using serial contrast-enhanced MRI. High-grade tumors were evident on T1 and T2 imaging and enhanced with gadolinium, facilitating use of contrast enhancement (Fig. 2A) as a surrogate for histological progression. At 3–5 months after induction, all mice (N = 9) developed focal, enhancing high-grade tumors (Fig. 2B–D) in addition to low-grade tumors undetected by MRI (Fig. 2E,F). Time from tumor induction to first appearance of high-grade tumors was variable (Fig. 2G), but thereafter, growth was relentless and ultimately proved uniformly fatal (Fig. 2H). These findings suggest that low-grade TRP tumors stochastically progress into rapidly proliferating, lethal high-grade tumors.
Fig. 2.
Low-grade tumors stochastically progress to rapidly proliferative, lethal high-grade tumors. A contrast-enhancing tumor (A) developed focally in a TRP+/− mouse. This high-grade (B), T121-immunoreactive (C) tumor showed histological features of GBM (B), including endothelial proliferation (D). Widespread low-grade (BE), T121-immunoreactive (CF) tumor was evident elsewhere in the brain. Boxes in BC indicate corresponding images in DEF. Quantification of serial T2-weighted MRIs from 9 TRP+/− mice showed logarithmic increases in high-grade tumor volume with mean doubling of 3 ± 1 days (G). Median time to first appearance of high-grade tumors, median survival, and mean time to death after first high-grade tumor appearance were 119 ± 7 (range 84–154), 122 ± 2, and 14 ± 3 days, respectively (H). Scale bars 1 mm (BC) and 50 µm (DEF).
CNA are a hallmark of human glioma.3,4 We hypothesized that similar alterations could drive malignant progression in TR±P mice. Therefore, we performed aCGH on tumors harvested before and after onset of malignant progression. The former was systematically harvested at 2 months. The latter was harvested from neurologically asymptomatic T and TP mice after 9–18 months and TR and TRP mice at neurological morbidity.
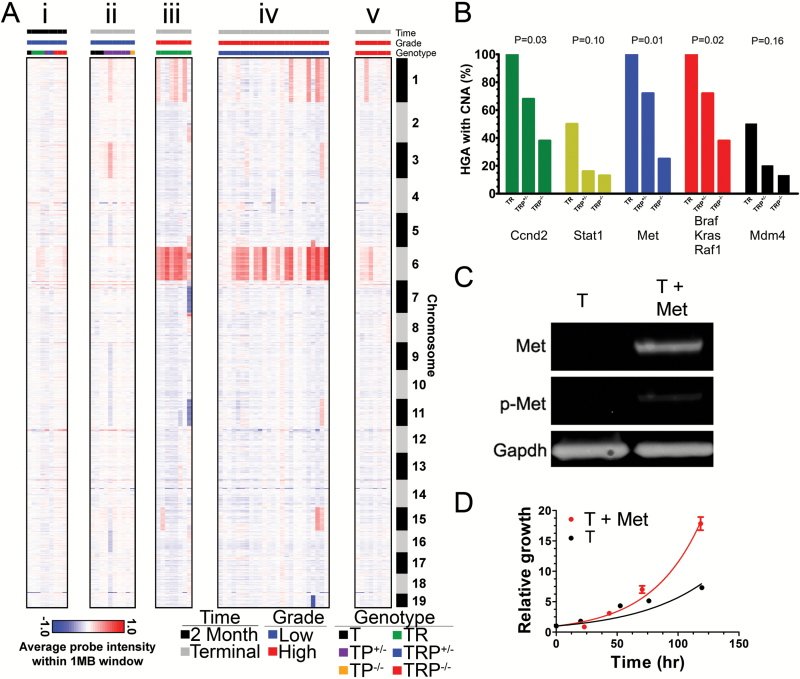
Lower-grade tumors harvested at 2 months possessed minimal and infrequent CNA (Fig. 3Ai). Furthermore, lower-grade tumors from T and TP mice contained few CNA even after 18 months (Fig. 3Aii), suggesting that CNA may be a consequence of KrasG12D-induced malignant progression. This hypothesis was supported by the fact that high-grade tumors from morbid TR (Fig. 3Aiii) and TRP (Fig. 3Aiv–v) mice developed widespread CNA, including frequent gains of chromosomes 1 and 6. Similar CNA were evident in all 3 high-grade tumor genotypes, but CNA were most frequent in TR and least frequent in TRP−/− tumors. Only 3 high-grade tumors (7%) had no CNA and all were from TRP−/− mice with short survival (1.9–2.1 mo). These results suggest that most high-grade TR and TRP tumors acquire CNA during malignant progression.
