ABSTRACT
HIV-1 infection of resting CD4 T cells plays a crucial and numerically dominant role during virus transmission at mucosal sites and during subsequent acute replication and T cell depletion. Resveratrol and pterostilbene are plant stilbenoids associated with several health-promoting benefits. Resveratrol has been shown to inhibit the replication of several viruses, including herpes simplex viruses 1 and 2, papillomaviruses, severe acute respiratory syndrome virus, and influenza virus. Alone, resveratrol does not inhibit HIV-1 infection of activated T cells, but it does synergize with nucleoside reverse transcriptase inhibitors in these cells to inhibit reverse transcription. Here, we demonstrate that resveratrol and pterostilbene completely block HIV-1 infection at a low micromolar dose in resting CD4 T cells, primarily at the reverse transcription step. The anti-HIV effect was fully reversed by exogenous deoxynucleosides and Vpx, an HIV-1 and simian immunodeficiency virus protein that increases deoxynucleoside triphosphate (dNTP) levels. These findings are consistent with the reported ability of resveratrol to inhibit ribonucleotide reductase and to lower dNTP levels in cells. This study supports the potential use of resveratrol, pterostilbene, or related compounds as adjuvants in anti-HIV preexposure prophylaxis (PrEP) formulations.
KEYWORDS: resting CD4 T cells, human immunodeficiency virus, pterostilbene, resveratrol, reverse transcription, stilbenoid, PrEP
INTRODUCTION
The stilbenoids resveratrol (RES) and pterostilbene (PTE) function as plant phytoalexins, i.e., defensive compounds generated in response to parasitic attack. Resveratrol is found in grape skins, small berries, peanuts, and some nuts and has been extensively studied as an active ingredient of red wine, contributing to the health benefits associated with the French paradox and other potential life-extending properties, including anti-inflammatory, anticancer, and antidiabetes activities (1). Pterostilbene is a natural dimethylated analog of resveratrol also found in grape skins, berries, peanuts, and almonds (2). Pterostilbene has anti-inflammatory activity (3, 4), is more lipophilic than resveratrol, and has greater stability in vitro and in vivo (5–8).
Resveratrol inhibits the replication of several viruses, including herpes simplex viruses (HSVs) 1 and 2, varicella-zoster virus, papillomaviruses, and influenza virus (9–12). Topical application of resveratrol has been found to inhibit HSV vaginal transmission in mice, suggesting that it might be useful as a topical microbicide (13). Alone, resveratrol does not inhibit wild-type (WT) human immunodeficiency virus type 1 (HIV-1) replication in activated T cells or in transformed T cell lines (14, 15), but it does potentiate inhibition of reverse transcription by nucleoside analog reverse transcriptase (RT) inhibitors (NRTIs), including tenofovir (TFV), didanosine, zidovudine (AZT), and emtricitabine (FTC) (14, 16). Resveratrol alone does, however, inhibit reverse transcription in NRTI-resistant RT mutants in activated T cells, such as mutants with the frequently occurring M184V mutation that sensitizes RT to reduced deoxynucleoside triphosphate (dNTP) levels (17), and TFV-resistant mutants in T cells and macrophages (18). Whereas resveratrol was observed to have no anti-HIV-1 effect in a transformed fibroblastic cell line (293T), pterostilbene at 10 μM showed 50% inhibition (19).
Resveratrol is an inhibitor of cellular ribonucleotide reductase (RNR), which converts ribonucleotides to deoxyribonucleotides for DNA synthesis during cell proliferation and for DNA repair (20, 21). A tyrosyl radical in the R2 subunit of RNR required for this conversion is destroyed by the radical-scavenging activity of resveratrol (20). Treatment of cells with resveratrol reduces cellular dNTP levels, particularly those of dATP and dGTP (22). The anti-HIV activities of resveratrol have been attributed to its inhibition of RNR, resulting in a reduction in dNTP levels, which enhances the competition of nucleoside analog drugs for cellular dNTPs (14, 17, 18, 23, 24). The failure of resveratrol alone to inhibit HIV-1 infection of activated and transformed T cells is thought to be the result of the inherently abundant levels of dNTPs in these cells, such that resveratrol cannot lower dNTP levels sufficiently to directly inhibit the reverse transcriptase of HIV-1 isolates with WT reverse transcriptase. Consistent with this idea, resveratrol alone has been found to partially inhibit HIV-1 infection of differentiated macrophages, a cell type with dNTP levels severalfold lower than those in activated T cells (14). Resveratrol has also been observed to inhibit Tat-dependent transactivation of the HIV-1 promoter in HeLa cells via activation of the Sirtuin 1 (SIRT1) deacetylase (25).
Although HIV-1 replicates optimally in activated CD4 T cells and transformed cell lines, in vivo, the cells most commonly infected following mucosal transmission of HIV and the related simian immunodeficiency virus (SIV) are T cells with a resting, nonproliferating phenotype (26–29). In situ analyses following vaginal infection of macaques found that ∼80% of the initial targets of SIV infection were intraepithelial or endocervical lamina propria resting CD4+ T cells (26, 27, 29) and Th17 cells (30). Vaginal, ectocervical, and endocervical tissue explant studies observed resting CD4 T cell infection to be confined to the mucosal stroma (31–35). In addition, infection of resting CD4 T cells contributes substantially to viral replication and immune depletion (27, 29, 36–39) and to formation of the latent reservoir (40, 41). Some studies of SIV infection in rhesus macaques have observed infection of non-CD3 cells as a minority population (30) or in substantial proportions, including Langerhans cells (42, 43) and dendritic cells (44).
Resting T cells are less permissive to productive HIV-1 infection, owing to several blocks, including the lack of transcription factors and the presence of restriction factors, such as SAMHD1 (45, 46); nevertheless, both in vivo and in vitro abundant productive infection in resting CD4 T cells is observed (29, 39, 47–49). Importantly, resting T cells contain dNTPs at levels 2 orders of magnitude lower than those in activated T cells (50), which slows the kinetics of reverse transcription (51) but does not prevent its eventual completion, resulting in productive infection and virus spread (49, 52). We thus revisited resveratrol and examined pterostilbene in the context of resting T cells.
RESULTS
Resveratrol inhibits HIV-1 infection of resting CD4 T cells but not activated CD4 T cells or cells of the immortalized CD4 T cell line Jurkat.
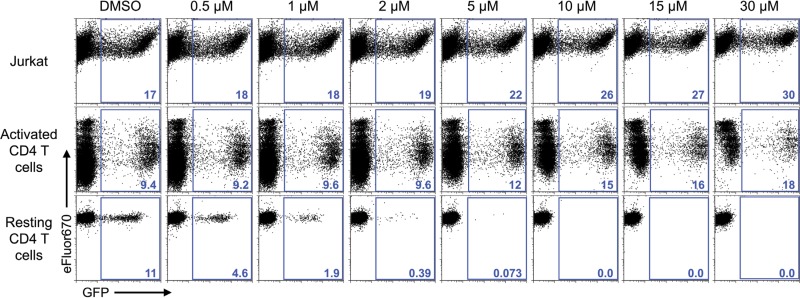
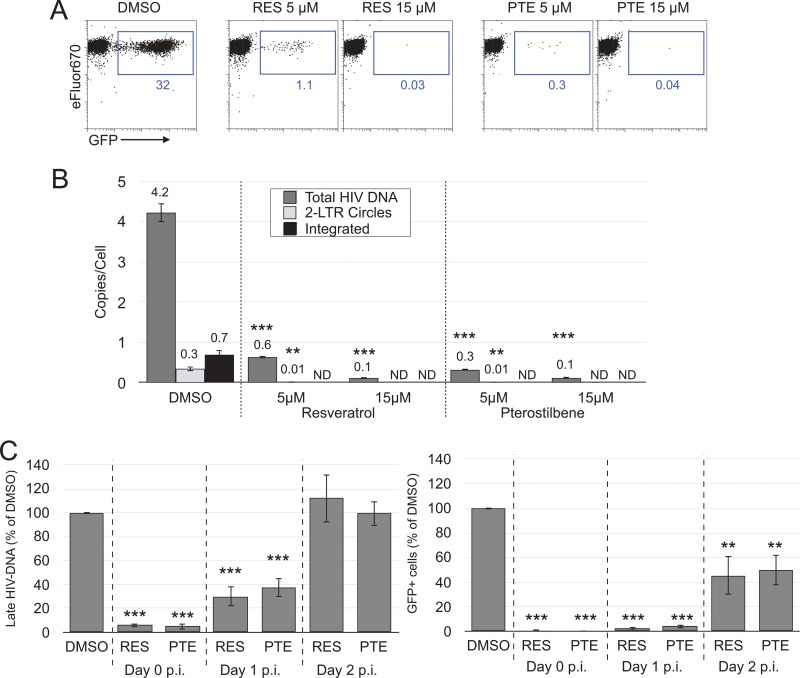
First, we sought to confirm previous reports regarding the failure to observe an anti-HIV effect of resveratrol in activated T cells. For this we employed an envelope-defective green fluorescent protein (GFP) reporter virus that was pseudotyped with the WT HIV-1 envelope. This virus can enter CD4+ cells but does not spread owing to the env gene defect that prevents de novo envelope protein expression in infected cells (53). Consistent with prior reports, resveratrol at concentrations up to 30 μM did not reduce HIV-1 infection in activated CD4 T cells or in cells of the transformed T cell line Jurkat (Fig. 1, top and middle rows) (14, 15). In fact, higher doses of resveratrol appeared to increase the percentage of GFP-positive (GFP+) cells in Jurkat and activated T cells. We hypothesized this apparent increase to be an artifact generated by resveratrol inhibiting the proliferation of uninfected and otherwise GFP-negative cells, whereas the proliferation of infected GFP-positive cells would already have been inhibited by HIV Vpr (54, 55). Resveratrol is known to inhibit lymphocyte proliferation (56). Confirming this idea, the dose-dependent increase in the percentage of GFP-positive cells correlated with a reduction in GFP-negative cell proliferation (see Fig. S1 in the supplemental material).
