Abstract
Background
Plantar heel pain can be managed with dry needling of myofascial trigger points (MTrPs); however, whether MTrP needling is effective remains controversial. Thus, we conducted this meta-analysis to evaluate the effect of MTrP needling in patients with plantar heel pain.
Materials and methods
PubMed, Embase, Web of Science, SinoMed (Chinese BioMedical Literature Service System, People’s Republic of China), and CNKI (National Knowledge Infrastructure, People’s Republic of China) databases were systematically reviewed for randomized controlled trials (RCTs) that assessed the effects of MTrP needling. Pooled weighted mean difference (WMD) with 95% CIs were calculated for change in visual analog scale (VAS) score, and pooled risk ratio (RR) with 95% CIs were calculated for success rate for pain and incidence of adverse events. A fixed-effects model or random-effects model was used to pool the estimates, depending on the heterogeneity among the included studies.
Results
Extensive literature search yielded 1,941 articles, of which only seven RCTs met the inclusion criteria and were included in this meta-analysis. The pooled results showed that MTrP needling significantly reduced the VAS score (WMD =−15.50, 95% CI: −19.48, −11.53; P<0.001) compared with control, but it had a similar success rate for pain with control (risk ratio [RR] =1.15, 95% CI: 0.87, 1.51; P=0.320). Moreover, MTrP needling was associated with a similar incidence of adverse events with control (RR =1.89, 95% CI: 0.38, 9.39; P=0.438).
Conclusion
MTrP needling effectively reduced the heel pain due to plantar fasciitis. However, considering the potential limitations in this study, more large-scale, adequately powered, good-quality placebo-controlled trials are needed to provide more trustworthy evidence in this area.
Keywords: plantar heel pain, myofascial trigger points, dry needling
Introduction
Plantar fasciitis is the most common cause of interior heel pain and affects approximately 10% of the general population.1 It is characterized by heel pain and tenderness centered on the medial tubercle of the calcaneus on weight bearing, especially immediately after rest, such as getting out of bed in the morning.2 Although plantar fasciitis is regarded as a self-limiting disease,3,4 it can take up to months or even years to recover.5
The etiology of plantar fasciitis is still unclear. Simons et al6 proposed that the presence of myofascial trigger points (MTrPs) within the plantar intrinsic foot musculature and muscles proximal to the foot might play an important role in plantar heel pain. MTrPs have been defined as hyperirritable points located in taut bands of skeletal muscle or fascia.7 The spot is tender when pressed and can give rise to characteristic referred pain, motor dysfunction, and autonomic phenomena.7
Current conventional treatments include rest, administration of nonsteroidal anti-inflammatory drugs, foot taping, physical therapy, stretch exercise, and steroid injection.8 Steroid injection is one of the most popular methods used in this condition, and it has been reported to be effective in the short term;8 however, it may lead to serious adverse events such as a recognized risk of subsequent plantar fascia rupture.9,10 Dry needling and acupuncture are increasingly being used to treat heel pain, as well as many other musculoskeletal pain conditions.11
Dry needling and acupuncture are alternative and less-invasive procedures, which stimulate the MTrPs.12,13 It has been reported that dry needling can alter the biochemical environment surrounding an MTrP and reduce spontaneous electrical activity within the MTrP regions of skeletal muscle.14,15 Moreover, this method is safe with minimal side effects when treating chronic pains. Several studies have assessed the effects of MTrP dry needling in the treatment of plantar heel pain.3,12,13 However, their results remain controversial. Therefore, we conducted this meta-analysis to assess the effects of MTrP dry needling in patients with heel pain due to plantar fasciitis.
Materials and methods
Literature search
We performed this meta-analysis in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement guidelines.16 Two independent investigators conducted a comprehensive literature review. PubMed, Embase, Web of Science, SinoMed (Chinese BioMedical Literature Service System, People’s Republic of China), and CNKI (National Knowledge Infrastructure, People’s Republic of China) were systematically searched to identify relevant articles up to March 12, 2017. The following structured search strategies were used: (Plantar[All Fields] AND (“heel”[MeSH Terms] OR “heel”[All Fields]) AND (“pain”[MeSH Terms] OR “pain”[All Fields])) AND (myofascial[All Fields] AND (“trigger points”[MeSH Terms] OR (“trigger”[All Fields] AND “points”[All Fields]) OR “trigger points”[All Fields])) AND ((dry[All Fields] AND needling[All Fields]) OR (“acupuncture”[MeSH Terms] OR “acupuncture”[All Fields] OR “acupuncture therapy”[MeSH Terms] OR (“acupuncture”[All Fields] AND “therapy”[All Fields]) OR “acupuncture therapy”[All Fields])). The search was limited to human subjects, and no language restriction was imposed. In addition, we also manually searched the reference lists of the included studies and previous reviews until no potentially eligible studies could be identified.
