Abstract
Acute-on-chronic liver injury is characterized by an important inflammatory response frequently associated with endotoxemia. In this context, acute phase proteins such as Pentraxin-3 (PTX3) are released, however, little is known about their role in chronic liver disease. The aim of this study was to elucidate the role of PTX3 in liver injury. Role of PTX3 was evaluated in cultured human cells, liver tissue slices and mice with acute-on-chronic liver injury. PTX3 expression was assessed in tissue and serum samples from 54 patients with alcoholic hepatitis (AH). PTX3 expression was up-regulated in animal models of liver injury and strongly induced by lipopolysaccharide (LPS). Liver cell fractionation showed that macrophages and activated hepatic stellate cells (HSC) were the main cell types expressing PTX3 in liver injury. Ex vivo, and in vivo studies showed that PTX3 treatment attenuated LPS-induced liver injury, inflammation and cell recruitment. Mechanistically, PTX3 mediated HSC wound-healing response. Moreover, PTX3 modulated LPS-induced inflammation in human primary liver macrophages and peripheral monocytes by enhancing a TRIF-dependent response and favoring a macrophage IL-10-like phenotype. Additionally, hepatic and plasma PTX3 levels were up-regulated in patients with AH, a prototypic acute-on-chronic condition and its expression correlated with disease severity scores, endotoxemia, infections and short-term mortality. Thus, suggesting that expression of PTX3 found in patients could be a counterregulatory response to injury.
Conclusion
Experimental and human evidences suggest that in addition to being a potential biomarker for AH, PTX3 participates in wound-healing response and attenuates LPS-induced liver injury and inflammation. Therefore, administration of PTX3 could be a promising therapeutic strategy in acute-on-chronic conditions, particularly those associated with endotoxemia.
Keywords: pentraxins, inflammation, alcoholic hepatitis, lipopolysaccharide
Background
Chronic liver diseases are characterized by a persistent inflammatory response as well as a progressive wound-healing process that usually lead to fibrosis (1). Advanced liver diseases are associated with increased gut permeability leading to increased endotoxemia and lipopolysaccharide (LPS) levels in serum (2–4). Moreover, patients with advanced diseases are prone to developing acute events of inflammation and infections (5), which are the main cause of the development of acute-on-chronic liver failure (ACLF) (6,7). One of the triggers of ACLF is Alcoholic Hepatitis (AH), which is characterized by and important inflammatory response, hepatocellular injury, liver failure and is frequently associated with increased endotoxemia.
By binding to LPS, toll-like receptor (TLR) 4 is one of the main receptor driving inflammatory response (8,9). TLR4 response to LPS is dependent on accessory proteins, among them MD2 and CD14 (10). TLR4 downstream signaling and cytokine production is mainly governed by myeloid differentiation primary response 88 (MyD88) (11), with the early activation of NF-κB and production of pro-inflammatory cytokines (12), and TIR-domain-containing adapter-inducing interferon (TRIF), essential for late activation of NF-κB , IRF-3 and production of IFN-inducible genes (9,12,13). Activation of TLR4 has been shown to enhance cytokine production, macrophage polarization (14), inflammatory cell recruitment and hepatic stellate cell (HSC) activation (15).
Pentraxin-3 (PTX3) is a member of the humoral arm of innate immunity and is involved in the modulation of inflammation and tissue remodeling (16). Moreover, PTX3 can also act in response to pathogens by acting as a soluble pattern-recognition receptor (16) or binding to pathogens (16–19). PTX3 is the long member of the pentraxin family together with C-reactive protein and the serum amyloid P component (20,21). Short components of this family are mainly produced by hepatocytes in response to inflammation and act as acute phase response proteins. Conversely, PTX3 is broadly expressed and can be produced in different organs after injury, infection or inflammation mainly by mesenchymal and inflammatory cells (20,21).
PTX3 exerts its pleiotropic functions through interaction with a variety of soluble ligands; however, although PTX3 can interact with Fc receptors, no specific receptors have been identified. (22)(19). PTX3 was shown to be protective in models of tissue injury mediated by infection, increased LPS as well as in sterile inflammation (23–28). Recently, the role of PTX3 in tissue repair and remodeling was reported. (29). PTX3-deficiency was found to be associated with dysfunctional tissue repair and with increased collagen deposition and scar formation in liver and other organs (29). In the context of liver diseases, circulating levels of PTX3 were found to be elevated in non-alcoholic steatohepatitis and associated with disease progression (30,31). However, the expression and role of PTX3 in liver injury and in response to inflammation are not well understood.
In this study we show that PTX3 expression and serum levels are strongly associated with liver disease progression and endotoxemia. PTX3 is locally produced by activated HSC in chronic liver injury, and administration of PTX3 attenuated LPS-induced liver injury and inflammation by modulating cytokine production induced by TLR4 activation.
Materials and Methods
Patients
Samples from 54 patients with clinical, analytical and histological features of AH were selected. These cases are part of our historic cohort of patients with AH and cirrhotic controls admitted to the Liver Unit of the Hospital Clinic of Barcelona from July 2009 to January 2015. The study was approved by the Ethics Committee of the Hospital Clinic of Barcelona and informed consent was obtained from all patients before inclusion. Inclusion and exclusion criteria have been described previously (4).
In vitro studies
Human primary HSC were isolated from control patients, and cultured as previously described (32). For gene expression analysis by qPCR, total RNA was isolated using RNeasy Mini Kit (Quiagen) as described manufacturer's protocol.
Effect of PTX3 in human monocyte inflammatory responses and polarization
Buffy coats, provided by the Blood and Tissue Bank (Barcelona, Spain), were obtained from healthy blood donors following the institutional standard operating procedures for blood donation and processing. Peripheral blood (PB) mononuclear cells were isolated from donors as previously described (33). The percentage of CD14+ cells (Peripheral Blood monocytes) routinely obtained were 5% (+/- 3%).
Experimental mouse models of liver damage
Experimental models were performed using 6 to 12-week-old male mice C57BL/6 (Charles River, l'Arbresle, France). All animal studies were approved by the Ethics Committee of the University of Barcelona. To induce chronic liver injury, mice were treated with carbon tetrachloride (CCl4) injected intraperitoneally at a dose of 0.5mL/kg twice a week; control mice were injected with corn oil. In order to study the effects of acute-on-chronic liver injury, we performed a model combining the damage of chronic CCl4 with administration of LPS (Sigma-Aldrich), mimicking the outcome of endotoxemia in the situation of chronic liver disease as previously used (34).
The role of PTX3 during acute-on-chronic liver injury was investigated in vivo in chronic CCl4 (0.5mL/kg twice a week during two weeks) treated mice. Intraperitoneal administration of rPTX3 (5mg/kg body weight) was performed 2 hours before intravenous injection of LPS (2,5mg/kg); control mice were intraperitoneally injected with corn oil and intravenously with vehicle. Mice were sacrificed 24 hours after LPS injection and blood, livers, lungs, and kidneys were removed for subsequent analysis.
Results
PTX3 expression is increased in experimental models of chronic and acute-on-chronic liver injury
PTX3 is rapidly induced after injury in several tissues; therefore, we evaluated the expression of PTX3 in animal models of chronic liver disease and acute-on-chronic liver injury. Administration of carbon tetrachloride (CCl4) to mice, a well-established animal model of chronic liver injury and fibrosis, induced a time-dependent increase in PTX3 liver expression (Fig. 1A). Liver cirrhosis is frequently associated with increased gut permeability and endotoxemia with elevated circulating levels of LPS (2,3). Therefore, we evaluated the effect of LPS administration on PTX3 expression. Interestingly, while the administration of LPS to healthy animals did not induce PTX3 expression (Fig. 1B), LPS infusion in CCl4 treated animals, a model of acute-on-chronic liver injury, induced a marked increase in hepatic PTX3 expression (Fig. 1C). Interestingly, Tlr4 hepatic expression was increased in CCl4 treated animals, but not further increased by LPS stimulation (Supp Fig. 1). These results suggest that preexisting liver injury may be necessary to mediate LPS-induced PTX3 expression.