Fig. 3.
Initiating mutations influence CNA acquisition during malignant progression. Heatmaps of aCGH data showed minimal acquisition of CNA in lower-grade tumors, either at 2 months (Ai) or >1 year (Aii) after induction. All high-grade TR tumors showed gains of chromosome 6 (Aiii), but only 64%–72% of high-grade TRP+/− tumors show similar gains (Aiv, Fisher P = 0.15). Half of TR, but only 16%–20% of high-grade TRP+/− tumors showed chromosome 1 gains (Fisher’s P = 0.17). High-grade TRP−/− tumors acquired the least CNA and had the lowest frequency (13%–25%) of chromosome 6 gains (Av, Fisher P ≤ 0.04). Initiating mutations (B) significantly influenced the development of CNA in recurrently altered (>20% of samples, Supplementary Table S5) Rb, RTK/MAPK/PI3K, and p53 pathway genes (Fisher’s P ≤ 0.16). T astrocytes transfected with lentiviral vectors encoding Met showed increased receptor expression and phosphorylation (p-Met) by immunoblot (C). Met significantly increased T astrocyte proliferation by MTS assay in vitro (D).
Among the core GBM pathways, Ccnd2, Stat1, Met, Braf, Kras, Raf1, and Mdm4 genes were gained in >20% (Fig. 3B, Supplementary Figure S7). Other notable, but less frequent, CNA were gains of Egfr, Erbb2, Pdgfrb, and Pik3ca oncogenes and loss of Pten, Cdkn2a, and Trp53 tumor suppressors (Supplementary Table S5A–E). Chromosomal gains are frequently associated with oncogene overexpression in gliomas, including CMET.28 We therefore tested whether c-Met overexpression would potentiate T astrocyte proliferation in vitro. Increased receptor expression and phosphorylation were noted (Fig. 3C) and increased proliferation was evident (Fig. 3D).
Deletion of p53 at Tumor Initiation Affects the CNA Landscapes of High-Grade Murine Tumors upon Malignant Progression
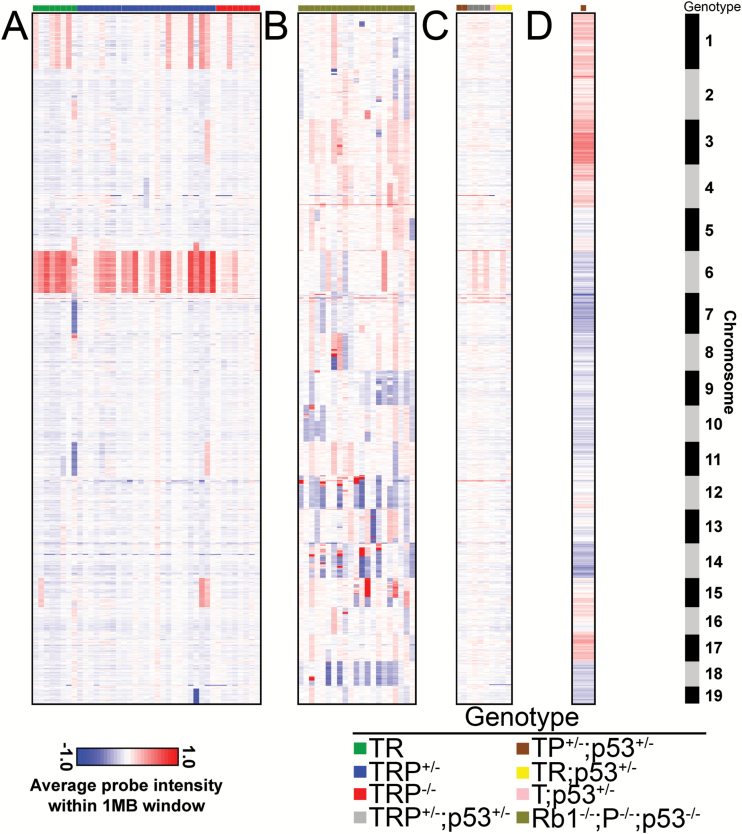
The role of p53 in maintenance of genomic integrity is well established and is frequently mutated in human GBM.3,29 To our knowledge, the only previous report that analyzed CNA landscapes in high-grade tumors utilized a GEM model initiated by knockout (KO) of p53 as well as Rb1/Pten in adult astrocytes.18 Whereas the chromosomal pattern of CNA was restricted in TR±P tumors (Fig. 4A), Rb1/Pten/p53 KO tumors harbored CNA in all autosomes (Fig. 4B). We therefore hypothesized that p53 deletion contributed