Study selection
We included full-text publications when the following inclusion criteria were met: 1) study design: randomized controlled trial (RCT); 2) population: adult patients aged 18 years or older who were diagnosed with plantar heel pain (plantar fasciitis); 3) intervention: dry needing/acupuncture of the MTrPs; 4) control: placebo or other treatment; and 5) outcome measure: changes in visual analog scale (VAS) score, success rate for pain, and adverse events. Studies were excluded if the needles in the active treatment group were inserted superficially over the site of an MTrP or into traditional acupuncture points. The rationale for this decision was based on the fact that the relationship between traditional acupuncture points and MTrP is unclear.17
Data extraction
We used a data extraction sheet to extract data from the included studies. The following information was extracted: first author’s name, year of publication, country, number of patients in each group, patient characteristics, methods used for randomization, concealment, blinding, and the main outcomes (changes in the VAS score, success rate for pain, and incidence of adverse events). We also contacted the corresponding author for further information when the data needed clarification or were not presented in their studies.
Risk of bias assessment and grading quality of evidence
We used the method recommended by the Cochrane Collaboration18 to assess the risk of bias in RCTs. This method consists of the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias.19 Each study was classified as of “high”, “unclear”, or “low” risk of bias according to these domains.19
The quality of evidence for outcome measures was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.20 The quality of each outcome was classified as very low, low, moderate, or high.20 A summary table was constructed using the GRADEprofiler (GRADEpro, version 3.6; http://www.gradeworkinggroup.org/).
Statistical analysis
The current meta-analysis was performed using Stata 12.0 (Stata Corporation, College Station, TX, USA). For continuous outcomes, they were expressed as weighted mean difference (WMD) with 95% CIs; for dichotomous outcomes, they were expressed as risk ratio (RR) with 95% CI. Before the data were pooled, we used the Cochrane Q chi-square test and I2 statistic to test the heterogeneity across studies, in which P-value <0.1 or I2>50% were considered to be significant.21 A random-effects model (DerSimonian–Laird method)22 was used to pool data when significant heterogeneity was identified; otherwise, a fixed-effects model (Mantel–Haenszel method)23 was used. We also conducted sensitivity analysis and subgroup analysis (based on different durations) to explore the potential sources of heterogeneity whenever significant heterogeneity was present. The presence of publication bias was assessed by the Begg24 and Egger’s tests.25 A two-tailed P–value <0.05 was considered statistically significant, except where a certain P-value had been specified.
Results
Search results
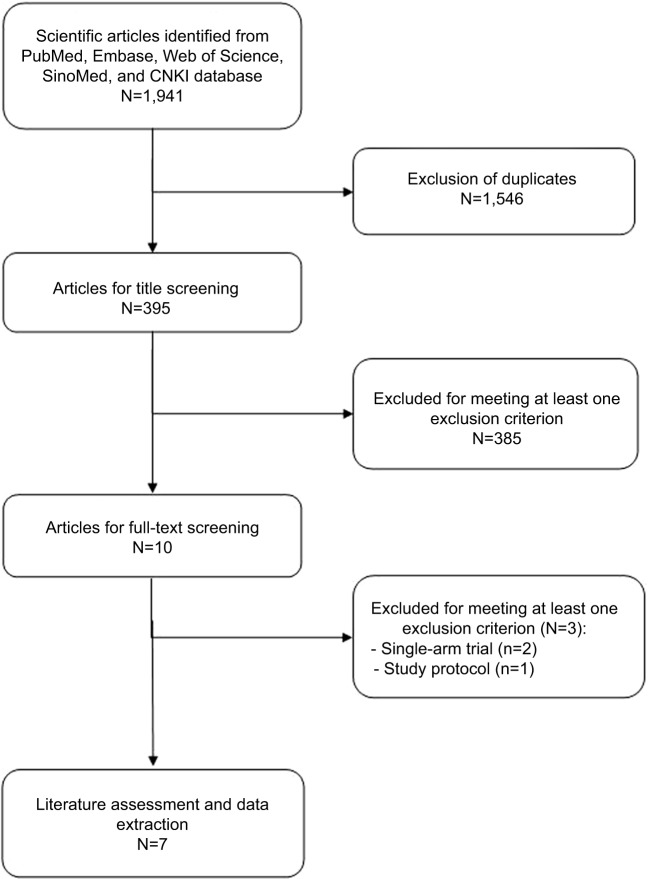
The initial search yielded 1,941 publications, of which 1,546 were excluded because of presence of duplicate records. Then, 385 were excluded because of various reasons (reviews, case series, non-RCTs, or not relevant with our topics) after the review of titles/abstract. Ten potential studies were identified for full-text information, but three were excluded because two were single-arm studies,26,27 and one was a protocol article.11 Finally, seven RCTs met the inclusion criteria and were included in this meta-analysis (Figure 1).28–34
Figure 1.