Figure 1. Ptx3 expression and cell source in experimental models of chronic and acute-on-chronic liver injury.
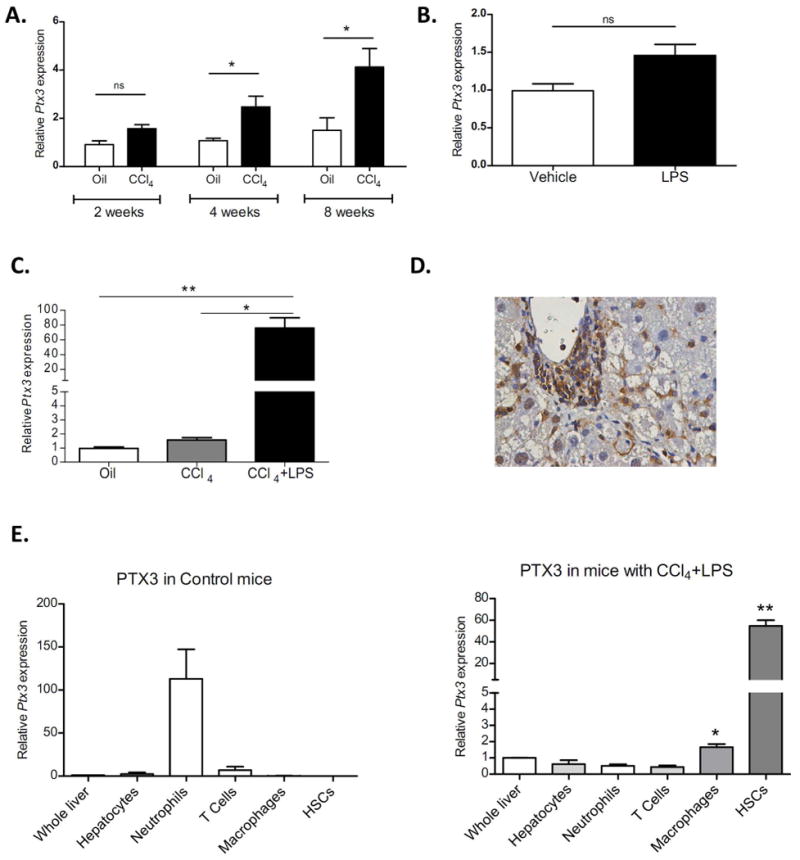
a) Hepatic gene expression of Ptx3 in mice treated with carbon tetrachloride (CCl4) during two weeks (n=4), four weeks (n=6) and eight weeks (n=4) (*p<0,05 compared with their appropriate oil treated control group mice). b) Hepatic expression of PTX3 in mice treated with LPS (10mg/Kg) (n=4) or vehicle (DPBS) (n=4). c) Hepatic gene expression of Ptx3 in mice treated with oil+vehicle, CCL4 and CCL4+LPS (n=6 per group) (**p<0,01 compared with control and CCL4 mice groups). d) Immunostaining of liver sections (x200 magnification) from CCL4+LPS treated mice show that PTX3 is expressed in inflammatory and non-parenchymal cells. e) Ptx3 gene expression in FACS-sorted hepatic cells population by immunoselection: neutrophils (Ly6G+), macrophages (F4/80+), T cells (CD3+), hepatocytes, and HSC (VitA+). Hepatic cells were compared with whole liver of oil control or CCl4+LPS respectively (*p<0,05; **p<0,01 compared with whole liver) (hepatic cells sorted from mice n=3).
In order to elucidate the cell source of PTX3 in chronic liver injury, we evaluated PTX3 expression by immunohistochemistry. As shown in Figure 1D, PTX3 expression in mice with CCl4-induced chronic liver injury plus LPS was mainly localized at inflammatory areas and sinusoidal cells. Furthermore, we assessed Ptx3 gene expression in different liver cell sorted populations, namely hepatocytes, HSC, macrophages, neutrophils and T cells isolated from healthy mice and those with acute-on-chronic liver injury. Interestingly, while the main producing cell type in healthy livers was neutrophils, macrophages and particularly HSC were the main cell types expressing PTX3 in injured livers (Fig. 1E). In order to evaluate the purity of sorted populations, we evaluated the gene expression of Alb, Mpo, Cd3e, F4/80 and Col1a1 in each of the populations obtained, which showed high enrichment (Supp Fig. 2).
PTX3 enhances HSC activation and is expressed in activated HSC
HSC are the main cell type producing PTX3 in mouse models of liver injury. Therefore, we assessed the expression and effects of PTX3 in human HSC, the main cell type responsible for the wound-healing response of the liver (35). We first evaluated the expression of PTX3 in human quiescent HSC freshly isolated from normal livers, followed by their activation in culture. Upon culture activation, HSC strongly increased the expression of PTX3 compared to quiescent HSC (Fig. 2A). Moreover, pro-inflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β) and LPS enhanced PTX3 expression in human primary activated HSC as compared to vehicle (p<0,05) (Fig. 2B).
Figure 2. Expression and role of PTX3 in hepatic stellate cells.
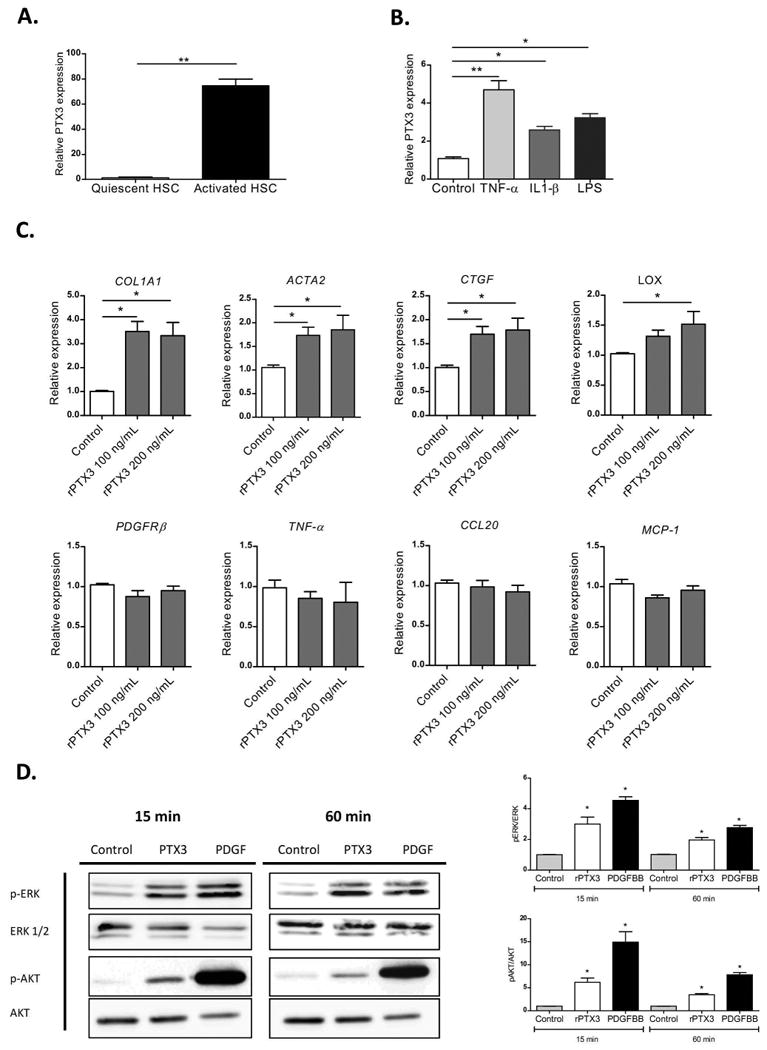
a) PTX3 gene expression in freshly isolated human quiescent HSC and after activation in vitro (n=4) (**p<0,01). b) Induction of PTX3 by pro-inflammatory mediators: cultured HSC incubated for 24h with tumor necrosis factor α (TNF-α) 20 ng/mL and interleukin 1β (IL-1β) 20 ng/mL and lipopolysaccharide (LPS) 100 ng/mL, (**p<0,01; *p<0,05 compared with control). c) Expression of HSC markers of activation, pro-inflammatory and pro-fibrogenic mediators in HSC after stimulation with rPTX3 100 ng/mL and 200 ng/mL. (*p<0,05 compared with control). d) Representative western blot of phosphorylated and total ERK and AKT in HSC stimulated with rPTX3 200 ng/mL and platelet derived growth factor (PDGFBB) (25 ng/mL) as positive control.