FIG 1.
Resveratrol inhibits single-round HIV-1 infection of resting CD4 T cells but not infection of activated CD4 T cells or Jurkat T cells. Cells of the Jurkat T cell line, primary CD4 T cells activated by PHA-L and IL-2, and resting CD4 T cells treated with IL-4 were infected with the HIV-1 GFP reporter virus G1ESI (115). Resveratrol was added immediately after infection. Flow cytometric analysis was performed at day 2 p.i. for activated CD4 T cells and Jurkat T cells and at day 5 p.i. for resting CD4 T cells. Data are representative of those from 3 independent experiments. The numbers in blue represent the percentage of GFP+ cells.
Next, we tested the effects of resveratrol in peripheral blood resting CD4 T cells. To improve the survival of the cells following infection, thus increasing the overall infection frequency (49), we treated the cells with interleukin-4 (IL-4), which, our prior studies indicated, maintains the resting phenotype, including small cell size, a lack of proliferation, and low levels of to no expression of activation markers, including CD25, CD38, CD62L, and CD69 (48, 57, 58). In contrast to the inability of resveratrol to inhibit productive HIV-1 infection of activated and transformed T cells, resveratrol completely prevented productive infection of resting CD4 T cells in a dose-dependent manner (Fig. 1, bottom row). Greater than 99% inhibition was found at 5 μM, and complete inhibition was achieved at ≥10 μM. This finding stands in contrast to that of a previous study which concluded that resveratrol by itself does not inhibit HIV-1 infection in resting T lymphocytes (14). These experiments were performed utilizing an HIV-1 strain that enters cells via interaction with CD4 and the coreceptor CXCR4 (59), which provides infection of most cell types substantially more efficient than that by strains utilizing CCR5 as a coreceptor. In vivo, however, most circulating strains utilize CCR5. We tested RES and PTE inhibition of infection of resting CD4 T cells via CCR5, finding 99.5% to 100% inhibition (data not shown), consistent with these compounds acting at a replication stage after virus entry.
Anti-HIV activity of related stilbenoids in resting CD4 T cells.
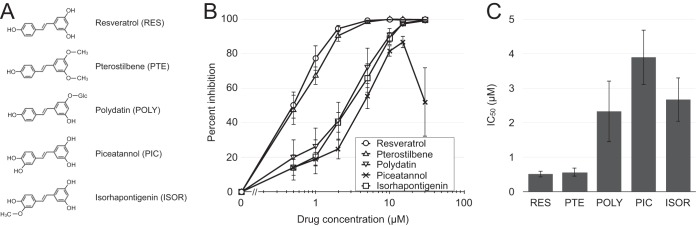
We next tested the anti-HIV-1 activity in resting T cells of four stilbenoids with structures similar to the structure of resveratrol: pterostilbene (PTE) (2); polydatin (POLY), also called piceid, a glucoside of resveratrol first isolated from the Chinese medicinal plant Polygonum cuspidatum (60); piceatannol (PIC), an analog of resveratrol with an additional hydroxyl group which, like resveratrol, is also found in red wine and grapes (61); and isorhapontigenin (ISOR), a metabolite of piceatannol (62) (Fig. 2A). Polydatin and isorhapontigenin completely inhibited productive infection at 30 μM, while piceatannol was somewhat less potent at equivalent concentrations, and its effectiveness also declined at concentrations above 10 μM (Fig. 2B and C). Pterostilbene, on the other hand, was as effective as resveratrol at all concentrations. None of these four stilbenoids inhibited infection of activated CD4 T cells or Jurkat cells at the doses tested (data not shown). No toxicity was observed at the concentrations used in the following studies (≤15 μM); in fact, all compounds conferred a slight increase in cell viability at these concentrations (Fig. S2). We chose pterostilbene and resveratrol for further study.
FIG 2.
Inhibition of resting T cell infection by five stilbenoids. (A) Molecular structures of the stilbenoids utilized in this study. (B) IL-4-treated resting CD4 T cells were infected with G1ESI and cultured in the presence of the indicated concentrations of resveratrol (RES), pterostilbene (PTE), polydatin (POLY), piceatannol (PIC), and isorhapontigenin (ISOR). Flow cytometric analysis was performed on day 5 p.i. Percent inhibition was calculated by dividing the number of GFP+ cells for each treatment by the number of GFP+ cells in the DMSO control treatment. (C) Mean IC50 calculated using nonlinear regression curve fit of the dose-response curve in GraphPad Prism software (version 5). (B and C) Data represent the means and standard deviations from 3 independent experiments using cells from different donors.
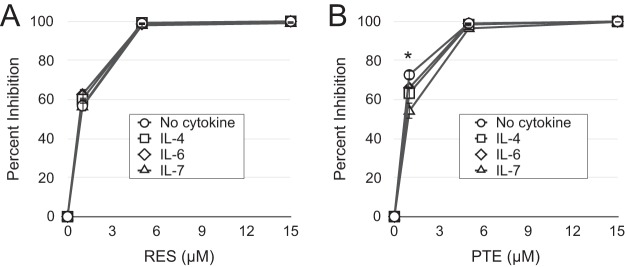
In this study, we treated the purified resting CD4 T cells with IL-4, which increases the yield of infected cells without inducing T cell activation (48, 63). Apart from IL-4, other cytokines, such as IL-6 and IL-7, have been shown to support the infection of resting CD4 T cells in vitro (48, 49, 63, 64). We have previously demonstrated that common gamma-chain cytokines, such as IL-4 and IL-7, increase HIV-1 infection of resting CD4 T cells by preventing the apoptosis that infection triggers rather than increasing the activation status of the cells (48, 57, 58) or the efficiency of infection itself (49). IL-4 enhances HIV-1 infection in lymphoid tissues and thus likely contributes to HIV-1 replication of resting T cells in vivo (65). We next examined the possibility that the antiviral effects of resveratrol and pterostilbene are influenced by the choice of cytokine used to enhance infection. We tested the resveratrol and pterostilbene dose-responses in the presence of IL-4, IL-6, IL-7, or no cytokine (Fig. 3). The various cytokines did not influence resveratrol's HIV-1 inhibition, whereas at the lowest dose of pterostilbene tested (1 μM), there was a statistically significant decrease in efficacy in the presence of each cytokine compared with that with no cytokine (IL-4 reduced the percent inhibition by 13% [P = 0.014], IL-6 reduced the percent inhibition by 9.2% [P = 0.03], IL-7 reduced the percent inhibition by 25.6% [P = 0.005]).
FIG 3.
Influence of cytokine treatment on the inhibition of HIV-1 infection by resveratrol and pterostilbene. Resveratrol (A) or pterostilbene (B) was added at the indicated concentrations immediately after infection of cells that had been incubated with no cytokine, IL-4, IL-6, or IL-7. Flow cytometry was performed on day 5 p.i. Percent inhibition was calculated by dividing the number of GFP+ cells for each treatment by the number of GFP+ cells in the DMSO control using the appropriate cytokine. There were no statistically significant differences in percent inhibition at each dose of stilbenoid with the exception of 1 μM pterostilbene. *, IL-4 reduced the percent inhibition by 13% (P = 0.014), IL-6 reduced the percent inhibition by 9.2% (P = 0.03), and IL-7 reduced the percent inhibition by 25.6% (P = 0.005). P values were calculated by unpaired Student's two-tailed t test. Data represent the means and standard deviations from 3 independent experiments using cells from different donors.
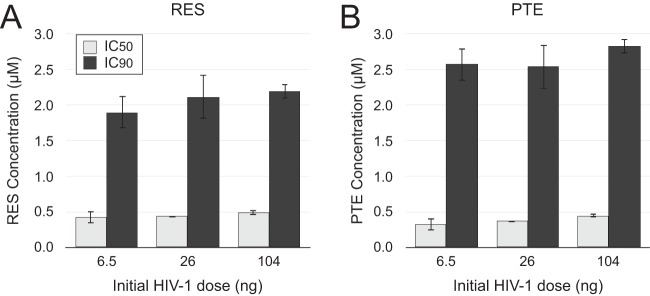
Next, we investigated whether the effectiveness of resveratrol and pterostilbene is influenced by the amount of virus infecting the cells. We infected resting CD4 T cells with 3 different doses of HIV-1 spanning a 16-fold difference in virus input, resulting in 1% to 33% productive infection in untreated control cells. The abilities of resveratrol and pterostilbene to inhibit infection were not altered by the dose of virus (Fig. 4).
