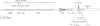Abstract
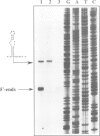
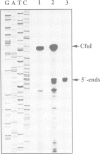
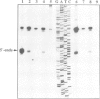
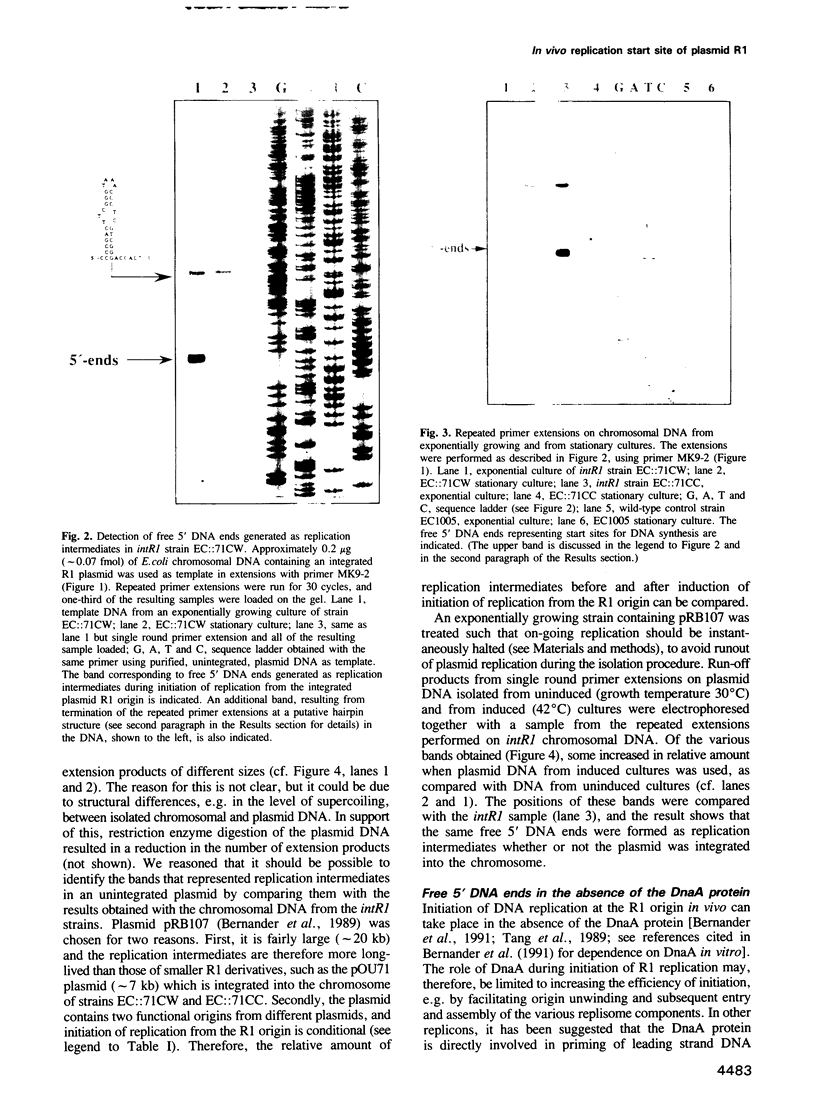
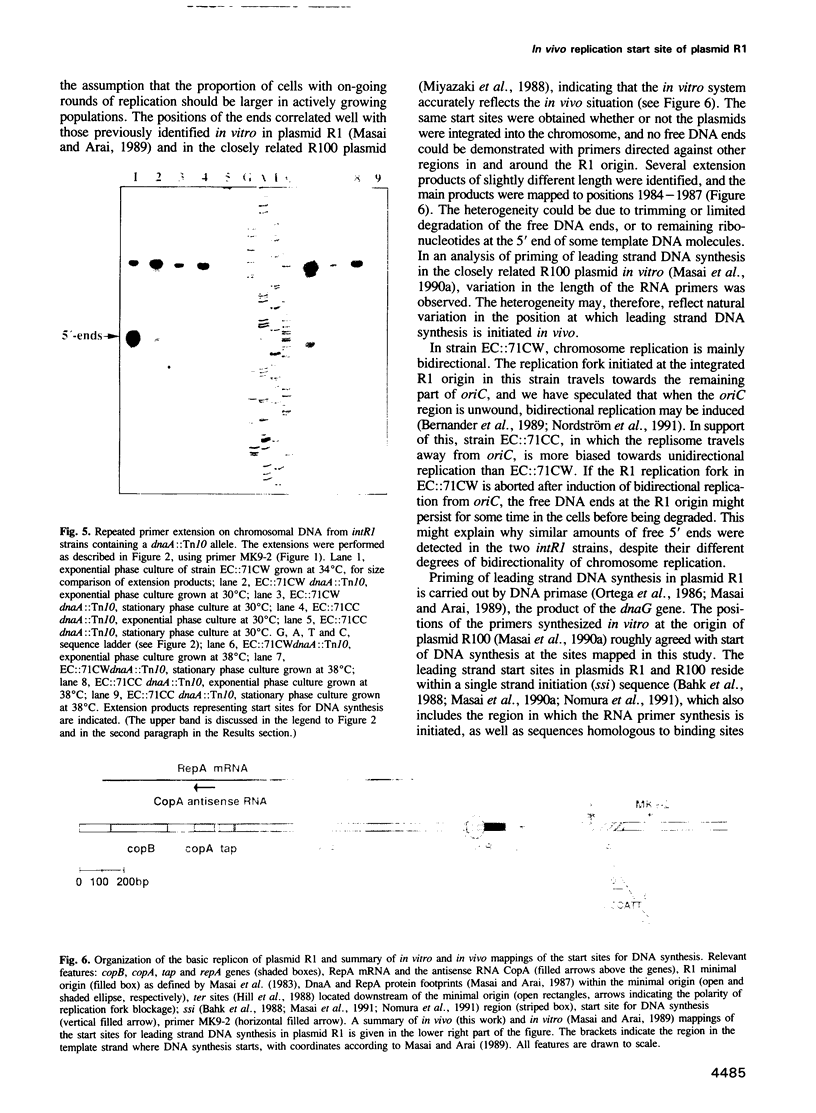
We have previously constructed Escherichia coli strains in which an R1 plasmid is integrated into the origin of chromosome replication, oriC. In such intR1 strains, oriC is inactive and initiation of chromosome replication instead takes place at the integrated R1 origin. Due to the large size of the chromosome, replication intermediates generated at the R1 origin in these strains are considerably more long-lived than those in unintegrated R1 plasmids. We have taken advantage of this and performed primer extensions on total DNA isolated from intR1 strains, and mapped the free 5' DNA ends that were generated as replication intermediates during R1 replication in vivo. The sensitivity of the mapping was considerably improved by the use of a repeated primer extension method (RPE). The free DNA ends were assumed to represent normal in vivo start sites for leading strand DNA synthesis in plasmid R1. The ends were mapped to a short region approximately 380 bp away from the R1 minimal origin, and the positions agreed well with previous in vitro mappings. The same start positions were also utilized in the absence of the DnaA protein, indicating that DnaA is not required for determination of the position at which DNA synthesis starts during initiation of replication at the R1 origin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahk J. D., Kioka N., Sakai H., Komano T. A runaway-replication plasmid pSY343 contains two ssi signals. Plasmid. 1988 Nov;20(3):266–270. doi: 10.1016/0147-619x(88)90033-9. [DOI] [PubMed] [Google Scholar]
- Bernander R., Dasgupta S., Nordström K. The E. coli cell cycle and the plasmid R1 replication cycle in the absence of the DnaA protein. Cell. 1991 Mar 22;64(6):1145–1153. doi: 10.1016/0092-8674(91)90269-5. [DOI] [PubMed] [Google Scholar]
- Bernander R., Merryweather A., Nordström K. Overinitiation of replication of the Escherichia coli chromosome from an integrated runaway-replication derivative of plasmid R1. J Bacteriol. 1989 Feb;171(2):674–683. doi: 10.1128/jb.171.2.674-683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg P., Nordström K., Wagner E. G. Replication control of plasmid R1: RepA synthesis is regulated by CopA RNA through inhibition of leader peptide translation. EMBO J. 1992 Jul;11(7):2675–2683. doi: 10.1002/j.1460-2075.1992.tb05333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Bruand C., Ehrlich S. D., Jannière L. Unidirectional