Stimulation of human primary activated HSC with recombinant PTX3 (rPTX3) induced an up-regulation of HSC activation marker and extracellular matrix genes (COL1A1, ACTA2, CTGF, LOX) (Fig 2C) but did not significantly change the expression of pro-inflammatory genes (TNF-α, CCL20, MCP-1) (Fig.2C). Moreover, rPTX3 induced ERK ½ and AKT phosphorylation in HSC, suggesting that PTX3 induces intracellular signaling in HSC (Fig. 2D). To further investigate the biological effect of PTX3 on HSC, we performed a wound-healing assay with primary HSC. As a positive control, cells were stimulated with PDGFBB, a main mitogen for HSC. As shown in Figure 3A, incubation with rPTX3 increased HSC migration and wound closure. Next, we evaluated the effects of silencing PTX3 in LX2 human HSC cell line by siRNA. As shown in Figure 3B, transfection of HSC with a siRNA targeting PTX3 reduced PTX3 mRNA and protein expression as compared to scramble siRNA. HSC treated with PTX3 siRNA significantly reduced the expression of activation genes such as COL1A1, ACTA2 and LOX, but did not reduce the expression of inflammatory mediators (Fig. 3C). Results from recombinant PTX3 stimulation and silencing of endogenous PTX3 expression suggest that both exogenous as well as endogenous PTX3 induce HSC activation. In order to elucidate if PTX3 might exert its role on HSC through Fc gamma receptors (FcγRs), we investigated their expression in primary human HSC. Gene expression of FcγR-1 and FcγR-2 was not detected in cultured HSC. The expression of FcγR-3, the FcγR with the highest affinity for PTX3 (22), was assessed by flow cytometry, showing no expression in cultured HSC (Supp. Fig. 3A).Taken together, these results indicate that PTX3 is expressed in HSC and is involved in their activation, reinforcing its potential role in liver wound-healing response.
Figure 3. Biological effect of PTX3 in hepatic stellate cells.
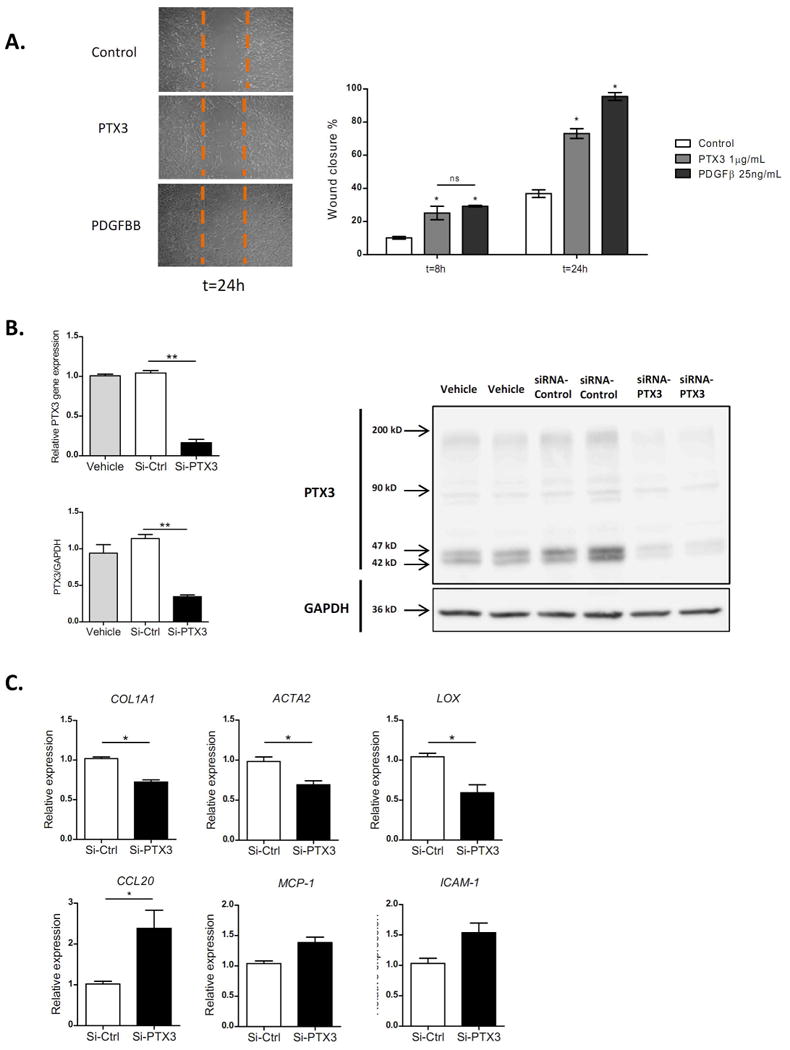
a) Representative pictures of Wound-healing assay (x200 magnification), with a dotted line indicating the wound size at time 0. Effect of PTX3 on HSC wound-healing response, incubated both rPTX3 (1 μg/mL) and PDGFBB (25 ng/mL). Wound closure is expressed as wound closure percentage with respect to time 0 after scratch (*p<0.01). b) Evaluation of siRNA efficiency. Expression of PTX3 mRNA in HSC transfected with siRNA (**p<0,001 compared with siRNA-Control). Representative image of western blot of three independent experiments, siRNA-PTX3 decreased PTX3 protein expression at predicted molecular weight (42-47 kD) and at multimeric forms of PTX3 with a molecular ranging from 42 to 200 kD. Proteins from total cell lysate of HSC treated for 48 hours with vehicle, siRNA negative Control and siRNA for PTX3. c) Expression of HSC activation markers in HSC transfected with siRNA-PTX3 and si-control for 24h.
PTX3 modulates LPS-induced liver injury and inflammation
PTX3 was highly expressed in LPS-induced acute-on-chronic liver injury. Therefore, we explored the potential role of PTX3 in LPS-induced inflammation in a context of chronic injury. First, we evaluated the effect of PTX3 in high precision-cut liver slices from mice treated with CCl4. Liver slices were incubated in the presence or absence of rPTX3 and stimulated with LPS. As shown in Figure 4A, rPTX3 alone did not induce any significant effect; however, incubation with rPTX3 was able to attenuate the expression of inflammatory mediators such as Ccl20, Ccl5, Mcp-1 induced by LPS. Moreover, incubation with rPTX3 also reduced lactate dehydrogenase (LDH) and aspartate transferase (AST) levels in tissue culture supernatant (Fig. 4B). Taken together, these results suggest that PTX3 may protect against LPS-induced liver damage by attenuating the expression of pro-inflammatory cytokines and hepatocellular damage.