FIG 4.
The anti-HIV activity of resveratrol and pterostilbene is independent of the initial dose of virus. IL-4-treated resting CD4 T cells were infected with the indicated amounts of HIV-1 (G1ESI) and treated with different doses of either resveratrol (RES) (A) or pterostilbene (PTE) (B). Cells were analyzed by flow cytometry at day 5 p.i. IC50s and IC90s were calculated using GraphPad Prism software (version 5), as described in Materials and Methods. Mean IC50 and IC90 values and standard deviations from 4 independent experiments using cells from different donors are shown. There were no statistically significant differences between the IC50 or IC90 of RES and PTE with different doses of virus, as determined by unpaired Student's two-tailed t test (P > 0.05). The percentage of GFP+ cells among cells receiving the DMSO control infections ranged from 1% for a 6.5-ng p24 dose to 33% for a 104-ng p24Gag dose across the 4 experiments.
Resveratrol and pterostilbene inhibit HIV-1 reverse transcription in resting CD4 T cells.
The anti-HIV properties of resveratrol have been attributed to its inhibition of ribonucleotide reductase and the resulting reduction in dNTP levels (14, 18, 20, 22, 23). The reduced dNTP levels are thought to allow nucleoside analog drugs to more effectively compete with endogenous nucleotides for incorporation into the nascent viral DNA during reverse transcription. We presumed that the already low dNTP levels in resting T cells allowed resveratrol and pterostilbene to reduce dNTP levels below the ability of reverse transcriptase to function. To test this notion, we examined the ability of HIV-1 to generate reverse transcripts in resting T cells in the presence of resveratrol or pterostilbene. We measured by quantitative PCR (qPCR) the amount of full-length HIV-1 DNA, the amount of integrated proviral DNA, and the amount of 2-LTR circles, an abortive component of the total full-length viral DNA that nevertheless may contribute to HIV-1 replication in resting T cells (48, 57). Treatment of infected cells with either stilbenoid resulted in a dramatic, dose-dependent reduction in all three types of HIV-1 DNA: compared to the results obtained with the dimethyl sulfoxide (DMSO)-treated control, 5 and 15 μM resveratrol reduced the amount of total full-length HIV DNA per cell by 85% (P = 0.0035) and 98% (P = 0.0032), respectively, whereas pterostilbene at 5 μM and 15 μM lowered the amount of full-length HIV-1 DNA per cell by 93% (P = 0.0034) and 98% (P = 0.0032), respectively (Fig. 5B); both resveratrol and pterostilbene at 5 μM reduced the number of 2-LTR circles by 97% (P = 0.0168 and 0.0174, respectively), and at a higher dose, both stilbenoids reduced the number of 2-LTR circles to below the detection limit of 1 copy per 1,000 cells; resveratrol and pterostilbene at concentrations of 5 μM and above were able to reduce the integrated provirus level to below the detection limit of our Alu PCR of 1 copy per 300 cells. The PCR results strongly correlated with the reduction in the number of GFP-expressing cells (Fig. 5A and B), which supports the concept that resveratrol and pterostilbene inhibited HIV-1 at the reverse transcription step.
FIG 5.
Resveratrol and pterostilbene inhibit reverse transcription in resting CD4 T cells. IL-4-treated CD4 T cells were infected with HIV-1 (G1ESI) and treated with the indicated concentrations of RES or PTE. (A) Flow cytometry dot plots of treated and untreated cells at day 5 p.i. The numbers in blue represent the percentage of GFP+ cells. (B) Cell samples collected on day 3 p.i. were analyzed by qPCR for total full-length, 2-LTR, and integrated HIV DNA. The means from triplicate PCRs and standard deviations are shown. Data are representative of those from 3 independent experiments using cells from different donors for total full-length and 2-LTR DNA and 2 independent experiments for integrated DNA. ND, none detected. The lower limit of detection was 1 copy of total full-length DNA per 1,000 cells and 1 copy of integrated DNA per 300 cells. **, P ≤ 0.05; ***, P ≤ 0.01. P values were determined by unpaired Student's two-tailed t test and were not available when DNA was undetectable. (C) RES and PTE halt reverse transcription following virus entry into cells at any time prior to completion. IL-4-treated CD4 T cells were infected with HIV-1, washed to remove free virus as in all experiments whose results have been presented, and treated with 5 μM RES or PTE immediately (day 0 p.i.) or at 1 or 2 days after infection. Cell samples collected on day 3 p.i. were analyzed by qPCR for late (full-length) HIV-1 DNA. Percent GFP+ cells for each treatment at day 5 p.i. was normalized against the percent GFP+ of the DMSO control. Means and standard deviations from 3 different independent experiments with cells from different donors are shown.
To further examine resveratrol and pterostilbene targeting of reverse transcription, we added each drug either immediately after infection, as in the assay whose results are presented in Fig. 1 to 5B, or 1 or 2 days after infection. Virus entry was complete prior to the day 1 dosing, while reverse transcription in resting CD4 T cells requires ≥2 days for completion (49), significantly slower than that in activated T cells and cell lines. We analyzed viral DNA 3 days after infection and GFP expression 5 days after infection (Fig. 5C). When added immediately after infection, each drug blocked the appearance of GFP+ cells by >99% and the production of full-length reverse transcripts by 94% (P = 0.001 versus DMSO-treated control cells) and 95% (P = 0.002), respectively, similar to the findings presented in Fig. 5A and B. However, when the stilbenes were added 1 day after infection, during which reverse transcription was partially completed or completed for only a subset of viruses, 70% inhibition of reverse transcription by resveratrol (P = 0.049) and 62.5% inhibition of reverse transcription by pterostilbene (P = 0.057) were observed. Each drug was ineffective at reducing HIV-1 DNA levels when it was added 2 days after infection, corresponding to the prior completion of reverse transcription. Interestingly, there was a 2-fold reduction in the number of GFP+ cells when the drugs were applied at 2 days postinfection (p.i.), and also a greater reduction in the number of GFP+ cells than the amount of viral DNA when they were applied at 1 day postinfection. This suggests an additional mechanism by which each drug can suppress HIV-1 replication. A prior study observed resveratrol inhibition of HIV-1 Tat-mediated transactivation of the viral promoter in transfected HeLa fibroblastic cells (25). Further studies will be required to determine if a similar mechanism operates in primary resting CD4 T cells infected with HIV-1. Alternatively, dNTP levels influence viral mutation rates (66), so it is possible that resveratrol and pterostilbene increase the generation of defective genomes incapable of proper gene expression.
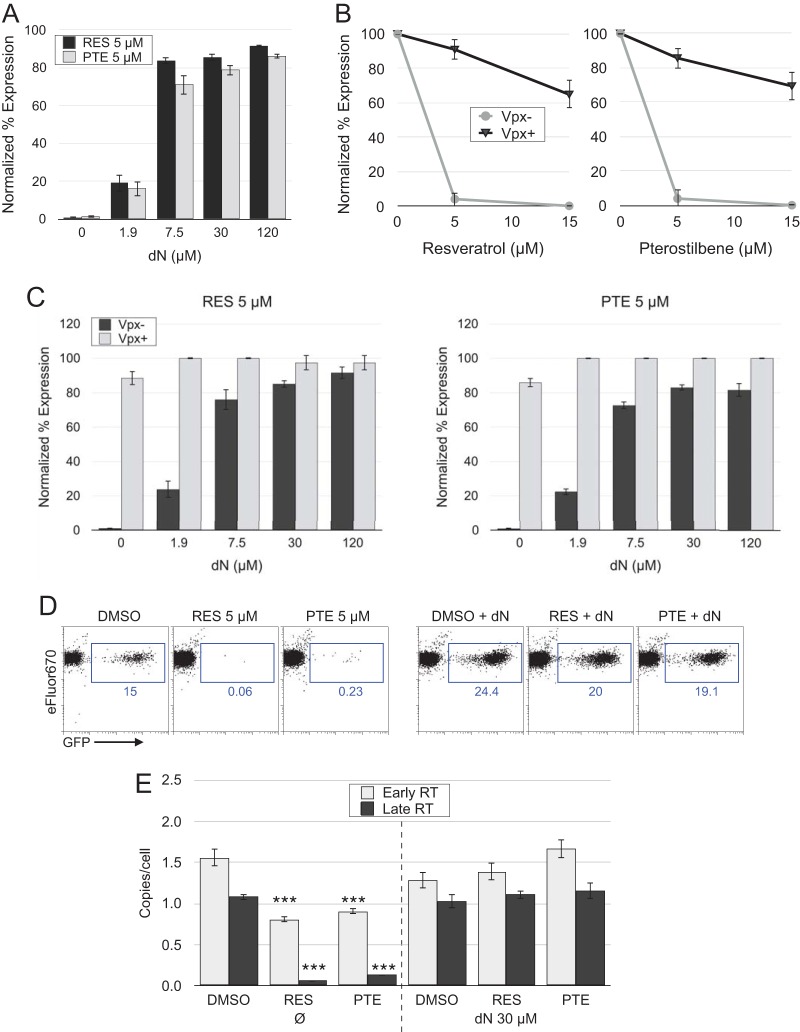
Resveratrol and pterostilbene inhibition of HIV-1 infection in resting CD4 T cells is reversed by exogenous dN or Vpx.