Eligibility of studies for inclusion in meta-analysis.
Abbreviation: CNKI, National Knowledge Infrastructure, People’s Republic of China.
Study characteristics
The main characteristics of the included studies are shown in Table 1. These studies were published between 2011 and 2017. The number of participants ranged from 20 to 108. Of the seven studies, four were conducted in People’s Republic of China,,29,31,33,34 one in Australia,28 one in Iran,30 and one in Thailand.32 Among the seven RCTs, five were published in English language,28–32 and two in Chinese language.33,34 The diagnosis of MTrP varied greatly among the included studies. Some used the palpation of “tender spot in a taut band” and “local twitch response”,29,33,34 while some others added “patient recognition of pain on sustained compression over the tender point” as a confirmatory finding.28,30–32 All RCTs used a VAS to measure the intensity of pain. The success for pain was defined as a minimum decrease of 50% in VAS scores.
Table 1.
Baseline characteristics of patients in the trials included in the meta-analysis
| First author, reference | Country | Treatment regimen | No of patients | Male/female patients | Age (mean ± SD, years) | Duration of heel pain (mean ± SD, months) |
|---|---|---|---|---|---|---|
| Cotchett MP28 | Australia | Real trigger point dry needling | 41 | 17/24 | 54.4±12.4 | 13.4±14.1 |
| Sham trigger point needling | 43 | 27/16 | 57.8±12 | 13.7±17.3 | ||
| Zhang SP29 | People’s Republic of China | Acupuncture (PC7) | 28 | 8/20 | 47±2.2 | 44.9±8.8 |
| Acupuncture (LI4) | 25 | 6/19 | 50±2.0 | 22.9±8.8 | ||
| Eftekharsadat B30 | Iran | Dry needling | 10 | 3/7 | 50.3±9 | NR |
| NR | 10 | 4/6 | 50.9±8.9 | NR | ||
| Li SM31 | People’s Republic of China | Miniscalpel–needle | 31 | 10/19 | 54.74±10.16 | 8.81±2.79 |
| Steroid injection | 30 | 7/25 | 56.93±9.25 | 9.8±2.94 | ||
| Kumnerddee M32 | Thailand | Acupuncture | 15 | 3/12 | 52.4±10.5 | 15.5±19.4 |
| Conventional treatment | 15 | 0/15 | 53.8±8 | 11.7±5.3 | ||
| Wang LF33 | People’s Republic of China | Warm needling plus | 54 | 32/22 | 52.21±5.02 | 6.04±0.95 |
| Chinese herb fumigation | 54 | 31/23 | 61.25±7.25 | 7.36±1.26 | ||
| Qian SH34 | People’s Republic of China | Warm needling plus | 31 | 14/17 | 36–72 | 1–36 |
| Chinese herb fumigation | 30 | 13/17 | 38–69 | 2–26 |
Abbreviation: NR, not reported.
Risk of bias and evidence of quality
The details of risk bias are presented in Figure 2. Overall, two RCTs31,32 were regarded as being at low risk of bias, three29,33,34 at unclear risk of bias, and two28,30 at high risk of bias. The main reasons for the two RCTs being at high risk of bias were that the studies were not blinded to the participants or outcome assessors. The main reasons for the three RCTs being at unclear risk of bias were that they did not report the methods used for randomized sequence or allocation sequence concealment adequately.
Figure 2.