Figure 4. Effect of PTX3 in high precision-cut liver slices.
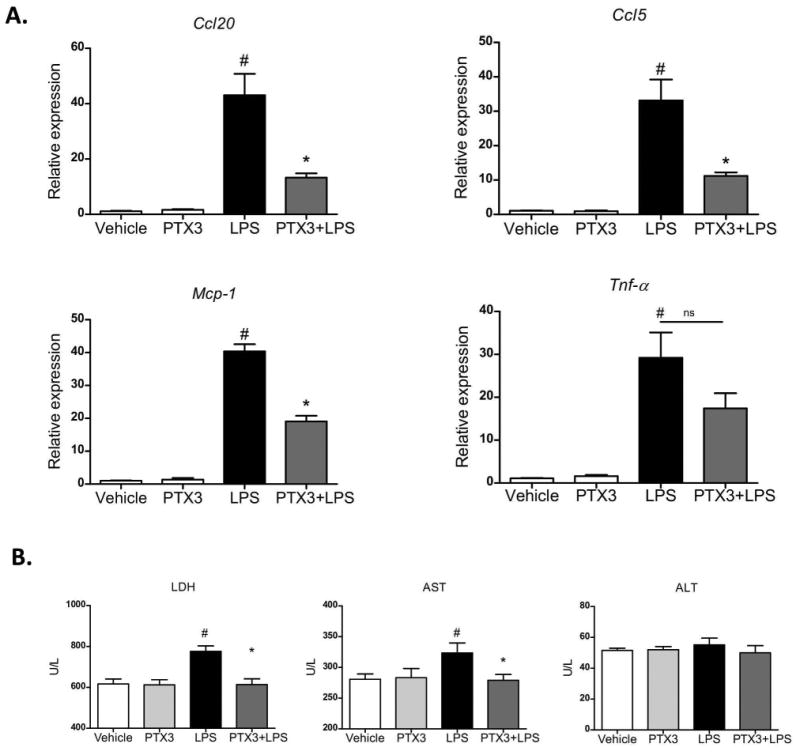
Liver slices from mice treated with CCl4 were incubated with or without PTX3 and stimulated with LPS. a) Gene expression of Ccl20, Ccl5, Mcp-1 and Tnf-α in liver slices after incubation for 1 hour with rPTX3 (500 ng/mL) (n=4) or vehicle with or without addition of LPS (25 μg/mL) (n=4) for an additional 6 hours. #p<0,05 compared with control; *p<0,05 compared with LPS induction. b) Tissue culture supernatant levels of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) in (n=4) from cultures of liver slices pre-incubated with rPTX3 or vehicle for 1 hour before LPS stimulation for 6 hours #p<0,05 compared with control; *p<0,05 compare with liver slices treated with LPS.
Next we evaluated the role of PTX3 in vivo in an acute-on-chronic hepatic injury model of CCl4-induced liver injury plus LPS (Fig. 5A). Treatment with rPTX3 before LPS stimulation substantially reduced liver damage as assessed by LDH, AST and alanine aminotransferase (ALT) serum levels (Fig. 5B). In addition, PTX3 treatment reduced inflammatory response by down-regulating Ccl20, Mcp-1, Ccl5, IL-1β, Tnf-α, Cxcl2 and Nos-2 gene expression (Fig. 5C). Moroever, macrophage M1 inflammatory mediators (i.e. Mcp-1, IL-1β, Tnf-α) but not M2 markers (Mrc1, Arg1 and IL4) were reduced in PTX3-treated animals (Fig. 5C). Moreover, histological evaluation showed a significant reduction of neutrophil and macrophage liver infiltration in mice treated with rPTX3 (Fig. 5D). This reduction in recruitment was not associated with changes in Fcγ receptor expression between experimental groups (Supp Fig. 3B). It is important to note that chronic liver injury and endotoxemia are associated with extra-hepatic organ injury (6). In this regard, rPTX3 administration significantly reduced the expression of LPS-induced inflammatory mediators and neutrophil infiltration in the kidney and the lung (Supp. Fig. 4A-C). Together, results from ex vivo and in vivo experiments suggest that PTX3 administration exerts a hepato-protective effect and modulates LPS-induced liver injury and inflammation.
Figure 5. Effect of PTX3 treatment in acute-on-chronic experimental model.
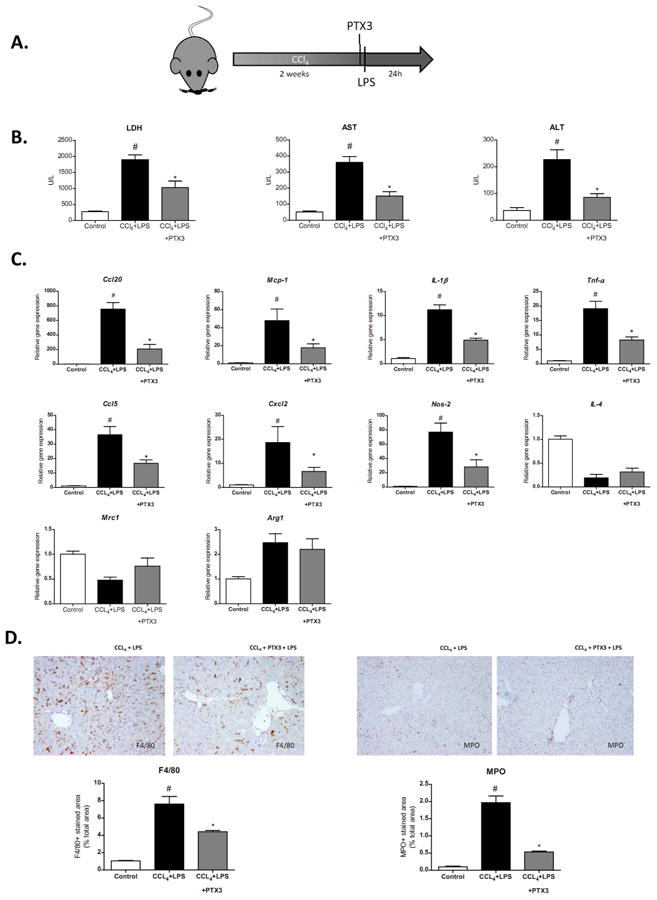
a) Scheme of experimental procedure. CCl4 treated mice were treated with rPTX3 two hours before the infusion of LPS. Mice were sacrificed 24h after LPS infusion. b) Lactate dehydrogenase (LDH), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels in control mice and in mice treated with CCl4+LPS (n=5) or CCl4+rPTX3+LPS (n=5) #p<0,01 compared with control group; *p<0,05 compared with CCl4+LPS treated group. c) Hepatic gene expression of Ccl20, Mcp-1, IL-1β, Tnf-α, Ccl5, Cxcl2, Nos-2, IL-4, Mrc1, and Arg1 in mice treated with CCl4+LPS (2,5mg/kg) (n=5) or CCl4+rPTX3 (5mg/kg)+LPS (n=5) #p<0,05 compared with control mice group; *p<0,05 compared with CCl4+LPS treated mice. d) Representative pictures of F4/80 and myeloperoxidase (MPO) immunostaining of liver sections of mice treated with CCl4+LPS or CCl4+rPTX3+LPS (×200 magnification). The graphs show quantification of the percentage of positive stained area.