We sought to confirm that reduced dNTP levels were primarily responsible for the antiviral effects of resveratrol and pterostilbene. Addition of exogenous deoxynucleosides (dN) to resting T cells is known to increase the kinetics of reverse transcription and fosters productive infection (51, 67), so we applied exogenous dN to the cells at the time of infection to restore dNTP levels within the cells. Nucleosides reversed 91.6% and 86.2% of the inhibition by 5 μM resveratrol and pterostilbene, respectively (Fig. 6A). In another test, we delivered the SIVmac239 Vpx protein within virus-like particles (VLPs) 1 day prior to infection in order to increase dNTP levels. Vpx is a virion-incorporated protein found in both HIV-2 and several SIV strains that degrades the cellular protein SAMHD1, which would otherwise lower dNTP levels in resting T cells and dendritic cells (68). By countering SAMHD1 activity, Vpx enhances HIV and SIV replication in these cell types. Vpx reversed 65% and 69% of the 15 μM resveratrol and pterostilbene inhibition of infection, respectively (Fig. 6A and B). Importantly, the combination of Vpx and dN application completely reversed resveratrol and pterostilbene inhibition at all dN concentrations, including those that alone were only weakly effective (Fig. 6C).
FIG 6.
Exogenous deoxynucleosides (dN) and Vpx reverse resveratrol and pterostilbene inhibition of HIV-1 infection and reverse transcription in resting CD4 T cells. (A) IL-4-treated CD4 T cells were infected with HIV-1 and treated with resveratrol or pterostilbene plus the indicated dose of dN. P was ≤0.05 for 1.9 μM dN and P was ≤0.01 for 7.5 to 120 μM dN compared with the results for DMSO-treated control cells by unpaired Student's two-tailed t test. (B) Delivery of SIV Vpx protein in virus-like particles (VLPs) to IL-4-treated cells was performed 1 day before infection with HIV-1. Control cells received VLPs lacking Vpx. P was ≤0.01 for Vpx-positve (Vpx+) versus Vpx-negative (Vpx−) conditions by unpaired Student's two-tailed t test. (C) The combination of dN and Vpx completely reversed both resveratrol and pterostilbene inhibition of infection at each concentration of dN (the results were not statistically significantly different [P > 0.05] from those for no RES or PTE by unpaired Student's two-tailed t test). Cells were analyzed by flow cytometry at day 5 p.i. IL-4-treated CD4 T cells were infected with HIV-1 and treated with the indicated concentrations of RES or PTE without dN at 30 μM. (A to C) Data represent the means and standard deviations from three independent experiments using cells from different donors. (D) Flow cytometry of a separate experiment demonstrating the restoration of reverse transcription by dN. The numbers in blue represent the percentage of GFP+ cells. (E) Cells from the assay whose results are presented in panel D were collected on day 3 p.i. and were analyzed by qPCR for early and late (full-length) RT transcripts. Means from triplicate PCRs and standard deviations are shown. Data are representative of those from 2 independent experiments using cells from different donors. ***, P ≤ 0.01 versus DMSO-treated control cells by unpaired Student's two-tailed t test.
The generation of the HIV-1 proviral DNA from the virion-associated RNA template proceeds in an ordered process, with early short reverse transcripts appearing rapidly after infection or even prior to infection within virions. Thus, we anticipated that these early products would be less inhibited than longer RT products. Virus expression is possible only from completed reverse transcripts; thus, the expectation was that dN would restore these to normal levels in RES- or PTE-treated cells. We analyzed both early and late reverse transcripts by qPCR of cells treated with 5 μM resveratrol or pterostilbene in the presence or absence of dN. As expected, resveratrol and pterostilbene inhibited late transcripts (94% [P = 0.0002] and 87.5% [P = 0.0002] reductions, respectively) substantially more than early reverse transcripts (48% [P = 0.003] and 41.7% [P = 0.004] reductions, respectively) (Fig. 6E). The addition of dN restored GFP expression (Fig. 6D) and early and late transcript production in resveratrol- and pterostilbene-treated cells to at least the levels in untreated cells, consistent with the reduction in the levels of the intracellular dNTP stores by these drugs being the primary mechanism by which they inhibit HIV-1 infection of resting CD4 T cells. Intriguingly, deoxynucleosides enhanced HIV-1 expression, as measured by the number of GFP+ cells, by more than 60% (compared with that for DMSO-treated cells) without increasing the amount of full-length HIV-1 DNA. It is possible that, as noted above regarding the relationship between dNTP levels and mutation rates (66), addition of dN to cells reduces the generation of defective genomes. Testing of this concept awaits further study.
Resveratrol and pterostilbene do not increase proviral latency.
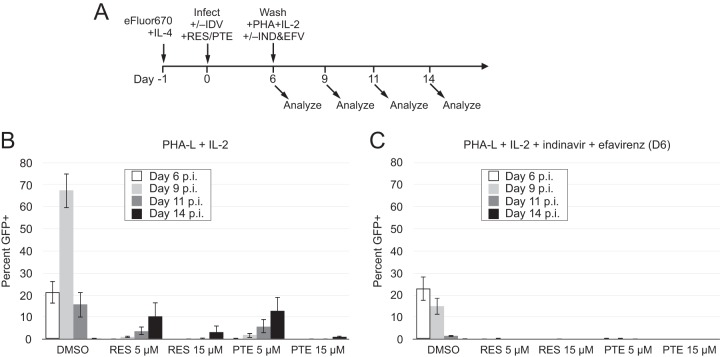
A prior study found that virus outgrowth could be achieved when a single 5 μM dose of resveratrol is applied to resting T cells at the time of infection and the cells are then washed and activated with phytohemagglutinin-L (PHA-L) for a further 8 days (14). We sought to determine if the virus outgrowth under these conditions resulted from activation of latent viruses or from de novo spread through the activated cells of any small amount of viruses that might have escaped the initial resveratrol treatment. To this end, we first repeated the conditions of that study, testing pterostilbene under these conditions as well (Fig. 7A). Similar to the findings of the prior study (14), there was an increase in productive infection over the 8 days after T cell activation that was nevertheless greatly diminished and delayed compared with the large and rapid burst of virus replication from cells not treated with a stilbenoid (Fig. 7B and S3A). Increasing the dose to 15 μM resveratrol or pterostilbene at the time of initial infection further decreased the subsequent expansion of the virus.
FIG 7.
The antiviral effect of resveratrol and pterostilbene is masked by de novo infection of activated CD4 T cells. (A) Scheme of infection and analysis. IL-4-treated CD4 T cells were infected with the replication-competent GFP reporter virus NLENG1-IRES (58) and were treated with RES and PTE immediately after infection. Following the protocol described previously (14), the infected cells were washed twice on day 6 p.i. and then activated with PHA-L and IL-2. (B) Cells were analyzed on the indicated days p.i. by flow cytometry. Representative flow cytometry data are shown in Fig. S3 in the supplemental material. (C) The protocol described in the legend to panel B was followed, except that indinavir (IDV) was added on the day of infection and again on day 6 p.i. and efavirenz (EFV) was added on day 6 p.i. to prevent de novo virus replication in the target cells. Data are representative of those from 3 independent experiments using cells from different donors.
In order to test whether the appearance of new GFP+ cells resulted from virus spread rather than activation of latent genomes, we prevented de novo virus transmission with the protease inhibitor indinavir and the nonnucleoside reverse transcriptase inhibitor (NNRTI) efavirenz, which were added at day 6 postinfection. Under these conditions, no expansion of GFP+ cells in the resveratrol- or pterostilbene-treated populations was seen, indicating that the increase in productive infection observed in Fig. 7B was not the result of latency reversal but, rather, was the result of de novo replication in activated T cells of the few viruses that escaped initial inhibition (Fig. 7C and S3B).
The effective concentrations of resveratrol and pterostilbene in resting T cells are lowered by TDF.