to the difference between genomic landscapes in these models. To test this hypothesis, we bred a floxed Trp53 allele into T±RP mice. At 2–6 months after induction, all T±RP;p53+/− mice harbored low-grade tumors and 4/14 had progressed to high-grade disease (Supplementary Table S6A). Similar to mice without p53 deletion (Fig. 4A), low-grade T±RP;p53+/− tumors harvested 2 months after induction were largely devoid of CNA (Fig. 4C). However, a T;p53+/− mouse developed GBM with widespread CNA by 11 months after induction (Fig. 4D), similar to Rb1/Pten/p53 triple KO tumors and in contrast to T±P mice, where low-grade tumors failed to progress (Supplementary Figure S6A) and lacked CNA even after 18 months (Fig. 3Aii). These data support the conclusion that heterozygous p53 deletion induces widespread genomic instability as tumors progress to high-grade disease.
Fig. 4.
Copy number landscapes of high-grade tumor models with and without p53 deletion are distinct. Heatmaps of aCGH data from 41 terminal high-grade TR±P tumors showed frequent CNA on chromosomes 1 and 6 (A). In contrast, 21 Rb1/Pten/p53 triple KO high-grade tumors (GSE22927, [5]) showed frequent CNA across the genome (B); aCGH heatmaps of 10 tumors from 7 TR±P mice that also harbored Trp53+/− deletion showed minimal CNA ~2 months after induction (C). However, GBM harvested from a terminally aged, neurologically symptomatic T;Trp53+/− mouse (D) showed a CNA pattern more similar to Rb1/Pten/p53 triple KO (B) than TR±P high-grade tumors (A).
Gene Expression Profiling Identifies 3 High-Grade Tumor Subtypes
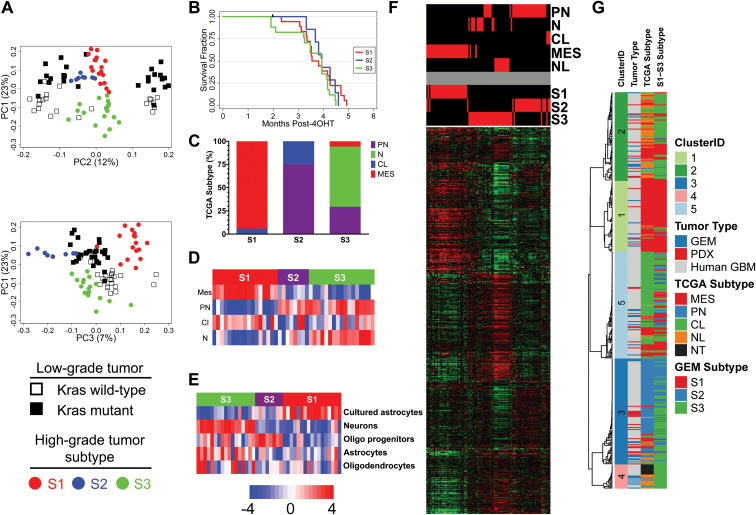
Because high-grade TR±P tumors harbored diverse CNA, we hypothesized that they would also harbor significant intertumor heterogeneity. We therefore compared the expression profiles of 43 terminal high-grade tumors using microarrays (Supplementary Table S6B). Three subtypes (S1–S3) were identified using unsupervised consensus clustering (Supplementary Figure S8A–C). SigClust confirmed that clusters were unique (data not shown) and silhouette width analysis identified 42 samples with profiles most representative of each subtype (Supplementary Figure S8D). We created a 600-gene classifier (Supplementary Table S7) that correctly predicted subtype with 0% cross validation and error rates (Supplementary Figure S8E).