PTX3 regulates inflammation by targeting monocytes and liver macrophages
Our data suggest that PTX3 is able to attenuate inflammatory responses to LPS in liver as well as the inflammatory cell infiltrate. However, since no direct anti-inflammatory effect was observed on HSC, we analyzed whether PTX3 could be targeting macrophages. First, we evaluated whether PTX3 was able to block TLR4-LPS response in peripheral blood monocytes isolated from healthy donors. Interestingly, while rPTX3 clearly reduced the early response to LPS by decreasing TNFα and IL-1β production, it increased the production of CCl5 (Fig. 6A). Second, we evaluated the effect of rPTX3 in human liver macrophages. As shown in monocytes, stimulation of liver macrophages with rPTX3 reduced LPS-induced TNF-α and IL-1β secretion in cell culture media, and induced a marked increase in CCL5 production (Fig. 6B). It has been shown that in macrophages PTX3 modulates the inflammatory response to infection by Aspergillus conidia by binding to MD2, the TLR4-accessory protein (26). We therefore studied the ability of PTX3 to interfere with MD2 activity in a widely used cellular system. We transfected human embryonic kidney (HEK)-293 cells stably expressing TLR4 or TLR4 plus CD14 with MD2. As shown in Supplementary Figure 5, rPTX3 did not significantly affect LPS response in TLR4 and TLR4/CD14 HEK cells transfected with MD2, suggesting that in this system PTX3 may not prevent MD2 contribution to LPS activation though TLR4, TLR4-CD14 complexes.
Figure 6. Effect of PTX3 on monocytes and macrophages.
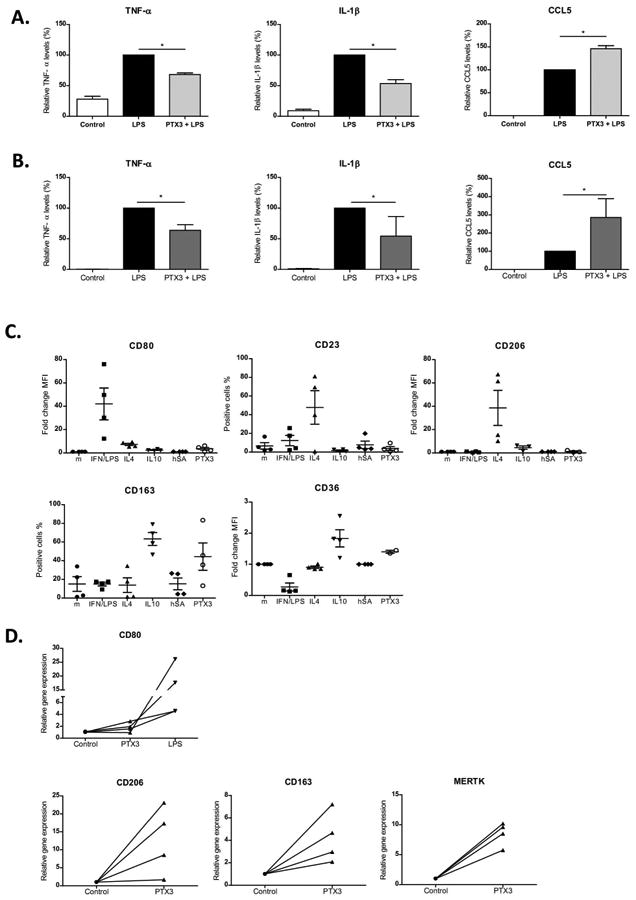
Peripheral blood monocytes isolated from healthy donors and human liver macrophages were incubated with rPTX3 followed by stimulation with LPS. a) human monocytes and b) human liver macrophages relative TNF-α, IL-1β and CCL5 levels in supernatant in the presence of LPS (10 ng/mL) and rPTX3 (1 μg/mL) compared with LPS (10 ng/mL) alone; n≥3 donors and patients. For normalization, cytokine production induced by stimulation with LPS of each experiment was set at 100; and relative cytokine production in the presence of rPTX3 or controls was calculated (*p<0,05). Peripheral blood monocytes isolated from four healthy donors were incubated (106 cells/well) during 3 days with INF/LPS (50/100 ng/mL), IL4 (40 ng/mL), IL10 (50 ng/mL), human albumin (hSA, 1 μg/mL) and rPTX3 (1 μg/mL) separately in RPMI with 5% FBS. c) Expression of macrophage polarization cell markers (CD80, CD23, CD206, CD163 and CD36) analyzed by flow cytometry (n=4). d) Human liver macrophages isolated from patients were incubated with rPTX3 (1 μg/mL). Gene expression of macrophages cell markers (CD80, CD206, CD163, Mertk) analyzed 24h after stimulation by real time PCR (n=4). As a positive control of CD80 (M1) induction liver macrophages were incubated with LPS (10 ng/mL).
Next, we evaluated whether PTX3 influenced the polarization of monocytes into macrophages. As control stimuli, we treated macrophages with: IFN/LPS to induce an M1 (M-IFN/LPS) phenotype (CD80 positive); IL4 (M-IL4) to induce a M2a phenotype (CD23 and CD206 positive); and with IL10 (M-IL10) to induce M2c (CD163 and CD36 positive) (36). As observed by comparison with all the different stimulations, rPTX3 modulated the expression of polarization markers in a similar way as IL10: it induced an increase in CD163 and CD36 expression, suggesting that it may enhance the acquisition of an IL10-like macrophage phenotype. Conversely, the use of human albumin as a negative control protein did not substantially alter the macrophage phenotype (Fig. 6C). Moreover, we also evaluated the potential of PTX3 to induce M2 phenotype in liver macrophages. Human liver macrophages stimulated with rPTX3 showed an increase in expression of M2 markers CD206, CD163 and Mertk and did not induce M1 marker CD80 (Fig. 6D).
Taking together, these results suggest that PTX3 may modulate LPS signaling response in monocytes and liver macrophages.
PTX3 is up-regulated in patients with acute-on-chronic liver disease and is associated with disease severity
Alcoholic hepatitis (AH) is an acute-on-chronic condition characterized by liver injury, fibrosis and an important inflammatory responses, in which endotoxemia is associated with a bad outcome (2,3). Therefore, PTX3 liver expression and plasma levels were evaluated in patients with alcoholic cirrhosis and AH.
As shown in Figure 7, PTX3 gene expression was increased in liver tissue from alcoholic cirrhotic patients and was strongly up-regulated in AH patients (p<0.01) (Fig. 7A), consistent with PTX3 expression in experimental models of liver injury (Fig. 1A-B) (37,38). Plasma levels of PTX3 were also increased in compensated alcoholic cirrhotic patients and were further increased in patients with both mild and severe AH (Fig. 7B). Interestingly, PTX3 hepatic gene expression and peripheral plasma levels showed a positive correlation, suggesting that the liver may be an important source of circulating PTX3 (Fig. 7C). PTX3 staining in alcoholic cirrhosis was localized in septa areas with fibrosis and inflammation (Fig. 7D). We next assessed the association of PTX3 plasma levels with clinical parameters and scores of disease severity. As shown in Figure 7E, we found a positive correlation of PTX3 plasma levels with the model for end-stage liver disease (MELD) (p<0.001) and age, serum bilirubin, international normalized ratio, and serum creatinine (ABIC) scores (p<0.01). Moreover, PTX3 plasma levels positively correlated with Maddrey score (p<0.01) and procalcitonin but not with ultra-sensitive C-reactive protein (usCRP) serum levels and the hepatic venous pressure gradient (HVPG). Importantly, PTX3 plasma levels correlated with LPS serum levels (Fig. 7F), indicating a potential association of PTX3 expression with endotoxemia. However, PTX3 plasma levels did not correlate with IL-10, suggesting that PTX3 may not be associated with IL-10 expression and immune exhaustion (Supp Fig. 6C). Additionally, PTX3 levels were higher in patients with infection at admission and in those who developed in-hospital infections as compared to non infected patients (Supp Fig. 6B). Next, we evaluated the association of PTX3 plasma level with 6-month mortality. PTX3 serum levels showed an AUROC of 0.76 (95% CI: 0.69-0.89; p=0.002) for predicting 6-month mortality. The Kaplan-Meier analysis showed that PTX3 levels above 10 ng/ml were associated with a higher mortality at 180 days (Fig. 7G). This cut-off showed a sensitivity of 78% and a specificity of 60%. Finally, to evaluate the potential prognostic impact of PTX3 on 180-day mortality, we fitted a bivariate Cox regression (HR -hazard ratio-) analysis adjusting PTX3 plasma levels for AH severity-scoring systems at admission (e.g. ABIC score, MELD and Lille Model). PTX3 plasma levels were independently associated with 180-day mortality after adjusting for the well-validated scoring systems (p<0.05 for all cases; Supplementary Table 2).