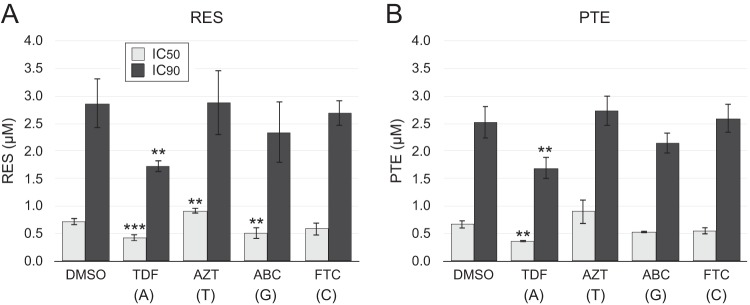
It has been demonstrated previously that resveratrol synergizes with the NRTI to inhibit HIV-1 infection (14, 18). Therefore, we investigated whether tenofovir disoproxil fumarate (TDF) or other NRTIs (AZT, FTC, and abacavir [ABC]) enhance the effectiveness of resveratrol and pterostilbene in resting CD4 T cells. We performed titrations of resveratrol or pterostilbene on infected resting CD4 T cells together in the background of a fixed concentration of each NRTI. The concentrations of NRTIs chosen provided ≤50% inhibition (Fig. S4) to reveal any synergistic interaction with the stilbenoids. The 50% inhibitory concentration (IC50) and IC90 values of each stilbenoid with and without an NRTI were calculated by nonlinear regression curve fitting after normalization of the antiviral effects of each NRTI as 0% inhibition. We observed that TDF, an analog of AMP, enhanced the antiviral activity of resveratrol by reducing the normalized IC50 by 41% (P = 0.003) and the normalized IC90 by 35.2% (P = 0.30) (Fig. 8A). ABC, a guanine analog, reduced the normalized IC50 of resveratrol by 29% (P = 0.047), but the reduction of the IC90 was not statistically significant. The cytosine analog FTC slightly reduced the IC50 and IC90 of resveratrol, but these reductions were not statistically significant (P > 0.05), while the addition of the thymidine analog AZT produced a small but nevertheless statistically significant increase in the normalized IC50 of resveratrol (26% increase; P = 0.014).
FIG 8.
Inhibition of HIV-1 infection with combinations of an NRTI and resveratrol or pterostilbene. HIV-1-infected resting CD4 T cells were treated with the indicated NRTI at a suboptimal dose and with resveratrol (A) or pterostilbene (B) at a range of concentrations. Resveratrol and pterostilbene were titrated in 3-fold dilutions from 15 μM to 0.06 μM. The effect of each NRTI on the IC50 and IC90 of each stilbenoid is presented. Tenofovir disoproxil fumarate (TDF) was used at 120 pM, zidovudine (AZT) was used at 18 μM, abacavir (ABC) was used at 16 nM, and emtricitabine (FTC) was used at 12 nM. The nucleoside corresponding to each NRTI is shown in parentheses. Mean IC50 and IC90 values and standard deviations derived from three independent experiments using cells from different donors are shown. ** and ***, P ≤ 0.05 and P ≤ 0.01, respectively, versus DMSO-treated control cells by unpaired Student's two-tailed t test. Raw data from 1 of 3 experiments are shown in Fig. S4.
DISCUSSION
Our study revisits the interaction between stilbenoids and HIV-1 to report their potent anti-HIV activity in primary resting CD4 T cells. This is the first study to demonstrate the nearly complete inhibition of HIV-1 infection by these compounds and to do so in a cell type that is the most abundant early target of infection in vivo. Both resveratrol and the related stilbenoid pterostilbene inhibited the generation of reverse transcripts following HIV-1 infection, consistent with the known ability of resveratrol to inhibit ribonucleotide reductase (20). This inhibition was completely reversed using exogenous deoxynucleosides and the SIV Vpx protein, confirming that these compounds acted via a reduction in dNTP levels. Host cell dNTPs are required for generation of the viral DNA by HIV-1 reverse transcriptase as well as for DNA repair during viral DNA integration (69, 70). Unlike activated T cells, which have high levels of dNTPs in preparation for cell replication, resting CD4 T cells maintain much lower dNTP levels (50, 68). As a result, HIV-1 infection of resting CD4 T cells is more vulnerable to disturbance of cellular dNTPs by pharmacologic means.
Resveratrol and, presumably, pterostilbene target cellular pathways other than the RNR pathway (3, 4, 71–77). Pterostilbene can inhibit HIV-1 integrase in vitro and partially inhibit HIV-1 infection of a transformed fibroblastic cell line, 293T (19, 78). Most prominent among the known resveratrol and pterostilbene targets is activation of Sirtuin 1 (SIRT1), an NAD+-dependent deacetylase (79). Resveratrol increases SIRT1 activity apparently through multiple mechanisms, including induction of SIRT1 transcription (72, 74, 80, 81). Sirtuins have been reported to have broad antiviral activity against viruses other than HIV but not via RNR inhibition (82). SIRT1 deacetylation of the HIV-1 transactivator Tat is required for its recycling back to the HIV-1 promoter after each cycle of transcriptional activation (83). Thus, inhibition or overactivation of SIRT1 could disturb this cycle, leading to inhibition of HIV-1 replication. Consistent with this, resveratrol inhibits HIV-1 expression in a HeLa cell line containing an integrated HIV-1 promoter-reporter module via the activation of SIRT1 (25).
The concentrations of resveratrol and pterostilbene used to achieve half-maximum inhibition of HIV-1 integration (78) and Tat transactivation (25) (47.5 μM and 50 μM, respectively) were up to 10 times higher than the concentration that we found completely inhibited HIV-1 in resting CD4 T cells (5 μM). Indeed, Fontecave et al. showed that 10 μM resveratrol can completely destroy the catalytic tyrosyl radical in a biochemical assay (20), and this concentration closely matched the IC90 of resveratrol in our model. These factors suggest that the anti-reverse transcription effect of the stilbenoids is potentially more important in resting T cells than SIRT activation or integrase inhibition. Indeed, the non-reverse transcription-targeting effects of resveratrol and pterostilbene were revealed only when the drugs were added at day 2 postinfection, after reverse transcription was largely completed.
In the body, most of the HIV-1 output during chronic infection comes from activated and proliferating CD4 T cells (84, 85); however, during acute SIV infection, it has been suggested that resting CD4 T cells provide the majority of virus output (37). Activated CD4 T cells are substantially more permissive to infection than resting CD4 T cells derived from peripheral blood. Hence the majority of studies on HIV-1 have been performed using primary activated CD4 T cells or transformed cell lines. On the other hand, the lentivirus genera of retroviruses are specifically adapted to replicate in nonproliferating cells, such as resting T cells and postmitotic macrophages. They achieve this through the presence of several genes that counteract antiviral components that are most active in resting or postmitotic cells, such as the HIV-2/SIV Vpx protein, which counteracts cellular SAMHD1 degradation of dNTPs (86, 87). Reduction of dNTP levels can be a strategy to inhibit retrovirus and DNA virus infection (50, 88). Importantly, for the present study, the HIV-1 reverse transcriptase has evolved to efficiently synthesize viral DNA at low dNTP levels and has a lower Km for dNTPs than the reverse transcriptase from oncoretroviruses (88). On the basis of the ability of resting T cells to be productively infected, SAMHD1 fails to lower dNTP levels to below those required for RT to function, though its kinetics are markedly decreased; thus, the combination of SAMHD1 and inhibition of RNR is required to lower the amount of dNTPs to a level that RT is unable to function at all. A recent paper on Vpx has suggested that Vpx additionally enhances HIV infection by an SAMHD1/dNTP-independent mechanism (89). While this additional mechanism may be operating in our system, the restoration of dNTP levels by Vpx would appear to be necessary to overcome resveratrol and pterostilbene inhibition.
Early studies utilizing quiescent peripheral blood CD4 T cells suggested that HIV-1 is unable to complete its replication cycle in resting T cells (90); however, later work has shown that while the kinetics of several events, such as reverse transcription and gene expression, are reduced in these cells, they do support productive infection (51). The great majority of HIV-1 replication does not occur in the blood but, rather, occurs in lymphoid and mucosal tissues, areas where cytokines, chemokines, and stromal cells provide support for infection of resting T cells (36, 48, 57, 63, 65, 91). Among these, both IL-4, which enhances HIV-1 replication in lymphoid tissues, and IL-7, which enhances HIV-1 replication in mucosal tissues, block HIV-1-induced apoptosis soon after infection of resting T cells (49). As stated above, much work has demonstrated that infection of resting CD4 T cells occurs early following transmission (27–29) as well as during all stages of HIV-1 infection (26). In particular, the ability of HIV-1 to replicate in nonproliferating host cells is vital to its successful spread from the genital mucosa to the rest of the body (29, 92). Interruption of resting CD4 T cell HIV-1 infection by resveratrol or pterostilbene at the time of exposure or soon thereafter might reduce the rate of successful transmission.
The development of topical microbicides (TM) to protect against sexual mucosal transmission is a priority in HIV research. In the absence of a vaccine, the promise of anti-HIV TM is to allow receptive sexual partners, especially women in developing countries, greater control over their protection from HIV and other sexually transmitted diseases (93, 94). A cervicovaginally applied gel has received the most attention thus far, and intravaginal rings and films dispensing antivirals over extended periods are also in development (95, 96). The formulations of TM remain a difficult problem owing to issues such as localized irritation and inflammation-promoting activities (97).