High-grade tumor transcriptomes were distinct from their genotype-matched low-grade counterparts (Fig. 5A). This finding confirms that, despite initiation from identical TR±P mutations, tumor progression is associated with significant transcriptome evolution. Subtype did not correlate with initiating mutations (Supplementary Table S6), a finding consistent with the lack of a Pten deletion-related effect in low-grade tumor transcriptomes. These results suggest that Pten deletion does not significantly contribute to transcriptome heterogeneity, either before or after malignant progression in this model system. Although initiating genotype correlated with survival (Supplementary Figure S6A), subtype did not (Fig. 5B).
Fig. 5.
High-grade murine glioma transcriptomes are heterogeneous. Separation of low-grade tumors with (black boxes) and without mutant KrasG12D (open boxes) and S1 (red), S2 (blue), and S3 (green) high-grade tumor subtypes (circles) were preserved when transcriptome datasets from low- and high-grade TRP tumors were combined (A). In contrast to initiating mutations (Supplementary Figure S6), high-grade glioma subtype did not influence survival (B, log-rank P = 0.4). S1–S3 subtype correlated with human TCGA GBM subtype based on ClaNC (C, Fisher’s P = 2.2 × 10–12) as well as ssGSEA (D); ssGSEA also showed enrichment of distinct neural cell lineage signatures. Cultured astrocyte, oligodendrocyte precursor cell (OPC), and neuron signatures were enriched in S1, S2, and S3 tumors, respectively (E). Hierarchical clustering of an independent test set composed of 3 adult high-grade glioma GEM models with different initiating mutations showed that the 600-gene classifier accurately clustered samples according to their predicted S1–S3 subtypes (F). Consensus hierarchical clustering of high-grade TRP tumors with human patient-derived xenografts (PDX) and GBM (TCGA dataset) using 8916 overlapping genes identified 5 clusters (G). Sample annotation tracks include ClusterID, tumor type—GEM, PDX, GBM, and TCGA subtype for human GBM.
In order to further characterize these subtypes, we examined differentially expressed genes using SAM (Supplementary Table S8A–C) and defined their biological functions using GO analyses (Supplementary Table S9A–C).24 Immune and cytokine response, nuclear factor-kappaB pathway, and extracellular matrix genes were significantly expressed in S1 tumors, suggesting that this subtype was similar to human mesenchymal GBM.5,8 We therefore classified the human GBM subtype of individual murine high-grade tumors using the 840-gene TCGA classifier. Nearly all high-grade S1 tumors (94%) were classified as mesenchymal GBM (Fig. 5C). S1 tumors were also enriched in mesenchymal GBM (Fig. 5D) and cultured murine astrocyte signatures (Fig. 5E) by ssGSEA.5,19
Cell cycle, proliferation, and metabolism genes were significantly expressed in S2 tumors. The majority (75%) were classified as proneural GBM (Fig. 5C). S2 tumors also expressed proneural GBM (Fig. 5D) and proliferation and murine oligodendrocyte precursor (Fig. 5E) signatures.5,19,30
Genes highly expressed in S3 tumors were enriched in synaptic transmission and other neuronal processes. TCGA classifier predicted 65%, 29%, and 6% of S3 tumors as human neural, proneural, and mesenchymal GBM, respectively, and all were enriched in a murine neuronal signature (Fig. 5C–E).19 These results imply that the transcriptomes of S3 tumors are heterogeneous, but are most similar to human neural GBM.
S1–S3 Subtypes Are Present in High-Grade Gliomas from Other Adult GEM Models and Patient-Derived Xenografts
S1–S3 subtypes were validated in an independent test set of transcriptome data from 3 adult high-grade GEM tumor models driven by different initiating mutations (Supplementary Table S10). These include PDGF;Pten±Trp53,21Trp53 plus HrasV12,20 as well as Rb1;Pten;Trp53.18 The 600-gene classifier showed similar expression in both the discovery and validation sets (data not shown). Furthermore, validation samples clustered by both predicted TCGA GBM and mouse S1–S3 subtypes (Fig. 5F). Similar to the results with TR±P tumors, S1, S2, and S3 tumors in the validation dataset were primarily predicted as human mesenchymal, proneural, and neural, respectively (Supplementary Table S10). These results support the notion that high-grade tumors converge on similar transcription profiles despite being initiated by diverse oncogenic mutations.
Consensus hierarchical clustering of high-grade TR±P tumors with human PDX and TCGA GBM confirmed the heterogeneity of GEM tumors and identified closely matching human PDX and GBM counterparts in all 4 TCGA subtypes (Fig. 5G, Supplementary Figure S9, Supplementary Table S11). This validation suggests that expression profiles are conserved in murine and human high-grade gliomas.