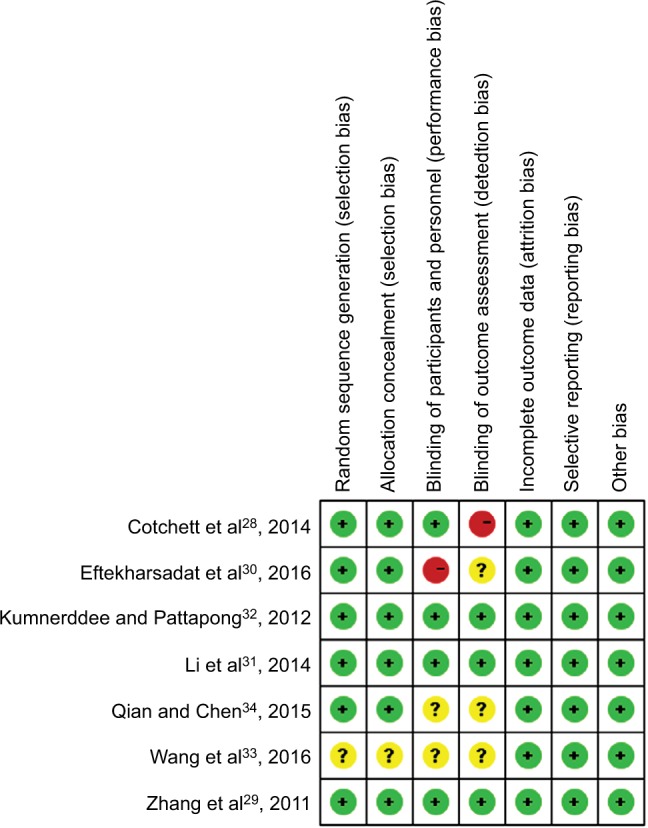
Risk of bias summary.
Notes: Red indicates high risk of bias, green indicates low risk of bias, and yellow indicates unclear risk of bias.
The GRADE evidence profiles for these outcomes are shown in Table 2. The quality of evidence was moderate for change in VAS scores and success rate for pain, but low for adverse events.
Table 2.
GRADE evidence profile
| Quality assessment
|
No of patients
|
Effect
|
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative (95% CI) | Absolute | ||||
| Changes in VAS scores (better indicated by lower values) | ||||||||||||
|
| ||||||||||||
| 7 | Randomized trials | Seriousa | Seriousb | No serious indirectness | No serious imprecision | Strong associationc | 210 | 207 | – | WMD-15.5 lower (−19.48 higher to −1 1.53 lower) |
Moderate | Critical |
| Success rate for pain | ||||||||||||
| 3 | Randomized trials | No serious risk of bias | Seriousd | No serious indirectness | No serious imprecision | None | 85/100 (85%) – |
71/99 (71.7%) 74.7% |
RR 1.15 (0.87–1.51) | 108 more per 1,000 (from 93 fewer to 366 more) 112 more per 1,000 (from 97 fewer to 38 1 more) |
Moderate | Important |
| Adverse events | ||||||||||||
| 3 | Randomized trials | Seriouse | Seriousf | No serious indirectness | No serious imprecision | None | 83/262 (31.7%) – |
18/140 (12.9%) 40% |
RR 1.89 (0.38 to 9.39) | 114 more per 1,000 (from 80 fewer to 1.000 more) 356 more per 1,000 (from 248 fewer to 1.000 more) |
Low | Important |
Notes:
Two trials were judged to be at high risk of bias.
Moderate heterogeneity (I2 =56.8%) was found.
A total of 417 patients were enrolled.
Substantial heterogeneity (I2=78.0%) was found.
One trial was judged to be at high risk of bias.
Substantial heterogeneity (I2 =85.0%) was found. ‘–’ indicates no data.
Abbreviations: GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio; WMD, weighted mean difference; VAS, visual analog scale.