The CAPRISA 004 clinical trial of a cervicovaginally applied gel containing 1% tenofovir obtained 39% protection from heterosexual transmission but, encouragingly, 61% protection in the most adherent women (98). Inhibition of HSV-2 transmission was observed to be similar to that of HIV-1 protection in this trial, and a subsequent TFV TM trial (99). Additional studies failed to obtain protection against HIV-1 (94, 100), likely explained by poor adherence, while the recent VOICE trial obtained 14.5% protection, again, with suboptimal adherence (99). It might be useful to include within topical microbicide formulations agents that increase the potency of the primary antiviral compound and that also have antiviral activities of their own against HIV and other sexually transmitted viruses, such as HSV, that increase the transmission risk (101–103). Anti-inflammatory activity could additionally be useful to decrease localized inflammation and the attendant increased transmission risk. Resveratrol or pterostilbene might meet the requirements of an adjuvant to boost TM efficacy in combination with standard antiviral compounds, including NNRTIs, with which RES and PTE have additive or synergistic activity in cells, such as activated T cells. Indeed, in mice, topical resveratrol has been shown to block vaginal HSV-1 transmission (13) presumably via inhibition of ribonucleotide reductase (104). Both resveratrol and pterostilbene have anti-inflammatory properties (4, 105, 106), including decreased T cell activation (74) related to SIRT1 activation (75, 107). Promotion of T cell quiescence would both reduce the inherent susceptibility to infection and promote sensitivity to the infection blockade by RNR inhibition. An important consideration in TM use is to test RES and PTE in non-T cell types, such as macrophages, dendritic cells, and Langerhans cells, which are also early targets of immunodeficiency virus infection of mucosal tissues. Partial inhibition of macrophage infection has been reported, and the potential to synergize with NNRTIs will need to be evaluated in diverse cell types.
The manipulation of the cellular dNTP pool for anti-HIV purposes has been previously attempted using systemic hydroxyurea (50, 108); however, hydroxyurea can potentiate the toxicity of some NRTIs, such as didanosine, and caused serious systemic adverse effects in vivo (109). Both resveratrol and pterostilbene are sold as nutritional supplements, and no in vivo toxicity even at high doses has been observed in controlled studies (110–112). While pterostilbene is more bioavailable than resveratrol and has a longer half-life in vivo, topical use of resveratrol might avoid the problem of low bioavailability (113). The results of the studies presented here support the concept of using resveratrol or pterostilbene as an adjuvant to improve inhibition of resting CD4 T cell infection within a topical preexposure prophylaxis strategy.
MATERIALS AND METHODS
Viruses.
The Env-defective NLENG1-ES-IRES (G1ESI) and replication-competent NLENG1-IRES (G1I) HIV-1 GFP reporter viruses have been described previously (48, 57). These constructs utilize the HIV-1 NL4-3 backbone and its native CXCR4-tropic envelope protein. Virus-like particles (VLPs) delivering SIVmac239 Vpx to cells have also been described previously (49). Infectious viruses were generated by transfection of 293T cells with a plasmid expressing the reporter virus (G1I) or cotransfection with a plasmid expressing an env-defective reporter virus (G1ESI) with the plasmid expressing the CXCR4-tropic HIV-1 NL4-3 envelope, as described previously (48). Virus stocks were filtered through a 0.45-μm-pore-size filter and treated with Benzonase nuclease (25 U/ml; EMD Millipore Biosciences) to remove residual plasmid remaining from the transfections (48). Virus stock titers were routinely determined by TaqMan RT-qPCR for HIV-1 RNA and normalized as previously described (48).
Cells, reagents, and infections.
Peripheral blood mononuclear cells (PBMCs) were purified from HIV-1-negative adults and were purchased as deidentified buffy coats from the New York Blood Center. Cells of the Jurkat cell line (clone E6) were obtained from the American Type Culture Collection (ATCC). Isolation and culture of primary CD4 T cells, IL-4 treatment, eFluor670 staining, and infection by spinoculation have been described previously (48, 57). Infections of PHA-L (3 μg/ml; Sigma-Aldrich)-activated CD4 T cells and Jurkat cells were also performed by spinoculation but were performed for only 30 min instead of the 2 h used for resting T cells. For experiments utilizing Vpx VLPs, at 1 day prior to infection with G1ESI, resting CD4 T cells were spinoculated with VLPs delivering Vpx or VLPs devoid of Vpx for 2 h.
After spinoculation, infected cells were washed once and then plated at 40,000 cells per well in a 96-well plate. These cells were incubated with the treatment compounds for 5 days, and then GFP expression was analyzed by flow cytometry, unless otherwise stated. Resveratrol (RES) was obtained from Santa Cruz Biotechnology. Pterostilbene (PTE) was from Cayman Chemical. Polydatin (POLY) and piceatannol (PIC) were from Abcam. Isorhapontigenin (ISOR) and the four deoxynucleosides (dN) were from Sigma-Aldrich. All small-molecule drugs as well as dN were added immediately after spinoculation or at the time points indicated above and at the doses indicated in the figure legends. The HIV-1 protease inhibitor indinavir (IDV) was dissolved in water and used at 2 μM, while the NNRTI efavirenz (EFV) was dissolved in DMSO and used at 1 μg/ml. The nucleoside/nucleotide reverse transcriptase inhibitors zidovudine (AZT), tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and abacavir (ABC) were used at suboptimal concentrations (at or below the IC50s) of 18 μM, 120 pM, 16 nM, and 12 nM, respectively, in combination with resveratrol or pterostilbene to find potential synergistic interactions between the two families of compounds. All antiretroviral compounds were obtained from the NIH AIDS Research and Reference Reagent Program.
Flow cytometry and IC50 and IC90 analysis.
Flow cytometry was performed in a FACSort flow cytometer (Becton-Dickinson) upgraded to 3 lasers and 5 color channels as previously described (48). Flow cytometry data were analyzed using FlowJo software. Percent inhibition was calculated by dividing the percentage of GFP+ cells in each treatment group by the percentage of GFP+ cells in the appropriate DMSO control. All analyses were carried out on live cells using a forward and side scatter gate in FlowJo software. The IC50 was calculated using a nonlinear regression curve fit of the dose-response curves of each compound or treatment condition in GraphPad Prism software (version 5.0). The IC90 was determined using the IC50 and Hill slope factor from the curve fit in the online GraphPad calculator for effective concentration (http://www.graphpad.com/quickcalcs/Ecanything1/).
PCR quantification of HIV-1 DNA.
Cellular DNA extraction, early HIV reverse transcript PCR, late (full-length) HIV-RT transcript HIV PCR, 2-LTR HIV PCR, and integration Alu PCR were performed using the primers and TaqMan probes described previously (48, 57, 114). Briefly, cell DNA was extracted using a DNeasy blood and tissue kit (Qiagen). Total and 2-LTR HIV-1 DNA was quantified by qPCR using the USB VeriQuest probe qPCR master mix (Affymetrix). Integrated HIV-1 DNA was quantified by Alu PCR as described previously (48) using the QuantiTect Probe PCR master mix (Qiagen). Early viral reverse transcripts (R-U5 of the long terminal repeat [LTR]) were quantified using primers ZXF forward and AA55 reverse and the ZXF probe, while full-length viral DNA (LTR-gag) was quantified using primers ZXF forward and ZXF reverse and the ZXF probe (48, 57, 114).
Statistical analysis.
The statistical significance of the difference between different experimental conditions was calculated using the unpaired Student's t test (two-tailed) with an alpha value of 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants R01AI093998, R01AI078783A, and R56AI118453 to D.N.L.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00408-17.