High-Grade Murine Tumors Show Evidence of Divergent Evolution upon Malignant Progression
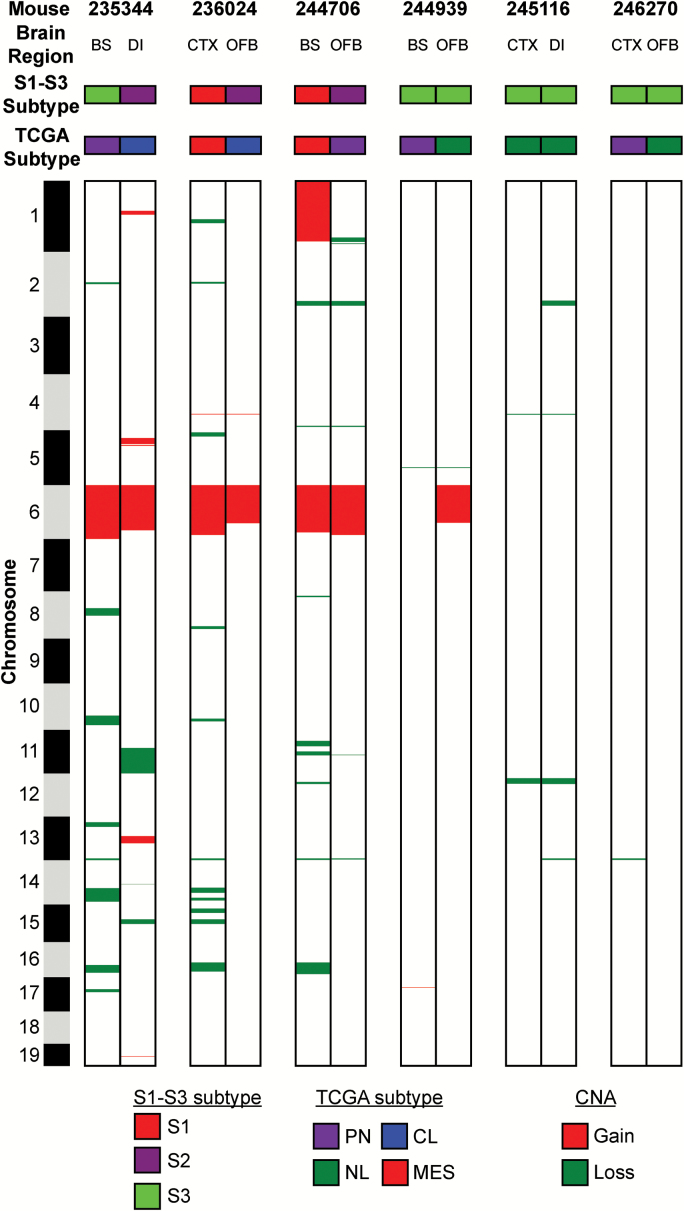
Seven TR±P mice developed 2 distinct high-grade tumors in different regions of the brain (Supplementary Table S6). To determine whether these tumors evolved separately, we analyzed their transcriptome and CNA profiles. Four had different S1–S3 and 5 had different TCGA transcriptome subtypes (Fig. 6). Of the 6 pairs analyzed by aCGH, none contained identical genomic copy number landscapes (Fig. 6, Supplementary Table S12). Together, these data suggest that high-grade tumors underwent divergent evolution during the course of malignant progression and further that CNA significantly contributed to their transcriptome heterogeneity.
Fig. 6.
High-grade murine tumors pair show evidence of divergent genomic evolution. Two tumors harvested from distinct brain regions from 6 different mice showed different S1–S3 transcriptome subtypes, TCGA transcriptome subtypes, and CNA.
Discussion
Human gliomas are molecularly heterogeneous tumors. Comprehensive molecular analyses have indicated that mutations in multiple genes and biological pathways are likely required for tumorigenesis.3–5,7,31 Driver mutations have thus been proposed as a major source of cancer heterogeneity.32 These findings are consistent with the results from a variety of GEM models, each developed with distinct methodologies.11 Indeed, we have previously developed a series of germline GEM models where driver mutations that dysregulate the Rb, MAPK, and/or PI3K pathways were targeted to adult murine astrocytes.12–15,23 This model system provided a unique opportunity to investigate evolution of tumor heterogeneity during malignant progression.