theta replication of the structurally stable Enterococcus faecalis plasmid pAM beta 1. EMBO J. 1991 Aug;10(8):2171–2177. doi: 10.1002/j.1460-2075.1991.tb07752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Staudenbauer W. L. Origin and direction of mini-R1 plasmid DNA replication in cell extracts of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1077–1084. doi: 10.1128/jb.150.3.1077-1084.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. The E. coli dnaA initiation protein: a protein for all seasons. Trends Genet. 1989 Oct;5(10):319–321. doi: 10.1016/0168-9525(89)90118-2. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Pelletier A. J., Tecklenburg M. L., Kuempel P. L. Identification of the DNA sequence from the E. coli terminus region that halts replication forks. Cell. 1988 Nov 4;55(3):459–466. doi: 10.1016/0092-8674(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Tohdoh N., Jiang X. W., Okazaki T. The distribution and properties of RNA primed initiation sites of DNA synthesis at the replication origin of Escherichia coli chromosome. Nucleic Acids Res. 1985 Oct 11;13(19):6847–6866. doi: 10.1093/nar/13.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H., Kornberg A. Replication deficiencies in priA mutants of Escherichia coli lacking the primosomal replication n' protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3029–3032. doi: 10.1073/pnas.88.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Arai K. Leading strand synthesis of R1 plasmid replication in vitro is primed by primase alone at a specific site downstream of oriR. J Biol Chem. 1989 May 15;264(14):8082–8090. [PubMed] [Google Scholar]
- Masai H., Arai K. RepA and DnaA proteins are required for initiation of R1 plasmid replication in vitro and interact with the oriR sequence. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4781–4785. doi: 10.1073/pnas.84.14.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Arai K. RepA protein- and oriR-dependent initiation of R1 plasmid replication: identification of a rho-dependent transcription terminator required for cis-action of repA protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6493–6514. doi: 10.1093/nar/16.14.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Kaziro Y., Arai K. Definition of oriR, the minimum DNA segment essential for initiation of R1 plasmid replication in vitro. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6814–6818. doi: 10.1073/pnas.80.22.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Nomura N., Arai K. The ABC-primosome. A novel priming system employing dnaA, dnaB, dnaC, and primase on a hairpin containing a dnaA box sequence. J Biol Chem. 1990 Sep 5;265(25):15134–15144. [PubMed] [Google Scholar]