REFERENCES
- 1.Baur JA, Sinclair DA. 2006. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. 2004. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J Agric Food Chem 52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 3.Paul S, Rimando AM, Lee HJ, Ji Y, Reddy BS, Suh N. 2009. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev Res (Phila) 2:650–657. doi: 10.1158/1940-6207.CAPR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikhil K, Sharan S, Palla SR, Sondhi SM, Peddinti RK, Roy P. 2015. Understanding the mode of action of a pterostilbene derivative as anti-inflammatory agent. Int Immunopharmacol 28:10–21. doi: 10.1016/j.intimp.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. 2011. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol 68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo SC, Ho PC, Lin HS. 2013. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol Nutr Food Res 57:1015–1025. doi: 10.1002/mnfr.201200651. [DOI] [PubMed] [Google Scholar]
- 7.Azzolini M, La Spina M, Mattarei A, Paradisi C, Zoratti M, Biasutto L. 2014. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol Nutr Food Res 58:2122–2132. doi: 10.1002/mnfr.201400244. [DOI] [PubMed] [Google Scholar]
- 8.Xiao J, Hogger P. 2015. Stability of dietary polyphenols under the cell culture conditions: avoiding erroneous conclusions. J Agric Food Chem 63:1547–1557. doi: 10.1021/jf505514d. [DOI] [PubMed] [Google Scholar]
- 9.Docherty JJ, Sweet TJ, Bailey E, Faith SA, Booth T. 2006. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res 72:171–177. doi: 10.1016/j.antiviral.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Campagna M, Rivas C. 2010. Antiviral activity of resveratrol. Biochem Soc Trans 38:50–53. doi: 10.1042/BST0380050. [DOI] [PubMed] [Google Scholar]
- 11.Abba Y, Hassim H, Hamzah H, Noordin MM. 2015. Antiviral activity of resveratrol against human and animal viruses. Adv Virol 2015:184241. doi: 10.1155/2015/184241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T, Li S, Zhang X, Pang X, Lin Q, Cao J. 2015. Resveratrol, sirtuins, and viruses. Rev Med Virol 25:431–445. doi: 10.1002/rmv.1858. [DOI] [PubMed] [Google Scholar]
- 13.Docherty JJ, Fu MM, Hah JM, Sweet TJ, Faith SA, Booth T. 2005. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res 67:155–162. doi: 10.1016/j.antiviral.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Heredia A, Davis C, Redfield R. 2000. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J Acquir Immune Defic Syndr 25:246–255. doi: 10.1097/00126334-200011010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Clouser CL, Chauhan J, Bess MA, van Oploo JL, Zhou D, Dimick-Gray S, Mansky LM, Patterson SE. 2012. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorg Med Chem Lett 22:6642–6646. doi: 10.1016/j.bmcl.2012.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Date AA, Destache CJ. 2016. Natural polyphenols: potential in the prevention of sexually transmitted viral infections. Drug Discov Today 21:333–341. doi: 10.1016/j.drudis.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Heredia A, Davis C, Amin MN, Le NM, Wainberg MA, Oliveira M, Deeks SG, Wang LX, Redfield RR. 2014. Targeting host nucleotide biosynthesis with resveratrol inhibits emtricitabine-resistant HIV-1. AIDS 28:317–323. doi: 10.1097/QAD.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heredia A, Davis CE, Reitz MS, Le NM, Wainberg MA, Foulke JS, Wang LX, Redfield RR. 2013. Targeting of the purine biosynthesis host cell pathway enhances the activity of tenofovir against sensitive and drug-resistant HIV-1. J Infect Dis 208:2085–2094. doi: 10.1093/infdis/jit395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pflieger A, Waffo Teguo P, Papastamoulis Y, Chaignepain S, Subra F, Munir S, Delelis O, Lesbats P, Calmels C, Andreola ML, Merillon JM, Auge-Gouillou C, Parissi V. 2013. Natural stilbenoids isolated from grapevine exhibiting inhibitory effects against HIV-1 integrase and eukaryote MOS1 transposase in vitro activities. PLoS One 8:e81184. doi: 10.1371/journal.pone.0081184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O. 1998. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett 421:277–279. doi: 10.1016/S0014-5793(97)01572-X. [DOI] [PubMed] [Google Scholar]
- 21.Aye Y, Li M, Long MJ, Weiss RS. 2015. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene 34:2011–2021. doi: 10.1038/onc.2014.155. [DOI] [PubMed] [Google Scholar]
- 22.Horvath Z, Saiko P, Illmer C, Madlener S, Hoechtl T, Bauer W, Erker T, Jaeger W, Fritzer-Szekeres M, Szekeres T. 2005. Synergistic action of resveratrol, an ingredient of wine, with Ara-C and tiazofurin in HL-60 human promyelocytic leukemia cells. Exp Hematol 33:329–335. doi: 10.1016/j.exphem.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Beach LB, Rawson JM, Kim B, Patterson SE, Mansky LM. 2014. Novel inhibitors of human immunodeficiency virus type 2 infectivity. J Gen Virol 95:2778–2783. doi: 10.1099/vir.0.069864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawson JM, Roth ME, Xie J, Daly MB, Clouser CL, Landman SR, Reilly CS, Bonnac L, Kim B, Patterson SE, Mansky LM. 2016. Synergistic reduction of HIV-1 infectivity by 5-azacytidine and inhibitors of ribonucleotide reductase. Bioorg Med Chem 24:2410–2422. doi: 10.1016/j.bmc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HS, Zhou Y, Wu MR, Zhou HS, Xu F. 2009. Resveratrol inhibited Tat-induced HIV-1 LTR transactivation via NAD(+)-dependent SIRT1 activity. Life Sci 85:484–489. doi: 10.1016/j.lfs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 27.Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. 2004. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A 101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura Y, Brown CR, Mattapallil JJ, Igarashi T, Buckler-White A, Lafont BA, Hirsch VM, Roederer M, Martin MA. 2005. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc Natl Acad Sci U S A 102:8000–8005. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152. [DOI] [PubMed] [Google Scholar]
- 30.Stieh DJ, Matias E, Xu H, Fought AJ, Blanchard JL, Marx PA, Veazey RS, Hope TJ. 2016. Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe 19:529–540. doi: 10.1016/j.chom.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol 74:5577–5586. doi: 10.1128/JVI.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK. 2002. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, Moore JP, Pope M, Shattock RJ. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med 199:1065–1075. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maher D, Wu X, Schacker T, Horbul J, Southern P. 2005. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci U S A 102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckstein DA, Penn ML, Korin YD, Scripture-Adams DD, Zack JA, Kreisberg JF, Roederer M, Sherman MP, Chin PS, Goldsmith MA. 2001. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity 15:671–682. doi: 10.1016/S1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- 37.Reilly C, Wietgrefe S, Sedgewick G, Haase A. 2007. Determination of simian immunodeficiency virus production by infected activated and resting cells. AIDS 21:163–168. doi: 10.1097/QAD.0b013e328012565b. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura Y, Sadjadpour R, Mattapallil JJ, Igarashi T, Lee W, Buckler-White A, Roederer M, Chun TW, Martin MA. 2009. High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc Natl Acad Sci U S A 106:8015–8020. doi: 10.1073/pnas.0903022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pahar B, Lala W, Kuebler D, Liu D. 2017. A significant productive in vivo infection of resting cells with simian immunodeficiency virus in a macaque with AIDS. J Med Primatol 46:59–62. doi: 10.1111/jmp.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, Theodosopoulos T, O'Doherty U. 2005. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol 79:14179–14188. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavez L, Calvanese V, Verdin E. 2015. HIV latency is established directly and early in both resting and activated primary CD4 T cells. PLoS Pathog 11:e1004955. doi: 10.1371/journal.ppat.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, Gardner MB, Miller CJ. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol 74:6087–6095. doi: 10.1128/JVI.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller CJ, Hu J. 1999. T cell-tropic simian immunodeficiency virus (SIV) and simian-human immunodeficiency viruses are readily transmitted by vaginal inoculation of rhesus macaques, and Langerhans' cells of the female genital tract are infected with SIV. J Infect Dis 179(Suppl 3):S413–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vatakis DN, Nixon CC, Zack JA. 2010. Quiescent T cells and HIV: an unresolved relationship. Immunol Res 48:110–121. doi: 10.1007/s12026-010-8171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan X, Baldauf HM, Keppler OT, Fackler OT. 2013. Restrictions to HIV-1 replication in resting CD4+ T lymphocytes. Cell Res 23:876–885. doi: 10.1038/cr.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai J, Agosto LM, Baytop C, Yu JJ, Pace MJ, Liszewski MK, O'Doherty U. 2009. Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. J Virol 83:4528–4537. doi: 10.1128/JVI.01910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trinité B, Ohlson EC, Voznesensky I, Rana SP, Chan CN, Mahajan S, Alster J, Burke SA, Wodarz D, Levy DN. 2013. An HIV-1 replication pathway utilizing reverse transcription products that fail to integrate. J Virol 87:12701–12720. doi: 10.1128/JVI.01939-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinité B, Chan CN, Lee CS, Levy DN. 2015. HIV-1 Vpr- and reverse transcription-induced apoptosis in resting peripheral blood CD4 T cells and protection by common gamma-chain cytokines. J Virol 90:904–916. doi: 10.1128/JVI.01770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao WY, Cara A, Gallo RC, Lori F. 1993. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A 90:8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Brien WA, Namazi A, Kalhor H, Mao SH, Zack JA, Chen IS. 1994. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J Virol 68:1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agosto LM, Yu JJ, Dai J, Kaletsky R, Monie D, O'Doherty U. 2007. HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology 368:60–72. doi: 10.1016/j.virol.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelderblom HC, Vatakis DN, Burke SA, Lawrie SD, Bristol GC, Levy DN. 2008. Viral complementation allows HIV-1 replication without integration. Retrovirology 5:60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy DN, Refaeli Y, Weiner DB. 1995. The vpr regulatory gene of HIV. Curr Top Microbiol Immunol 193:209–236. [DOI] [PubMed] [Google Scholar]