Meta-analysis of outcome measures
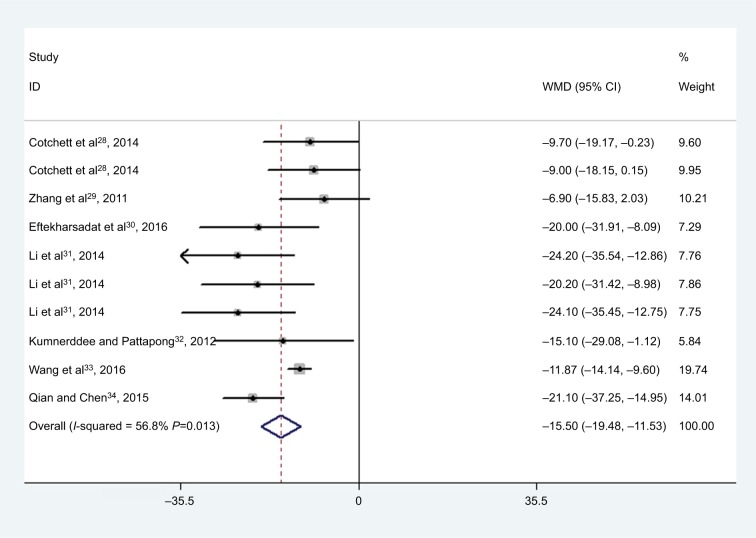
All the studies reported the data on changes in VAS scores.28–34 The aggregated results of these studies showed that MTrP needling was associated with a significantly greater reduction in VAS score (WMD =−15.50, 95% CI: −19.48, −11.53; P<0.001) (Figure 3). There was moderate heterogeneity among the included studies (I2=56.8%, P=0.013). Subgroup analysis was conducted based on different durations. At 1, 6, and 12 months, MTrP needling significantly reduced VAS score (at 1 month: WMD =−15.04, 95% CI: −20.14, −9.94; P<0.001; at 6 months: WMD =−18.20, 95% CI: −26.95, −9.45; P<0.001; at 12 months: WMD =−24.10, 95% CI: −35.45, −12.75; P<0.001).
Figure 3.
Forest plot showing the effect of MTrP needling on the VAS score.
Notes: Weights are from random-effects analysis. The multiple results of the same references are the results of subgroup population in the same reference.
Abbreviations: MTrP, myofascial trigger point; VAS, visual analog scale; WMD, weighted mean difference.
In the MTrP needling group, VAS scores for morning pain and active pain were significantly decreased compared with control (morning pain: WMD =−42.46, 95% CI: −58.79, −26.12; P<0.001; activity pain: WMD =−38.92, 95% CI: −55.16, −22.67; P<0.001).
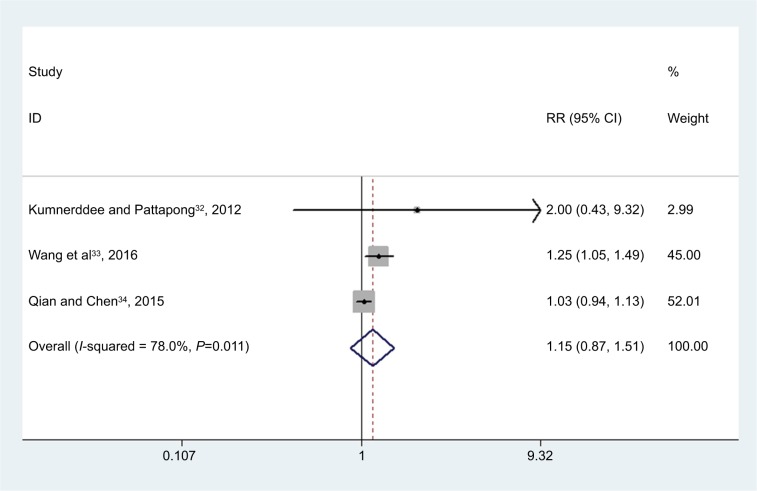
Three studies reported the success rate for pain.32–34 The aggregated results of these studies suggested that MTrP needling was associated with a success rate for treatment of pain comparable with that of the control (RR =1.15, 95% CI: 0.87, 1.51; P=0.320) (Figure 4).
Figure 4.
Forest plot showing the effect of MTrP needling on the success rate for treatment of pain.
Note: Weights are from random-effects analysis.
Abbreviations: MTrP, myofascial trigger point; RR, risk ratio.
Three studies reported the adverse events.28,29,32 These immediate adverse events were related to needle site pain and were transient in nature. The aggregated results of these studies suggested that the incidence of adverse events was similar between the MTrP needling and control groups (RR =1.89, 95% CI: 0.38, 9.39; P=0.438).