Figure 7. PTX3 expression and correlation with clinical parameters in patients with liver disease.
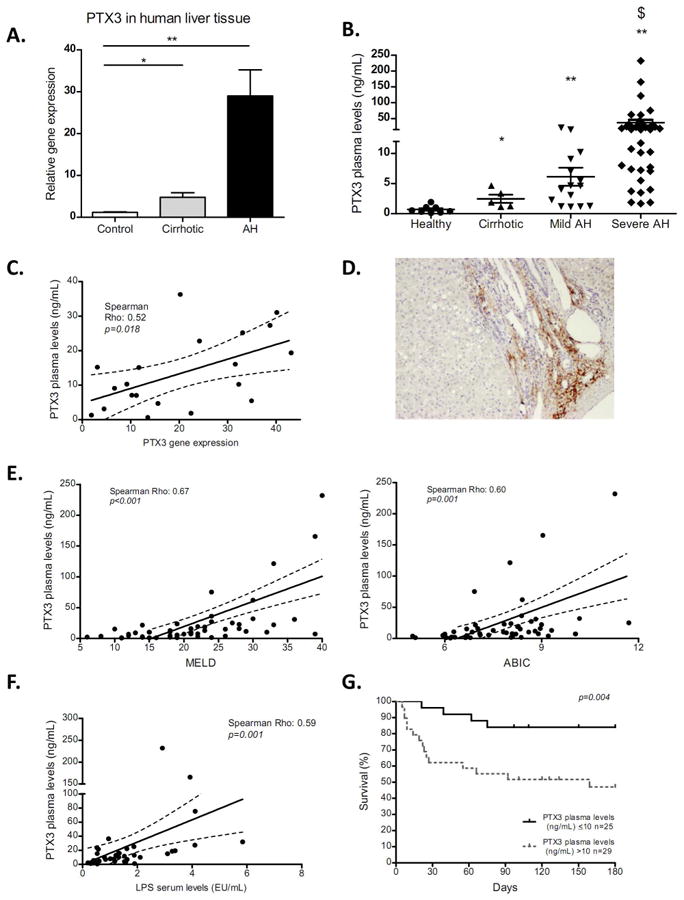
a) PTX3 hepatic gene expression in cirrhotic alcoholic liver disease patients (Cirrhotic) (n=5), patients with alcoholic hepatitis (AH) (n=30) compared with healthy controls (n=5) (*p<0,05; **p<0,01). b) PTX3 plasma levels were significantly increased in patients with compensated cirrhosis (n=5) and both mild (n=15) and severe AH (n=39), compare with healthy patients (n=8); *p<0,05 compare with controls; **p<0,01 compare with controls; $ p<0,01 compare with other groups. c) correlation of PTX3 plasma levels and hepatic gene expression in patients with AH (n=23). d) Representative picture of PTX3 immunohistochemistry in liver sections of cirrhotic alcoholic liver disease patients (x200 magnification). Correlation of PTX3 plasma level with disease severity score, e) Model for End-stage Liver Disease (MELD) score, f) Age, serum Bilirubin, INR, and serum Creatinine (ABIC) score in patients with AH (n=54). g) Correlation of LPS serum level and PTX3 plasma levels in patients with AH (n=40). h) Kaplan-Meier analysis showing prognostic 180 day mortality based on PTX3 plasma level in patients with AH (n=54). The cut-off with better sensitivity and specificity was identified as 10 ng/mL (p=0,004). Severe AH was defined as a MELD >21 and or ABIC >8.99.
These results suggest that PTX3 is strongly associated with disease severity, and its expression may be enhanced as a result of acute-on-chronic liver injury and endotoxemia.
Discussion
In this study, we show that PTX3 is highly up-regulated in chronic liver diseases and particularly in acute-on-chronic conditions such as AH in which correlates with disease severity, adverse outcome and endotoxemia. We have identified activated HSC as the main cell source of PTX3 in chronic liver disease. Moreover, PTX3 exerts a positive loop on HSC, enhancing HSC activation and thereby promoting PTX3 synthesis. Interestingly, besides its effects on wound-healing response, we show that PTX3 reduces LPS-induced acute-on-chronic tissue injury and inflammatory cell recruitment. Moreover, PTX3 has a modulatory effect on liver inflammation and particularly on LPS-induced responses.
Although there was a clear positive correlation of PTX3 with disease progression, our in vitro, ex vivo and in vivo data suggest that PTX3 exerts a hepatoprotective and a modulatory effect on acute-on-chronic inflammatory events. Taken into account that PTX3 protein and regulation are conserved between mice and humans (37,38). This apparently contradictory result suggests that PTX3 might be expressed as a local response to injury and infection by modulating an exacerbated inflammatory response. Moreover, high PTX3 levels in patients could be a counter-regulatory response to disease severity. In the context of chronic liver injury, the level of PTX3 expression may be determined by injury, inflammation and endotoxemia. Additionally, PTX3 levels were associated with infection. Although interesting, this latter result should be taken with caution since the number of patients is low and significance is likely mainly due to the small number of patients with very high plasma levels of PTX3. The current data suggest that PTX3 plasma levels may have potential as a prognostic biomarker in AH patients. However, future studies with larger cohorts of patients should specifically evaluate the potential of PTX3 as a biomarker for chronic liver diseases.
Interestingly, in patients with AH, there was a good correlation between liver expression and circulating levels of PTX3, suggesting that the liver may be the main source of PTX3 in chronic liver diseases. Although PTX3 production and release is local at the site of injury, PTX3 is secreted and detected in the circulation, suggesting that it may exert its effects at a systemic level. Indeed, from our therapeutic approach in which PTX3 was administered systemically, we could detect its effects in the liver but also in the lungs and kidneys. This is an important finding for the potential use of PTX3 administration as a therapeutic intervention since in acute-on-chronic conditions extra-hepatic organs are frequently affected. Moreover, infections and endotoxemia are the main cause of acute-on-chronic liver failure, a syndrome recently associated with multi-organ failure and high mortality (6).
