Abstract
Objective
To compare bladder sensitivity between pelvic pain and pain-free patients undergoing noninvasive, controlled bladder distension via diuresis. We also sought to measure potential mechanisms underlying bladder sensitivity.
Design
prospective observational study
Setting
community teaching hospital
Population
Reproductive-age women with non-bladder chronic pelvic pain (CPP, n=23), painful bladder syndrome (PBS, n=23) and pelvic pain-free controls (n=42)
Methods
Participants were compared on cystometric capacity, pelvic floor pressure-pain thresholds (PPTs), pelvic muscle function, O’Leary-Sant bladder questionnaire, and psychosocial instruments using Wilcoxon rank-sum tests. Multivariate regression was used to identify factors underlying bladder pain phenotypes.
Main outcome measures
self-reported bladder distension pain, pelvic floor pain thresholds
Results
Participants with PBS exhibited higher bladder distension pain than those with CPP, with both groups reporting pain higher than controls (p’s <0.05). No significant associations were found between bladder distension pain and pelvic muscle structure or pain sensitivity measures. However, bladder distension pain positively correlates with both vaginal PPTs adjacent to the bladder (r=0.46), and pain with transvaginal bladder palpation (r=0.56). Pain at maximal distension was less influenced by somatic sensitivity than bladder symptoms (r=0.35 vs. r=0.59, p<0.05). Multivariate regression identified three independent components of bladder symptoms in PBS: bladder distension pain, bladder sensation, and somatic symptoms.
Conclusions
Diuresis-induced bladder pain differentiates CPP from PBS. Experimental bladder pain is not predicted by pelvic floor sensitivity. Compared to patient-reported outcomes it appears less influenced by psychological factors. Further study is needed to determine screening for experimental bladder pain sensitivity could predict future risk of PBS.
Keywords: Pelvic Pain, Painful Bladder Syndrome, Pressure Pain Threshold, Quantitative Sensory Testing
Tweetable abstract
Controlled, water ingestion-provoked bladder pain can objectively identify visceral pain sensitivity
Introduction
Painful bladder syndrome/interstitial cystitis (PBS) is a poorly understood chronic pain state arising in part from an amalgam of disrupted peripheral and central pain regulatory circuits. Since few treatments are consistently effective, preventative strategies are urgently needed. A well-recognized finding in PBS is increased bladder pain with bladder filling.1 Although self-report of distress in PBS appears to reflect both urgency/frequency (International Continence Society [ICS] terms this increased bladder sensation) and pain, the physiological basis for these dual contributions is not precisely known.2 In a preliminary study, during a standardized cystometry task, PBS patients report prolonged and more intense discomfort compared to healthy controls.3
The induction of pain at low filling volumes in patients with PBS parallels findings showing that many irritable bowel syndrome patients also report pain at lower distension pressures during anal manometry.4 In functional bowel disorders, standard assays of visceral hyperalgesia are well-recognized research tools and have been used to characterize the relevant nerve pathways and molecular underpinnings of these symptoms.5,6 Targeting the bladder for visceral pain testing is limited by discomfort from urethral catheterization and infectious risk. Validating more comfortable, non-invasive tests could enhance research participation.
The present study extends our prior studies of non-invasive bladder distension in studying menstrual pain and cross-organ visceral sensitivity in otherwise healthy controls, with the objective of determining if bladder distension pain differs between CPP and PBS patients (vs. healthy controls).7 Since patients with PBS have widespread reduced pain tolerance and report more somatic symptoms, we also explored secondarily if non-specific factors and psychological distress might affect experimental bladder pain testing.1,8 In particular, we specifically assessed whether pelvic floor sensitivity predicts bladder distension pain, as pelvic physical therapy reduces bladder pain symptoms, but the underlying mechanism for this efficacy is unknown.9
Methods
Overview
This study was a planned analysis for one aim of an overall study of pelvic floor function and bladder pain sensitivity. We prospectively recruited chronic pelvic pain (CPP) and PBS patients, as well as pain-free controls prospectively for a cross-sectional study at Evanston Hospital (Evanston, Illinois) between July 2010 and September 2013 from nearby clinics and community advertisements.