Publication bias
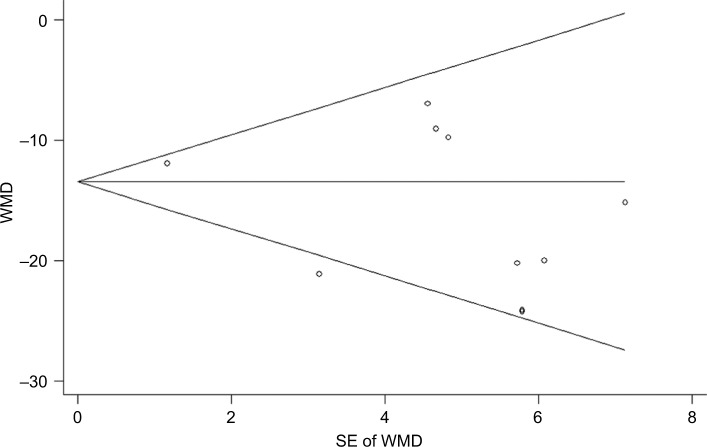
We performed the assessment of publication bias for VAS score using Egger’s and Begg’s tests. The results showed that there was no potential publication bias among the included studies (Egger’s test: P=0.169; Begg’s test: P=0.283) (Figure 5).
Figure 5.
Forest plot showing the test for publication bias for WMD of the changes in VAS score.
Note: Figure shows Begg’s funnel plot with pseudo 95% confidence limits.
Abbreviations: VAS, visual analog scale; WMD, weighted mean difference.
Discussion
The aim of this meta-analysis was to assess the effects of MTrP needling in the treatment of patients with plantar heel pain. The pooled estimates of all seven included trials using a random-effects model showed that MTrP needling significantly reduced the VAS score and was associated with a similar success rate for pain compared with control. Moreover, the improvements of pain relief were maintained throughout the 12-month follow-up, which indicated that this treatment offered long-term effectiveness. The incidence of adverse events was comparable between MTrP needling and control groups.
There have been three similar systematic reviews or meta-analyses35–37 that should be noted. Tough et al35 conducted a systematic review and meta-analysis of seven RCTs. Among these studies, four investigated patients with MTrP pain in the upper quadrant and three studied pain in the lower quadrant (lumbopelvic region).35 Meta-analysis of four studies using a placebo as control showed that needling did not appear to be significantly superior to placebo (standardized mean difference [SMD] =14.9, 95% CI: −5.81, 33.99).35 The authors concluded that dry needling had a comparable treatment effect as placebo on MTrP pain.35 However, this meta-analysis was conducted based on only four RCTs, and it suffered from limited sample size, poor quality, and substantial heterogeneity in the data pool, which precluded making definitive conclusions.35 Cotchett et al36 performed a systematic review of three quasi-experimental trials to assess the effectiveness of dry needling and/or injections for MTrPs associated with plantar heel pain. In that study, two trials found a reduction in pain for the use of MTrP dry needling combined with acupuncture, and the third trial observed a reduction in pain using 1% lidocaine injections in combination with physical therapy.36 However, because of the substantial heterogeneity and poor methodological quality of the three trials, the authors did not conduct the meta-analysis.36 In an updated systematic review conducted by Tough and White,37 the authors included six RCTs to investigate the effectiveness of acupuncture/dry needling for MTrP pain. In the four RCTs that were used to undertake a meta-analysis, the population group varied from patients with upper trapezius pain and young athletes with gluteal MTrP causing hamstring pain on the one hand to elderly patients with chronic neck pain and chronic low back pain on the other.37 Meta-analysis of the four RCTs showed that needling was statistically superior to placebo in reducing pain (WMD =16.67, 95% CI: 3.23, 30.11).37 However, the authors suggested that their results should be interpreted with caution since marked heterogeneity (I2=82.6%) was observed among the studies.37
Our meta-analysis showed that MTrP dry needling had beneficial effect in pain relief. Our results were consistent with the findings of several published studies. Tillu and Gupta26 evaluated the effect of a 4-week period of acupuncture, followed by 2 weeks of acupuncture combined with MTrP needling of the calf and heel regions.26 Their result showed significant reduction in plantar heel pain, as measured in a VAS.26 Perez-Millan and Foster38 investigated a 6-week period of MTrP needling combined with electroacupuncture of the heel and arch, and they found significant improvement in the plantar heel pain. Cotchett et al28 performed a parallel-group, participant-blinded, RCT to evaluate the effectiveness of dry needling for plantar heel pain. During the 6-week treatment, patients received the real or sham MTrP dry needling. They found that real dry needling was more effective than sham dry needling in pain reduction (adjusted mean difference, VAS pain score =−14.4, 95% CI: −23.5, −5.2), but real dry needling also resulted in a greater frequency of minor transitory adverse events than sham dry needling.28
The effect of MTrP needling could be explained by several mechanisms, although these mechanisms are largely derived from research involving traditional acupuncture. It has been proposed that dry needling could reduce the pain by affecting the biochemical environment and local blood flow surrounding an MTrP and, ultimately, the nervous system.28 Shah et al14 reported that the concentration of substance P and calcitonin gene-related peptide surrounding an MTrP was significantly decreased following the dry needling intervention. In an animal model, Hsieh et al39 found that a single dry needling procedure in the biceps femoris muscle significantly reduced the levels of substance P and increased the β-endorphin level in local tissue and serum, which indicated a short-term analgesic effect for dry needling. Cagnie et al40 found increased blood flow and oxygen saturation in the immediate vicinity of MTrPs for 15 minutes following a single dry needling intervention of the upper trapezius muscle. The increased blood flow to the region might remove the pain-inducing substances.14
Although the results of this study suggested that MTrP dry needing significantly reduced the foot pain beneath the heel, the inconvenience brought by it should not be neglected. It was noted from our study that dry needling resulted in minor adverse events (needle site pain or subcutaneous bleeding) and that the incidence of these events was similar to that in the control. In the study conducted by Cotchett et al,28 it was estimated that for every three patients treated with dry needling, one person will experience an immediate adverse event. Patients should be informed about the possibility of these relatively mild and transitory adverse events before the treatment, so that they can weigh the benefits and risks of dry needling.