We found that initiating mutations influence tumor initiation and LGG burden, as well as progression to lethal high-grade gliomas. Indeed, simultaneous activation of MAPK (KrasG12D, not Nf1 loss) and PI3K (Pten loss) in G1/S-defective astrocytes (T121, not Rb1 loss) potentiated tumor initiation and produced increasingly dense LGG that quickly progressed to GBM.13 High-grade tumors showed contrast enhancement on MRI, and their growth was rapid and lethal. We have previously shown that TRP tumors spontaneously acquire Pten loss of heterozygosity and Trp53 missense mutations during progression.12 We extend these findings here and show that they also acquire CNA, the frequency of which correlated with initiating genotype. Finally, we find that the transcriptomes of TR±P-induced tumors evolve during progression from low- to high-grade disease.
In addition to affecting tumorigenesis, initiating mutations—KrasG12D but not Pten deletion—were also reflected in LGG expression profiles. Distinct transcriptome profiles were evident in low-grade, Kras mutant tumors that were destined to progress to GBM versus tumors that did not progress. The former tumors also had transcriptome signatures similar to the isocitrate dehydrogenase (IDH) wild-type, PG subtype of lower-grade human gliomas that feature activating RTK mutations (eg, EGFR) and poor prognosis.6,7,9,10 These findings are consistent with recent genetic studies of human gliomas, suggesting that activating RTK and MAPK mutations drive malignant progression, while PI3K pathway mutations are a consequence of this process.33 One notable difference between TRP tumors and human LGG is the extent of genomic instability.10 Whereas the former have relatively quiescent genomes, regardless of initiating genotype or tumor latency, human IDH wild-type lower-grade gliomas have more widespread CNA, similar to GBM.7,9,10 This difference may be attributable to species differences or the relative paucity of anaplastic, grade III gliomas present in our GEM tumors harvested 2 months after induction.
High-grade tumor transcriptomes were distinct from their genotype-matched low-grade counterparts. This finding confirms that, despite tumor initiation from identical mutations, progression is associated with significant transcriptome evolution. These results are consistent with the transcriptome differences between non-genotype-matched human low- and high-grade tumors and suggest that secondary mutations acquired during malignant progression significantly influence glioma transcriptomes.34–36 This notion contrasts with a recent description of a proneural GEM that maintained similar expression at early and terminal time points.37
The effects of Pten gene dosage at tumor initiation had a clear effect on tumor aggressiveness, as TRP−/− mice had the shortest survival and most frequently developed GBM. However, high-grade TRP−/− tumors had fewer CNA than their TR and TRP+/− counterparts. These results are consistent with the notion that homozygous deletion of Pten at tumor initiation, together with T and R mutations, induced such rapid tumor growth that CNA were not selected for during their malignant evolution.
The genomic architecture of intratumoral heterogeneity in human GBM has been reconstructed by phylogenic analysis of deep sequencing data.33 Such studies have identified the likely chronology of mutations that develop as individual tumors evolve. However, computational reconstruction lacks the ability to determine whether early mutations are mechanistically linked to the mutations that arise late in the course of malignant progression. We provide evidence here that initiating (early) and progression (late) mutations are linked in KrasG12D, but not Trp53 deletion-driven tumors of astrocyte origin. Indeed, the vast majority of KrasG12D-driven tumors consistently developed progression-related CNA highlighted by chromosome 6 gains. Mouse chromosome 6 is largely syntenic with human chromosome 7, a frequent site of CNA in human GBM.3 The concept that initiating and progression mutations may be linked thus provides a mechanistic basis for the patterns of mutation co-occurrence observed in human GBM.3,38 While a mechanistic linkage remains unclear, identification of co-occurring mutations has already improved the molecular classification of IDH wild-type LGG, particularly the ATRX/TP53-mutant astrocytomas that develop CNA on chromosomes 7 and 10 and evolve to GBM.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
C.R.M. was a Damon Runyon-Genentech Clinical Investigator supported by grants from the Damon Runyon Cancer Research Foundation (CI-45-09), Department of Defense (W81XWH-09-2-0042), and UNC University Cancer Research Fund (UCRF). D.M.I. and R.S.M. are in the UNC Graduate Training Program in Translational Medicine supported by the Howard Hughes Medical Institute. D.M.I. was supported by NIH T32GM007092 and T32CA071341. R.S.M. is a Wagner Scholar in the UNC Pathobiology and Translational Science Graduate Program. H.D.D., S.K., and M.E.B. are supported by U01CA168397 and the Ben and Catherine Ivy Foundation. Translational Pathology Laboratory (TPL) and Small Animal Imaging Facility are supported by P30CA016086 and UCRF. TPL is supported by P30ES010126 and W81XWH-09-2-0042. The UNC Neuroscience Center is supported by P30NS045892.