PTX3 has been reported to participate in wound-healing response in different organs, including the liver (29). In this regard, we showed that while PTX3 is mainly expressed in neutrophils in healthy liver, in injury it is mainly expressed in HSC, the main cell type responsible for the liver wound-healing response and liver fibrosis (35). Human HSC do not express the main receptors identified for PTX3. This is not surprising, since the existence of potential PTX3 receptors have been extensively investigated in other cell types with little success. It has been proposed that unidentified receptors for PTX3 may exist, however, most reports suggest that PTX3 exerts its biological functions by indirect binding to other proteins such as TSG-6, FGF-2, C1q, Ficolin-1, Inter-α-trypsin-inhibitor (IαI) and not by direct interaction with a specific receptor (39–44). Besides the role of HSC in the production of extracellular matrix components fro wound-healing response, HSC are important players in the inflammatory response of the liver, mainly by promoting inflammatory cell recruitment and expression of inflammatory mediators. Although PTX3 does not modulate inflammatory gene expression in HSC, the important expression of PTX3 by activated HSC suggests that it may have paracrine effects to modulate liver inflammation and to control inflammatory responses in the liver. The differential expression of PTX3 in healthy and injured liver together with the data that show that PTX3 drives HSC activation, suggest that in response to injury PTX3 released by neutrophils may promote HSC activation. However, in chronic liver disease, activated HSC would become the main cell type producing PTX3, which may enhance wound-healing response and exert protective and immune modulatory effects. Neutrophils serve as a reservoir of PTX3 for a rapid release in response to microbial or inflammatory signals, when PTX3 acts as an acute phase protein (45). Once neutrophils activate, they stop or reduce PTX3 production. During injury, different cell types may also contribute to PTX3 production (46). In this study we describe that HSC, express PTX3 during activation and in response to injury and inflammatory signals. One of the transcription factors that regulate the constitutive expression of PTX3 is AP-1 (47), but no information is available regarding the regulation of PTX3 expression in liver injury. Further studies will need to address the regulation of PTX3 in HSC.
Our data together with previous reports showing the role of PTX3 in tissue repair (29) suggest that PTX3 expression may not only be associated with fibrogenesis but also with a correct tissue wound-healing response and modulation of inflammation. Further studies would have to determine the potential beneficial effects of long-term PTX3 treatment in chronic liver diseases and fibrogenesis.
Acute administration of PTX3 has a clear protective effect on hepatocellular injury as well as inflammatory response. The well-known pleiotropic nature of PTX3 suggests that PTX3 may exert this complex protective effect by a combination of mechanisms. In other organs it is reported that PTX3 binds to p-selectin preventing inflammatory cell recruitment and thereby attenuating inflammatory response (43). In addition, it is known that PTX3 opsonizes apoptotic cells and contributes to their clearance, preventing autoimmune reactions in inflamed tissues (18,39,48). Therefore, in the context of liver injury, PTX3 reduce inflammatory response by reducing inflammatory cell infiltration and potentially by directly targeting inflammatory cells modulating their phenotype and response. Remarkably, in this study we identified an unrecognized novel mechanism of action of PTX3 that may be of critical relevance in the context of chronic liver disease. We found that PTX3 modulates TLR4 downstream responses, reducing MyD88-dependent cytokines (TNF-α and IL-1β) (12) and enhancing the production of TRIF-dependent CCL5 (12) in human monocytes and in liver macrophages. This is in agreement with a previous report describing that PTX3 binds to MD2 and needs TRIF to promote TRIF-dependent protection in Aspergillosis (26). Moreover, our data suggest that PTX3 favors the acquisition of an anti-inflammatory IL10-like phenotype in human monocytes-derived macrophages and expression of M2 markers in isolated liver macrophages. These results suggest that PTX3 may target peripheral monocytes and liver macrophages modulateing inflammatory response and macrophage differentiation and polarization. Although we show a clear effect of PTX3 on macrophages, we cannot conclude that PTX3 effect on macrophages is the only or the main mechanisms conferring hepatoprotection. Altogether, these results suggest that the effects for PTX3 observed are certainly not exclusively mediated by a single mechanism but rather by a combination of biological roles leading to a hepatoprotective effect and attenuation of inflammatory recruitment and response.
The results of the present study show, for the first time, the liver expression of PTX3 by activated HSC, and the role of PTX3 in liver injury and inflammation. Besides its well recognized role on bacteria opsonization and clearance, we identified a novel mechanism of action of PTX3 in the context of LPS-induced liver injury and endotoxemia. Moreover, we describe its immunomodulatory effects in human macrophages in the context of liver injury and disease. These results, together with the human data provided, suggest that PTX3 may be an important local and also systemic player in chronic liver disease, and that PTX3 administration may be a promising therapeutic strategy in acute-on-chronic liver disease.
Supplementary Material
Acknowledgments
Acknowledgments: This work was performed in the Centre Esther Koplowitz. The authors wish to thank Cristina Millán for her excellent technical support. We are indebted to the Genomics Unit, Biobank Unit and Cytometry and Cell Sorting Facility of the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for their technical help.
Grant Support: This work was supported by grants from Fondo de Investigación Sanitaria Carlos III (FIS), co-financed by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa” (FIS PI14/00320, PI12/00330, FIS PI12/01265 to PS-B, PG, JC) and from the NIH on Alcohol Abuse and Alcoholism grants 1U01AA020821 and 1U01AA021908-01-33490. PS-B is funded by Instituto de Salud Carlos III, Miguel Servet (CP11/00071 and CON14/00129) and co-financed by Fondo Europeo de Desarrollo Europeo (FEDER), Unión Europea, “Una manera de hacer Europa”. PG is funded by Agencia de Gestió d'Ajuts Universitaris I de Recerca (AGAUR) 2014 SGR 708, Centro de Investigación en Red Enfermedades Hepaticas y Digestivas (CIBEReHD) and Institució Catalana de Recerca i Estudis Avançats (ICREA). MC, IG and MRS are funded by Instituto de Salud Carlos III, Sara Borrell, Rio Hortega and Miguel Servet CPII 14/00021 grants respectively and Fundació Marató de TV3, 2013-3610. JA was partially funded by (CONACyT, Mexico City, Mexico). BA and DR-T received a grant from the Ministerio de Educación, Cultura y Deporte, FPU program.
Abbreviation list
- AKT
protein kinase B
- ABIC
age, serum bilirubin, international ratio, and serum creatinine
- ACLF
acute-on-chronic liver failure
- ACTA2
Alpha-Actin-2
- AH
Alcoholic Hepatitis
- Arg1
Arginase 1
- CCL20
C-C Motif Chemokine Ligand 20
- CCl4
carbon tetrachloride
- Ccl5
C-C Motif Chemokine Ligand 5
- CD14
cluster of differentiation 14
- CD163
cluster of differentiation 163
- CD206
cluster of differentiation 206
- CD23
cluster of differentiation 23
- CD36
cluster of differentiation 36
- CD80
cluster of differentiation 80
- COL1A1
Collagen Type I Alpha
- CRP
C-reactive protein
- CTGF
Connective Tissue Growth Factor
- Cxcl2
C-X-C Motif Chemokine Ligand 2
- FcγR
Fc gamma receptor
- HE
hepatic encephalopathy
- HSC
hepatic stellate cell
- HVPG
hepatic venous pressure gradient
- IFN
Interferon
- IL10
Interleukin 10
- IL-1β
Interleukin 1 Beta
- IL4
Interleukin 4
- LDH
lactate dehydrogenase
- LOX
Lysyl Oxidase
- LPS
lipopolysaccharide
- MAPK/ERK
Mitogen-Activated Protein Kinase
- MCP-1
Monocyte Chemotactic Protein 1
- MD2
myeloid differentiation factor 2
- MELD
model for end-stage liver disease
- Mrc1
Mannose Receptor, C Type 1
- MyD88
myeloid differentiation primary response 88
- Nos-2
Nitric Oxide Synthase 2
- PB
Peripheral blood
- PDGFBB
Platelet Derived Growth Factor Subunit B
- PDGFRβ
Platelet Derived Growth Factor Receptor Beta
- PTX3
Pentraxin-3
- rPTX3
recombinant PTX3
- siRNA
small interference RNA
- SIRS
systemic inflammatory response syndrome
- TLR4
toll-like receptor 4
- TNF-α
Tumor Necrosis Factor
- TRIF
TIR-domain-containing adapter-inducing interferon
Footnotes
Disclosures: authors declare that they have no competing interests.
See the Supplementary Materials and Methods section for more details.
