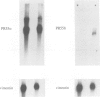
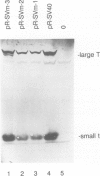
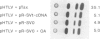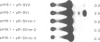Abstract
Previous results indicated that SV40 small t is essential for SV40-induced transformation of diploid cells but dispensable for the transformation of cells with a deletion on the short arm of chromosome 11 (del-11 cells). From these results we concluded that del-11 cells contain a cellular 'SV40 small t-like' factor, which is able to transactivate the HPV16 long control region (LCR) and to complement SV40 large T in transformation. Since SV40 small t and the regulatory 55 kDa subunit (PR55) of protein phosphatase 2A (PP2A), have been shown to inhibit the enzyme activity of PP2A, the PR55 beta subunit could be the putative 'small t-like' factor. In accordance with this hypothesis, we show that the PR55 beta subunit is highly expressed in del-11 but not in diploid cells and is able to trans-activate the HPV16 LCR in diploid cells. Moreover, inhibition of PP2A by okadaic acid resulted in trans-activation of the HPV16 LCR in diploid cells. Alignment of PR55 and SV40 small t showed a common four amino acid motif DKGG. We present evidence that the integrity of this motif is necessary for the PP2A-mediated ability of SV40 small t to trans-activate the HPV16 LCR.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Baker M. C. Deficiency of all or part of chromosome 11 in several types of cancer: significance of a reduction in the number of normal chromosomes 11. Cytogenet Cell Genet. 1988;47(1-2):106–107. doi: 10.1159/000132521. [DOI] [PubMed] [Google Scholar]
- Atkin N. B., Baker M. C. Nonrandom chromosome changes in carcinoma of the cervix uteri. I. Nine near-diploid tumors. Cancer Genet Cytogenet. 1982 Nov;7(3):209–222. doi: 10.1016/0165-4608(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Drews R. E., Chan V. T., Schnipper L. E. Oncogenes result in genomic alterations that activate a transcriptionally silent, dominantly selectable reporter gene (neo). Mol Cell Biol. 1992 Jan;12(1):198–206. doi: 10.1128/mcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hurlin P. J., Kaur P., Smith P. P., Perez-Reyes N., Blanton R. A., McDougall J. K. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):570–574. doi: 10.1073/pnas.88.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B., Rundell K. Association of simian virus 40 small-t antigen with the 61-kilodalton component of a cellular protein complex. J Virol. 1990 Nov;64(11):5649–5651. doi: 10.1128/jvi.64.11.5649-5651.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi M., Morita H., Yamada H., Satoh H., Barrett J. C., Oshimura M. Normal human chromosome 11 suppresses tumorigenicity of human cervical tumor cell line SiHa. Mol Carcinog. 1989;2(1):12–21. doi: 10.1002/mc.2940020103. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Mayer R. E., Hendrix P., Cron P., Matthies R., Stone S. R., Goris J., Merlevede W., Hofsteenge J., Hemmings B. A. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991 Apr 16;30(15):3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Weller W., Jaspers S., Miller T. B., Lane W. S., Roberts T. M. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J Virol. 1992 Feb;66(2):886–893. doi: 10.1128/jvi.66.2.886-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro G., Lee M., Morgan D., Defendi V. Evolution of in vitro transformation and tumorigenesis of HPV16 and HPV18 immortalized primary cervical epithelial cells. Am J Pathol. 1991 Jan;138(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Phillips B., Rundell K. Failure of simian virus 40 small t antigen to disorganize actin cables in nonpermissive cell lines. J Virol. 1988 Mar;62(3):768–775. doi: 10.1128/jvi.62.3.768-775.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonovich M., Jeang K. T. Activation of the human T-cell leukemia virus type I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate and by tax (p40x) occurs through similar but functionally distinct target sequences. J Virol. 1989 Jul;63(7):2987–2994. doi: 10.1128/jvi.63.7.2987-2994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Goh W. C., Dayton A. I., Lippke J., Haseltine W. A. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986 Feb 13;319(6054):555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Rundell K. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J Virol. 1987 Apr;61(4):1240–1243. doi: 10.1128/jvi.61.4.1240-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Major E. O., Lampert M. Association of cellular 56,000- and 32,000-molecular-weight protein with BK virus and polyoma virus t-antigens. J Virol. 1981 Mar;37(3):1090–1093. doi: 10.1128/jvi.37.3.1090-1093.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösl F., Dürst M., zur Hausen H. Selective suppression of human papillomavirus transcription in non-tumorigenic cells by 5-azacytidine. EMBO J. 1988 May;7(5):1321–1328. doi: 10.1002/j.1460-2075.1988.tb02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Stanbridge E. J. Introduction of human chromosome 11 via microcell transfer controls tumorigenic expression of HeLa cells. EMBO J. 1986 Dec 20;5(13):3461–3466. doi: 10.1002/j.1460-2075.1986.tb04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Tsukitani Y., Feramisco J. R. Transcriptional and post-transcriptional regulation of c-fos expression by the tumor promoter okadaic acid. Oncogene. 1991 Mar;6(3):423–430. [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H. L., Raadsheer E., Rood I., Mehendale S., Slater R. M., van der Noordaa J., ter Schegget J. Induction of anchorage-independent growth of human embryonic fibroblasts with a deletion in the short arm of chromosome 11 by human papillomavirus type 16 DNA. J Virol. 1988 Dec;62(12):4538–4543. doi: 10.1128/jvi.62.12.4538-4543.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P. H., Smits H. L., Jebbink M. F., ter Schegget J. The short arm of chromosome 11 likely is involved in the regulation of the human papillomavirus type 16 early enhancer-promoter and in the suppression of the transforming activity of the viral DNA. Virology. 1990 May;176(1):158–165. doi: 10.1016/0042-6822(90)90240-r. [DOI] [PubMed] [Google Scholar]
- Smits P. H., de Ronde A., Smits H. L., Minnaar R. P., van der Noordaa J., ter Schegget J. Modulation of the human papillomavirus type 16 induced transformation and transcription by deletion of loci on the short arm of human chromosome 11 can be mimicked by SV40 small t. Virology. 1992 Sep;190(1):40–44. doi: 10.1016/0042-6822(92)91190-6. [DOI] [PubMed] [Google Scholar]
- Srivatsan E. S., Benedict W. F., Stanbridge E. J. Implication of chromosome 11 in the suppression of neoplastic expression in human cell hybrids. Cancer Res. 1986 Dec;46(12 Pt 1):6174–6179. [PubMed] [Google Scholar]
- Vousden K. H. Human papillomaviruses and cervical carcinoma. Cancer Cells. 1989 Oct;1(2):43–50. [PubMed] [Google Scholar]
- Walter G., Carbone-Wiley A., Joshi B., Rundell K. Homologous cellular proteins associated with simian virus 40 small T antigen and polyomavirus medium T antigen. J Virol. 1988 Dec;62(12):4760–4762. doi: 10.1128/jvi.62.12.4760-4762.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J. J., Goudsmit J., Keulen W., Klaver B., Krone W., Tersmette M., de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992 Feb;66(2):757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ronde A., Sol C. J., van Strien A., ter Schegget J., van der Noordaa J. The SV40 small t antigen is essential for the morphological transformation of human fibroblasts. Virology. 1989 Jul;171(1):260–263. doi: 10.1016/0042-6822(89)90534-5. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M., Wagner D., Schneider A., Wesch H., Miklaw H., Wahrendorf J., Papendick U., zur Hausen H. Human papillomavirus infections in women with and without abnormal cervical cytology. Lancet. 1987 Sep 26;2(8561):703–706. doi: 10.1016/s0140-6736(87)91072-5. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Intracellular surveillance of persisting viral infections. Human genital cancer results from deficient cellular control of papillomavirus gene expression. Lancet. 1986 Aug 30;2(8505):489–491. doi: 10.1016/s0140-6736(86)90360-0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]