Participants
CPP was defined as pain lasting three months or longer in the area between the umbilicus and inguinal ligament. Symptoms could not solely be perceived on the skin, only involve the hip or back, or only occur with menses. PBS patients were defined by International Continence Society (ICS) criteria: complaint of pelvic pain related to bladder function, accompanied by other symptoms such as increased daytime and nighttime frequency, in the absence of proven urinary infection or other obvious pathology reported urgency or frequency symptoms,2 Controls were pelvic pain-free, PBS cohort age-matched patients (±5 years) and recruited from the same population. Cases were limited to ages 18–55 years old. Exclusion criteria included: pregnancy, active urogenital infection, prior urogenital malignancy, unexplained hematuria, active nephro/ureterolithiasis, vaginal prolapse exceeding second degree, and unwillingness to avoid short-acting opioids prior to examination. All participants received modest stipends. The NorthShore University Health System Institutional Review Board approved the study, and all participants gave informed consent.
Study procedure
All examinations and tests were performed in a research examination room. Participants were asked to complete a screen visit and two assessment visits.
Screen Visit
All participants signed consent before any study procedures were executed. A complete abdominopelvic examination was performed by the primary author. The exam included asking participants to rate pain from palpation at multiple sites using a 0–10 rating scale (0, no pain, to 10, worst imaginable pain). Vaginal tissue compliance, voluntary pelvic floor contractility, and pelvic floor gross muscle strength were quantified on exam using Likert scales (for more details see Appendix S1). Participants also completed the O’Leary-Sant Interstitial Cystitis Symptom (ICSI) and Problem Indices (ICPI) and University of Wisconsin (UW) Interstitial Cystitis questionnaires of bladder function.10,11 They also completed Patient Reported Outcomes Measurement Information System (PROMIS) computer adaptive tests for anxiety and depression.12 A somatic symptom score was derived as the total score of non-bladder symptoms (dizziness, chest pain, nausea, feelings of suffocation, and tingling in fingers and toes) each rated on a 0–6 Likert scale from selected UW reference scale questions, similar to those on the Brief Symptom Inventory assessing somatization.11
Assessment Visit 1
On the first assessment visit, external and internal pressure-pain thresholds (PPT) and bladder testing were performed using our prior published standardized protocols.13 The order of PPT and bladder testing was randomized, except for the PBS participants, who all underwent bladder filling first. For pressure-pain threshold assessment, we first tested the four external sites (shoulder, forehead, hip and knee), applying pressure at a rate between 0.5 and 1.0 kg/cm2/s using a pain pressure algometer with a 1 cm2 circular cap. The same approach was next applied to test four transvaginal pelvic floor sites (right and left iliococcygeus, anterior bladder, and posterior anorectal raphe) using a specially designed, fingertip-mounted algometer. Averaged thresholds from two trials were used for the final analysis. Extended details are presented in Appendix S1.
Participants were asked to hydrate with 12 ounces of water one hour before the visit and abstain from caffeine the day of testing. After an initial void, participants had baseline volume measurement of the bladder while supine. Bladder volume was measured using a Voluson 730 three-dimensional transabdominal 5.0 MHz ultrasound transducer (GE Healthcare, Wauwatosa, WI). Measurement of bladder volumes was performed using the scanner’s onboard Virtual Organ Computer-aided Analysis (VOCAL™) software with the transducer oriented sagittally above the symphysis pubis. Volume was calculated from the perimeter measurements of six serial plane sections separated by 30°. Validation and reliability of this method by our research team has been previously published.7
Following the initial scan, participants were asked to drink 20 ounces of water within 5 minutes to further encourage diuresis. While participating they were offered light reading and were asked not to do other distracting tasks such as making phone calls or engaging in regular conversation. Participants were asked to report awareness upon reaching three levels of bladder urgency: first sensation, first desire to void, and maximal capacity. At each of these three thresholds, we measured bladder volume and then asked the participant to rate their level of bladder pain and urgency (10 cm visual analogue scale [VAS]). The urgency scale was anchored at opposite ends with the descriptors “no urgency” and “worst urgency imaginable.” Similarly, the pain scale was anchored at opposite ends with the descriptors “no pain” and “worst pain imaginable.” Additionally, every 15 minutes from the time that participants finished drinking the priming dose of water, they were instructed to evaluate their current level of pain and urgency using the same VAS measures. If a participant did not reach maximum capacity by 45 and 60 minutes, she was asked to drink an additional ten ounces of water (maximum of twenty additional ounces) to encourage diuresis. The bladder testing was capped at two hours. Bladder filling rates were estimated by calculating the change in volumes estimated at each cystometric threshold, divided by the elapsed time.