- 55.Jowett JB, Planelles V, Poon B, Shah NP, Chen ML, Chen IS. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol 69:6304–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao X, Xu YX, Janakiraman N, Chapman RA, Gautam SC. 2001. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol 62:1299–1308. doi: 10.1016/S0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 57.Chan CN, Trinité B, Lee CS, Mahajan S, Anand A, Wodarz D, Sabbaj S, Bansal A, Goepfert PA, Levy DN. 2016. HIV-1 latency and virus production from unintegrated genomes following direct infection of resting CD4 T cells. Retrovirology 13:1. doi: 10.1186/s12977-015-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trinité B, Chan CN, Lee CS, Mahajan S, Luo Y, Muesing MA, Folkvord JM, Pham M, Connick E, Levy DN. 2014. Suppression of Foxo1 activity and down-modulation of CD62L (L-selectin) in HIV-1 infected resting CD4 T cells. PLoS One 9:e110719. doi: 10.1371/journal.pone.0110719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du QH, Peng C, Zhang H. 2013. Polydatin: a review of pharmacology and pharmacokinetics. Pharm Biol 51:1347–1354. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 61.Seyed MA, Jantan I, Bukhari SN, Vijayaraghavan K. 2016. A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J Agric Food Chem 64:725–737. doi: 10.1021/acs.jafc.5b05993. [DOI] [PubMed] [Google Scholar]
- 62.Setoguchi Y, Oritani Y, Ito R, Inagaki H, Maruki-Uchida H, Ichiyanagi T, Ito T. 2014. Absorption and metabolism of piceatannol in rats. J Agric Food Chem 62:2541–2548. doi: 10.1021/jf404694y. [DOI] [PubMed] [Google Scholar]
- 63.Unutmaz D, KewalRamani VN, Marmon S, Littman DR. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med 189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris JH III, Nguyen T, Nwadike A, Geels ML, Kamp DL, Kim BR, Boyer JD, Shen A. 2017. Soluble factors secreted by endothelial cells allow for productive and latent HIV-1 infection in resting CD4+ T cells. AIDS Res Hum Retroviruses 33:110–120. doi: 10.1089/aid.2016.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moutsopoulos NM, Vazquez N, Greenwell-Wild T, Ecevit I, Horn J, Orenstein J, Wahl SM. 2006. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J Leukoc Biol 80:1145–1155. doi: 10.1189/jlb.0306142. [DOI] [PubMed] [Google Scholar]
- 66.Sanjuan R, Domingo-Calap P. 2016. Mechanisms of viral mutation. Cell Mol Life Sci 73:4433–4448. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plesa G, Dai J, Baytop C, Riley JL, June CH, O'Doherty U. 2007. Addition of deoxynucleosides enhances human immunodeficiency virus type 1 integration and 2LTR formation in resting CD4+ T cells. J Virol 81:13938–13942. doi: 10.1128/JVI.01745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med 18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skalka AM, Katz RA. 2005. Retroviral DNA integration and the DNA damage response. Cell Death Differ 12(Suppl 1):971–978. doi: 10.1038/sj.cdd.4401573. [DOI] [PubMed] [Google Scholar]
- 70.Sloan RD, Wainberg MA. 2011. The role of unintegrated DNA in HIV infection. Retrovirology 8:52. doi: 10.1186/1742-4690-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, Bode AM, Dong Z. 2008. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog 47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. 2012. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villalba JM, Alcain FJ. 2012. Sirtuin activators and inhibitors. Biofactors 38:349–359. doi: 10.1002/biof.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou T, Yang Y, Xia F, Huang A, Gao X, Fang D, Xiong S, Zhang J. 2013. Resveratrol inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS One 8:e75139. doi: 10.1371/journal.pone.0075139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gambini J, Ingles M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM, Gomez-Cabrera MC, Vina J, Borras C. 2015. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev 2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosuru R, Rai U, Prakash S, Singh A, Singh S. 2016. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur J Pharmacol 789:229–243. doi: 10.1016/j.ejphar.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 77.Pavan AR, Silva GD, Jornada DH, Chiba DE, Fernandes GF, Man Chin C, Dos Santos JL. 2016. Unraveling the anticancer effect of curcumin and resveratrol. Nutrients 8:E628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thierry S, Benleulmi MS, Sinzelle L, Thierry E, Calmels C, Chaignepain S, Waffo-Teguo P, Merillon JM, Budke B, Pasquet JM, Litvak S, Ciuffi A, Sung P, Connell P, Hauber I, Hauber J, Andreola ML, Delelis O, Parissi V. 2015. Dual and opposite effects of hRAD51 chemical modulation on HIV-1 integration. Chem Biol 22:712–723. doi: 10.1016/j.chembiol.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stunkel W, Campbell RM. 2011. Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen 16:1153–1169. doi: 10.1177/1087057111422103. [DOI] [PubMed] [Google Scholar]
- 80.Tennen RI, Michishita-Kioi E, Chua KF. 2012. Finding a target for resveratrol. Cell 148:387–389. doi: 10.1016/j.cell.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 81.Cheng Y, Di S, Fan C, Cai L, Gao C, Jiang P, Hu W, Ma Z, Jiang S, Dong Y, Li T, Wu G, Lv J, Yang Y. 2016. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis 21:905–916. doi: 10.1007/s10495-016-1258-x. [DOI] [PubMed] [Google Scholar]
- 82.Koyuncu E, Budayeva HG, Miteva YV, Ricci DP, Silhavy TJ, Shenk T, Cristea IM. 2014. Sirtuins are evolutionarily conserved viral restriction factors. mBio 5:e02249-14. doi: 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, Hruby H, Jung M, Verdin E, Ott M. 2005. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol 3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 85.Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, McCarter M, Mawhinney S, Campbell TB, Lie Y, Coakley E, Levy DN, Connick E. 2011. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J Virol 85:10189–10200. doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amie SM, Noble E, Kim B. 2013. Intracellular nucleotide levels and the control of retroviral infections. Virology 436:247–254. doi: 10.1016/j.virol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baldauf HM, Stegmann L, Schwarz SM, Ambiel I, Trotard M, Martin M, Burggraf M, Lenzi GM, Lejk H, Pan X, Fregoso OI, Lim ES, Abraham L, Nguyen LA, Rutsch F, Konig R, Kim B, Emerman M, Fackler OT, Keppler OT. 2017. Vpx overcomes a SAMHD1-independent block to HIV reverse transcription that is specific to resting CD4 T cells. Proc Natl Acad Sci U S A 114:2729–2734. doi: 10.1073/pnas.1613635114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zack JA, Haislip AM, Krogstad P, Chen IS. 1992. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol 66:1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Introini A, Vanpouille C, Lisco A, Grivel JC, Margolis L. 2013. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog 9:e1003148. doi: 10.1371/journal.ppat.1003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haase AT. 2011. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 93.Shattock RJ, Rosenberg Z. 2012. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med 2:a007385. doi: 10.1101/cshperspect.a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alexandre KB, Mufhandu HT, London GM, Chakauya E, Khati M. 2016. Progress and perspectives on HIV-1 microbicide development. Virology 497:69–80. doi: 10.1016/j.virol.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 95.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, Husnik MJ, Soto-Torres L, Nel A, Johnson S, Richardson-Harman N, Rabe LK, Dezzutti CS. 2015. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 70:242–249. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, et al. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rushton M. 2015. Microbicide development hinges on new products administered in new ways. IAVI Rep 19:4–9. [PubMed] [Google Scholar]
- 98.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, CAPRISA 004 Trial Group . 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, Team VS. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rees H, Delany-Moretlwe SA, Lombard C, Baron D, Panchia R, Myer L, Schwartz JL, Doncel GF, Gray G. 2015. FACTS 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women, abstr 26LB Abstr Conf Retroviruses Opportunistic Infect (CROI) 2015, Seattle, WA. [Google Scholar]
- 101.Corey L, Wald A, Celum CL, Quinn TC. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr 35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 102.Kaul R, Prodger J, Joag V, Shannon B, Yegorov S, Galiwango R, McKinnon L. 2015. Inflammation and HIV transmission in sub-Saharan Africa. Curr HIV/AIDS Rep 12:216–222. doi: 10.1007/s11904-015-0269-5. [DOI] [PubMed] [Google Scholar]
- 103.Passmore JA, Jaspan HB, Masson L. 2016. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS 11:156–162. doi: 10.1097/COH.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL. 1999. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res 43:145–155. doi: 10.1016/S0166-3542(99)00042-X. [DOI] [PubMed] [Google Scholar]
- 105.de la Lastra CA, Villegas I. 2005. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res 49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 106.Das S, Das DK. 2007. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 107.Estrela JM, Ortega A, Mena S, Rodriguez ML, Asensi M. 2013. Pterostilbene: biomedical applications. Crit Rev Clin Lab Sci 50:65–78. doi: 10.3109/10408363.2013.805182. [DOI] [PubMed] [Google Scholar]
- 108.Gao WY, Johns DG, Mitsuya H. 1994. Anti-human immunodeficiency virus type 1 activity of hydroxyurea in combination with 2′,3′-dideoxynucleosides. Mol Pharmacol 46:767–772. [PubMed] [Google Scholar]
- 109.Lisziewicz J, Foli A, Wainberg M, Lori F. 2003. Hydroxyurea in the treatment of HIV infection: clinical efficacy and safety concerns. Drug Saf 26:605–624. doi: 10.2165/00002018-200326090-00002. [DOI] [PubMed] [Google Scholar]
- 110.Ruiz MJ, Fernandez M, Pico Y, Manes J, Asensi M, Carda C, Asensio G, Estrela JM. 2009. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J Agric Food Chem 57:3180–3186. doi: 10.1021/jf803579e. [DOI] [PubMed] [Google Scholar]
- 111.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. 2010. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res 70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Riche DM, McEwen CL, Riche KD, Sherman JJ, Wofford MR, Deschamp D, Griswold M. 2013. Analysis of safety from a human clinical trial with pterostilbene. J Toxicol 2013:463595. doi: 10.1155/2013/463595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walle T. 2011. Bioavailability of resveratrol. Ann N Y Acad Sci 1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 114.Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, Birch CJ, Bell JE, Bannert N, Crawford K, Wang H, Schols D, De Clercq E, Kunstman K, Wolinsky SM, Gabuzda D. 2001. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol 75:10073–10089. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. 2004. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci U S A 101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.