This meta-analysis has several potential limitations that should be taken into account. First, we acknowledge that some of the included RCTs had a relatively small sample size (N<50). Small trials are more likely to result in an overestimated treatment effect compared with larger trials. Second, there was substantial heterogeneity among the included studies. However, this should not be surprising given the large variation in the treatment approaches, needling technique, diagnostic criteria for MTrP, and study design. These factors might increase the heterogeneity and have a potential impact on the treatment effect. Third, two of the included studies were classified as being at high risk of bias, which reduced the reliability of the original results.
Conclusion
This meta-analysis indicated that MTrP needling effectively reduced the heel pain due to plantar fasciitis. However, the findings should be interpreted with caution due to the limitations in terms of substantial heterogeneity, poor quality, and small sample size. More large-scale, adequately powered, good-quality placebo-controlled trials are needed to provide more trustworthy evidence in this area.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.DeMaio M, Paine R, Mangine RE, et al. Plantar fasciitis. Orthopedics. 1993;16:1153–1163. doi: 10.3928/0147-7447-19931001-13. [DOI] [PubMed] [Google Scholar]
- 2.Barrett SJ, O’Malley R. Plantar fasciitis and other causes of heel pain. Am Fam Physician. 1999;59(8):2200–2206. [PubMed] [Google Scholar]
- 3.Buchbinder R. Clinical practice. Plantar fasciitis. N Engl J Med. 2004;350:2159–2166. doi: 10.1056/NEJMcp032745. [DOI] [PubMed] [Google Scholar]
- 4.Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84(6):676–682. [PubMed] [Google Scholar]
- 5.Young CC, Rutherford DS, Niedfeldt MW. Treatment of plantar fasciitis. Am Fam Physician. 2001;63:467–474. [PubMed] [Google Scholar]
- 6.Simons D, Travell J, Simons L. Lower Half of the Body. 2nd ed. Vol. 2. Baltimore, MD: Williams & Wilkins; 1999. Myofascial pain and dysfunction: the trigger point manual. [Google Scholar]
- 7.Simons D, Travell J, Simons L. Upper Half of Body. Baltimore: Lippincott Williams & Wilkins; 1999. Travell & Simons’ myofascial pain and dysfunction The Trigger Point Manual; p. 1. [Google Scholar]
- 8.Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev. 2003;(3):Cd000416. doi: 10.1002/14651858.CD000416. [DOI] [PubMed] [Google Scholar]
- 9.Landorf KB, Menz HB. Plantar heel pain and fasciitis. BMJ Clin Evid. 2008;2:1111. [PMC free article] [PubMed] [Google Scholar]
- 10.Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19(2):91–97. doi: 10.1177/107110079801900207. [DOI] [PubMed] [Google Scholar]
- 11.Cotchett MP, Landorf KB, Munteanu SE, Raspovic A. Effectiveness of trigger point dry needling for plantar heel pain: study protocol for a randomised controlled trial. J Foot Ankle Res. 2011;4:5. doi: 10.1186/1757-1146-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh K, Katsumi Y, Hirota S, Kitakoji H. Randomised trial of trigger point acupuncture compared with other acupuncture for treatment of chronic neck pain. Complement Ther Med. 2007;15(3):172–179. doi: 10.1016/j.ctim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Hirota S, Katsumi Y, Ochi H, Kitakoji H. Trigger point acupuncture for treatment of knee osteoarthritis – a preliminary RCT for a pragmatic trial. Acupunct Med. 2008;26(1):17–26. doi: 10.1136/aim.26.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73(4):256–263. doi: 10.1097/00002060-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorsher PT. On the probability of trigger point-acupuncture point correspondences: an evidence-based rebuttal of Stephen Birch’s commentary. J Altern Complement Med. 2008;14:1183–1184. doi: 10.1089/acm.2008.0329. author reply 1184–1185. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Oxford: The Cochrane Collaboration; 2011. [Google Scholar]