Conflict of interest statement. The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
We thank Daniel Roth for mouse husbandry, Debbie Little for histology, and Hong Yuan for mouse imaging assistance. We thank Pablo Tamayo (Broad Institute) for ssGSEA R scripts and Suzanne Baker and Chunxu Qu (St Jude) for helpful discussions.
References
- 1. Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131(3):397–406. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 10. Gorovets D, Kannan K, Shen R, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18(9):2490–2501. [DOI] [PubMed] [Google Scholar]
- 11. Schmid RS, Vitucci M, Miller CR. Genetically engineered mouse models of diffuse gliomas. Brain Res Bull. 2012;88(1):72–79. [DOI] [PubMed] [Google Scholar]
- 12. Song Y, Zhang Q, Kutlu B, et al. Evolutionary etiology of high-grade astrocytomas. Proc Natl Acad Sci U S A. 2013;110(44):17933–17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irvin DM, McNeill RS, Bash RE, Miller CR. Intrinsic astrocyte heterogeneity influences tumor growth in glioma mouse models. Brain Pathol. 2017;27(1):36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmid RS, Simon JM, Vitucci M, et al. Core pathway mutations induce de-differentiation of murine astrocytes into glioblastoma stem cells that are sensitive to radiation but resistant to temozolomide. Neuro Oncol. 2016;18(7):962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitucci M, Karpinich NO, Bash RE, et al. Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis. Neuro Oncol. 2013;15(10):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akella NS, Ding Q, Menegazzo I, et al. A novel technique to quantify glioma tumor invasion using serial microscopy sections. J Neurosci Methods. 2006;153(2):183–189. [DOI] [PubMed] [Google Scholar]
- 17. Dong HW. Allen reference atlas: a digital color brain atlas of the C57Black/6J male mouse. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 18. Chow LM, Endersby R, Zhu X, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19(3):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedmann-Morvinski D, Bushong EA, Ke E, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338(6110):1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lei L, Sonabend AM, Guarnieri P, et al. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011;6(5):e20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNeill RS, Schmid RS, Bash RE, et al. Modeling astrocytoma pathogenesis in vitro and in vivo using cortical astrocytes or neural stem cells from conditional, genetically engineered mice. J Vis Exp. 2014;(90):e51763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98(9):5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marumoto T, Tashiro A, Friedmann-Morvinski D, et al. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15(1):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dasgupta B, Li W, Perry A, Gutmann DH. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 2005;65(1):236–245. [PubMed] [Google Scholar]
- 28. Burel-Vandenbos F, Ngo-Mai M, Dadone B, et al. MET immunolabeling is a useful predictive tool for MET gene amplification in glioblastoma. Neuropathol Appl Neurobiol. 2016; doi:10.1111/nan.12320. [DOI] [PubMed] [Google Scholar]
- 29. Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–228. [DOI] [PubMed] [Google Scholar]
- 30. Whitfield ML, Sherlock G, Saldanha AJ, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13(6):1977–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noushmehr H, Weisenberger DJ, Diefes K, et al. ; Cancer Genome Atlas Research Network. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–322. [DOI] [PubMed] [Google Scholar]
- 33. Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shai R, Shi T, Kremen TJ, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22(31):4918–4923. [DOI] [PubMed] [Google Scholar]
- 35. van den Boom J, Wolter M, Kuick R, et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. Am J Pathol. 2003;163(3):1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Godard S, Getz G, Delorenzi M, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63(20):6613–6625. [PubMed] [Google Scholar]
- 37. Sonabend AM, Bansal M, Guarnieri P, et al. The transcriptional regulatory network of proneural glioma determines the genetic alterations selected during tumor progression. Cancer Res. 2014;74(5):1440–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ciriello G, Cerami E, Sander C, Schultz N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 2012;22(2):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.