References
- 1.Bataller R, Brenner D. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res [Internet] 2000;24:48S–54S. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10803780. [PubMed] [Google Scholar]
- 3.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762–772. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology [Internet] 2010;139:1246–1256.e5. doi: 10.1053/j.gastro.2010.06.019. Available from: http://dx.doi.org/10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology [Internet] 2013;144:1426–1437.e9. doi: 10.1053/j.gastro.2013.02.042. Available from: http://dx.doi.org/10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, et al. Acute-on chronic liver failure. [cited 2016 Feb 22];J Hepatol [Internet] 2012 57:1336–48. doi: 10.1016/j.jhep.2012.06.026. Available from: http://www.journal-of-hepatology.eu/article/S0168827812005193/fulltext. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Kaisho T, Akira S. Toll-Like Receptors. Annu Rev Immunol [Internet] 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. Available from: http://dx.doi.org/10.1146/annurev.immunol.21.120601.141126%5Cnhttp://www.annualreviews.org/doi/pdf/10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med [Internet] 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2193086&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 12.Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, et al. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem Biophys Res Commun. 2005;328:383–392. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Takeuchi O, Fujita T, Inoue Ji, Muhlradt PF, Sato S, et al. Lipopolysaccharide Stimulates the MyD88-Independent Pathway and Results in Activation of IFN-Regulatory Factor 3 and the Expression of a Subset of Lipopolysaccharide-Inducible Genes. J Immunol [Internet] 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. Available from: http://www.jimmunol.org/content/167/10/5887.full. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Muhlbauer M, Weiss TS, Thasler WE, Gelbmann CM, Schnabl B, Scholmerich J, et al. LPS-mediated NFkappaB activation varies between activated human hepatic stellate cells from different donors. 2004 doi: 10.1016/j.bbrc.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 17.Cotena A, Maina V, Sironi M, Bottazzi B, Jeannin P, Vecchi A, et al. Complement dependent amplification of the innate response to a cognate microbial ligand by the long pentraxin PTX3. J Immunol [Internet] 2007;179:6311–6317. doi: 10.4049/jimmunol.179.9.6311. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17947708. [DOI] [PubMed] [Google Scholar]
- 18.Baruah P, Dumitriu IE, Peri G, Russo V, Mantovani A, Manfredi AA, et al. The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells. J Leukoc Biol [Internet] 2006;80:87–95. doi: 10.1189/jlb.0805445. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16617159. [DOI] [PubMed] [Google Scholar]
- 19.Moalli F, Doni A, Deban L, Zelante T, Zagarella S, Bottazzi B, et al. Role of complement and Fcγ receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 20.Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch a, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 21.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol [Internet] 1993;150:1804–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7679696. [PubMed] [Google Scholar]
- 22.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature [Internet] 2008;456:989–92. doi: 10.1038/nature07468. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-57749172475&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han B, Haitsma JJ, Zhang Y, Bai X, Rubacha M, Keshavjee S, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2011;37:334–342. doi: 10.1007/s00134-010-2067-2. [DOI] [PubMed] [Google Scholar]
- 24.Bonavita E, Gentile S, Rubino M, Maina V, Papait R, Kunderfranco P, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Moalli F, Paroni M, Véliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, et al. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol. 2011;186:5425–5434. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 26.Bozza S, Campo S, Arseni B, Inforzato A, Ragnar L, Bottazzi B, et al. PTX3 Binds MD-2 and Promotes TRIF-Dependent Immune Protection in Aspergillosis. J Immunol [Internet] 2014;193:2340–8. doi: 10.4049/jimmunol.1400814. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25049357. [DOI] [PubMed] [Google Scholar]
- 27.Han B, Ma X, Zhang J, Zhang Y, Bai X, Hwang DM, et al. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest [Internet] 2012;92:1285–96. doi: 10.1038/labinvest.2012.92. Available from: http://dx.doi.org/10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salio M, Chimenti S, Angelis NDe, Molla F, Maina V, Nebuloni M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 29.Doni a, Musso T, Morone D, Bastone a, Zambelli V, Sironi M, et al. An acidic microenvironment sets the humoral pattern recognition molecule PTX3 in a tissue repair mode. J Exp Med [Internet] 2015 doi: 10.1084/jem.20141268. Available from: http://www.jem.org/cgi/doi/10.1084/jem.20141268. [DOI] [PMC free article] [PubMed]
- 30.Yoneda M, Uchiyama T, Kato S, Endo H, Fujita K, Yoneda K, et al. Plasma Pentraxin3 is a novel marker for nonalcoholic steatohepatitis (NASH) BMC Gastroenterol [Internet] 2008;8:53. doi: 10.1186/1471-230X-8-53. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2621235&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boga S, Koksal AR, Alkim H, Yilmaz Ozguven MB, Bayram M, Ergun M, et al. Plasma Pentraxin 3 Differentiates Nonalcoholic Steatohepatitis (NASH) from Non-NASH. Metab Syndr Relat Disord [Internet] 2015;13:393–399. doi: 10.1089/met.2015.0046. Available from: http://online.liebertpub.com/doi/10.1089/met.2015.0046. [DOI] [PubMed] [Google Scholar]
- 32.Morales-Ibanez O, Domínguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology [Internet] 2013;58:1742–56. doi: 10.1002/hep.26521. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3877722&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanjurjo L, Am??zaga N, Aran G, Naranjo-G??mez M, Arias L, Armengol C, et al. The human CD5L/AIM-CD36 axis: A novel autophagy inducer in macrophages that modulates inflammatory responses. Autophagy. 2015;11:487–502. doi: 10.1080/15548627.2015.1017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Affò S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut [Internet] 2014:1–11. doi: 10.1136/gutjnl-2013-306098. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24415562. [DOI] [PMC free article] [PubMed]
- 35.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun [Internet] 2013;4:2823. doi: 10.1038/ncomms3823. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4059406&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity [Internet] 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. Available from: http://dx.doi.org/10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–1872. [PubMed] [Google Scholar]
- 38.Daigo K, Mantovani A, Bottazzi B. The yin-yang of long pentraxin PTX3 in inflammation and immunity. Immunol Lett. 2014;161:38–43. doi: 10.1016/j.imlet.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma YJ, Doni A, Romani L, Jürgensen HJ, Behrendt N, Mantovani A, et al. Ficolin-1-PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. J Immunol [Internet] 2013;191:1324–33. doi: 10.4049/jimmunol.1300382. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23817411. [DOI] [PubMed] [Google Scholar]
- 40.Ma YJ, Doni A, Hummelshøj T, Honoré C, Bastone A, Mantovani A, et al. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem. 2009;284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camozzi M, Rusnati M, Bugatti A, Bottazzi B, Mantovani A, Bastone A, et al. Identification of an antiangiogenic FGF2-binding site in the N terminus of the soluble pattern recognition receptor PTX3. J Biol Chem. 2006;281:22605–22613. doi: 10.1074/jbc.M601023200. [DOI] [PubMed] [Google Scholar]
- 42.Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 43.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. [cited 2014 Aug 22];Nat Immunol [Internet] 2010 11:328–334. doi: 10.1038/ni.1854. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20208538. [DOI] [PubMed] [Google Scholar]
- 44.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–86. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 45.Jaillon S, Peri G, Delneste Y, Frémaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaillon S, Bonavita E, Gentile S, Rubino M, Laface I, Garlanda C, et al. The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int Arch Allergy Immunol. 2014;165:165–178. doi: 10.1159/000368778. [DOI] [PubMed] [Google Scholar]
- 47.Basile A, Sica A, D'Aniello E, Breviario F, Garrido G, Castellano M, et al. Characterization of the Promoter for the Human Long Pentraxin PTX3. J Biol Chem. 1997;272:8172–8178. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 48.Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