- 19.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tillu A, Gupta S. Effect of acupuncture treatment on heel pain due to plantar fasciitis. Acupunct Med. 1998;16:66–68. [Google Scholar]
- 27.Imamura M, Fischer AA, Imamura ST, et al. Treatment of myofascial pain components in plantar fasciitis speeds up recovery: documentation by algometry. J Musculoskeletal Pain. 1998;6:91–110. [Google Scholar]
- 28.Cotchett MP, Munteanu SE, Landorf KB. Effectiveness of trigger point dry needling for plantar heel pain: a randomized controlled trial. Phys Ther. 2014;94(8):1083–1094. doi: 10.2522/ptj.20130255. [DOI] [PubMed] [Google Scholar]
- 29.Zhang SP, Yip TP, Li QS. Acupuncture treatment for plantar fasciitis: a randomized controlled trial with six months follow-up. Evid Based Complement Alternat Med. 2011;2011:154108. doi: 10.1093/ecam/nep186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eftekharsadat B, Babaei-Ghazani A, Zeinolabedinzadeh V. Dry needling in patients with chronic heel pain due to plantar fasciitis: a single-blinded randomized clinical trial. Med J Islam Repub Iran. 2016;30:401. [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Shen T, Liang Y, Zhang Y, Bai B. Miniscalpel-needle versus steroid injection for plantar fasciitis: a randomized controlled trial with a 12-month follow-up. Evid Based Complement Alternat Med. 2014;2014:164714. doi: 10.1155/2014/164714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumnerddee W, Pattapong N. Efficacy of electro-acupuncture in chronic plantar fasciitis: a randomized controlled trial. Am J Chin Med. 2012;40(6):1167–1176. doi: 10.1142/S0192415X12500863. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Guo J, Lin F, et al. Efficacy of warm needling plus Chinese Herb Fumigation in chronic plantar fasciitis: a randomized controlled trial. Modern J Integr Trad Chin West Med. 2016;25:416–417. [Google Scholar]
- 34.Qian S, Chen L. Efficacy of warm needling plus Chinese Herb Fumigation in patients with chronic heel pain due to plantar fasciitis: a randomized controlled trial. Shanghai J Acu Mox. 2015;34:362–363. [Google Scholar]
- 35.Tough EA, White AR, Cummings TM, Richards SH, Campbell JL. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain. 2009;13(1):3–10. doi: 10.1016/j.ejpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Cotchett MP, Landorf KB, Munteanu SE. Effectiveness of dry needling and injections of myofascial trigger points associated with plantar heel pain: a systematic review. J Foot Ankle Res. 2010;3:18. doi: 10.1186/1757-1146-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tough EA, White AR. Effectiveness of acupuncture/dry needling for myofascial trigger point pain. Phys Ther Rev. 2011;16:147–154. [Google Scholar]
- 38.Perez-Millan R, Foster L. Low frequency electroacupuncture in the management of refractory plantar fasciitis. Med Acupunct. 2011;13:1–6. [Google Scholar]
- 39.Hsieh YL, Yang SA, Yang CC, et al. Dry needling at myofascial trigger spots of rabbit skeletal muscles modulates the biochemicals associated with pain, inflammation, and hypoxia. Evid Based Complement Alternat Med. 2012;2012:342165. doi: 10.1155/2012/342165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cagnie B, Barbe T, De Ridder E, Van Oosterwijck J, Cools A, Danneels L. The influence of dry needling of the trapezius muscle on muscle blood flow and oxygenation. J Manipulative Physiol Ther. 2012;35(9):685–691. doi: 10.1016/j.jmpt.2012.10.005. [DOI] [PubMed] [Google Scholar]