- Masai H., Nomura N., Kubota Y., Arai K. Roles of phi X174 type primosome- and G4 type primase-dependent primings in initiation of lagging and leading strand syntheses of DNA replication. J Biol Chem. 1990 Sep 5;265(25):15124–15133. [PubMed] [Google Scholar]
- Miyazaki C., Kawai Y., Ohtsubo H., Ohtsubo E. Unidirectional replication of plasmid R100. J Mol Biol. 1988 Nov 20;204(2):331–343. doi: 10.1016/0022-2836(88)90580-3. [DOI] [PubMed] [Google Scholar]
- Nomura N., Masai H., Inuzuka M., Miyazaki C., Ohtsubo E., Itoh T., Sasamoto S., Matsui M., Ishizaki R., Arai K. Identification of eleven single-strand initiation sequences (ssi) for priming of DNA replication in the F, R6K, R100 and ColE2 plasmids. Gene. 1991 Dec 1;108(1):15–22. doi: 10.1016/0378-1119(91)90482-q. [DOI] [PubMed] [Google Scholar]
- Nordström K., Bernander R., Dasgupta S. Analysis of the bacterial cell cycle using strains in which chromosome replication is controlled by plasmid R1. Res Microbiol. 1991 Feb-Apr;142(2-3):181–188. doi: 10.1016/0923-2508(91)90028-9. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Light J. Control of replication of bacterial plasmids: genetics, molecular biology, and physiology of the plasmid R1 system. Plasmid. 1984 Sep;12(2):71–90. doi: 10.1016/0147-619x(84)90054-4. [DOI] [PubMed] [Google Scholar]
- Ortega S., Lanka E., Diaz R. The involvement of host replication proteins and of specific origin sequences in the in vitro replication of miniplasmid R1 DNA. Nucleic Acids Res. 1986 Jun 25;14(12):4865–4879. doi: 10.1093/nar/14.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Wagner E. G., Nordström K. Control of replication of plasmid R1: kinetics of in vitro interaction between the antisense RNA, CopA, and its target, CopT. EMBO J. 1988 Oct;7(10):3279–3288. doi: 10.1002/j.1460-2075.1988.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., Messer W. DnaA protein binding to the plasmid origin region can substitute for primosome assembly during replication of pBR322 in vitro. Cell. 1987 Jan 16;48(1):73–78. doi: 10.1016/0092-8674(87)90357-6. [DOI] [PubMed] [Google Scholar]
- Tang X. B., Womble D. D., Rownd R. H. DnaA protein is not essential for replication of IncFII plasmid NR1. J Bacteriol. 1989 Oct;171(10):5290–5295. doi: 10.1128/jb.171.10.5290-5295.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Sampathkumar P., Easton A. M., Luckow V. A., Rownd R. H. Transcription of the replication control region of the IncFII R-plasmid NR1 in vitro and in vivo. J Mol Biol. 1985 Feb 5;181(3):395–410. doi: 10.1016/0022-2836(85)90228-1. [DOI] [PubMed] [Google Scholar]