Assessment Visit 2
This visit was conducted approximately one month after assessment visit 1. All participants underwent internal and external pressure testing and completed a similar battery of questionnaires assessing pain levels and mental health as was collected during the screen visit.
Study Size
The study aim when the study was initially funded targeted a primary hypothesis that bladder sensation and pelvic floor sensitivity are positively associated. Prior published data suggested a Cohen’s d effect size of 1.1 for this association.1,3,14 A power analysis estimated that to achieve that effect size with 80% power, we would need 38 CPP and/or PBS patients (28 enhanced bladder sensation, and 10 with normal bladder sensation) to significantly resolve a group difference (p<0.05). An additional aim was to assess if there were differences in self-reported bladder sensation between pain groups and pain-free controls.
Statistical Analysis
For this paper we addressed three primary pre-defined contrasts: between diagnostic groups we compared bladder pain at maximum capacity and a time series variable capturing overall change in bladder pain during the experimental bladder task. We also compared average pelvic floor pressure pain thresholds between pain patients exhibiting lower vs. higher first sensation thresholds (≥100 mL cutoff).14 We had complete case data for all bladder testing and accompanying ratings. Based on Shapiro-Wilk determinations of normality of variables, group differences were evaluated with Wilcoxon rank-sum tests (followed by post-hoc Dunn’s tests with Holm-Sidak corrections for multiple comparisons), repeated measures ANOVA on the ranks, or chi-squared tests with STATA 13.0 (College Station, TX). Relationships between bladder distension pain, somatic pain sensitivity, the ICSI and other candidate contributing factors (pelvic floor tone, strength, flexibility and voluntary control; anxiety; depression; and somatic symptoms) were analyzed with Spearman rank-order correlation. Significant differences between correlation coefficients were verified with Fisher-to-z transforms. To verify whether bladder distension pain or other factors were independently related to bladder pain phenotypes, we performed multivariate linear regression and determined receiver operating characteristic curves.
Results
Demographic Profile
As expected, the PBS group reported higher bladder distress on both the UW and ICSI bladder-specific measures compared to pain-free controls (p’s<0.01, Table 1). Women with CPP had intermediate UW and ICSI scores that were significantly higher than healthy controls, but lower than participants with PBS (p<0.05). Diary data supported that both CPP and PBS patients had more voids per day compared to healthy controls (p<0.05). Consistent with prior published findings, both pain groups had significant duration of ongoing symptoms, significant rates of comorbid diagnoses (IBS, endometriosis, fibromyalgia, abuse history), and heightened levels of depression and anxiety.
Table 1.
Sociodemographic and medical history profiles between controls and women with chronic pelvic pain (CPP) or painful bladder syndrome (PBS).
| Variable | Controls n=42 |
CPP n=23 |
p for CPP vs Control |
PBS n=23 |
p for PBS vs Control |
p for PBS vs CPP |
|---|---|---|---|---|---|---|
| Age | 32 (23–44) | 35 (29–44) | .279 | 31 (27–39) | .694 | .185 |
| Weight (lb) | 146 (127–192) | 170 (140–199) | .244 | 145 (128–183) | .814 | .390 |
| Married/Committed | 20/42 (48%) | 15/23 (65%) | .519 | 14/23 (61%) | .31 | .999 |
| Caucasian | 30/41 (73%) | 15/23 (65%) | .999 | 21/23 (91%) | .219 | .096 |
| Parous | 12/42 (29%) | 10/23 (43%) | .684 | 11/23 (48%) | .369 | .999 |
| UW Bladder | 0 (0–2) | 11 (4.5–17) | .001 | 22 (19–31)* | .001 | .025 |
| UW Reference | 2 (0–5) | 6.5 (3–19) | .001 | 21 (4–30.5) | .001 | .468 |
| ICSI | 2 (1–3) | 7 (3–9) | .001 | 13 (10–14)* | .001 | .007 |
| ICPI | 0 (0–0) | 4 (2–10) | .001 | 11 (9–13)* | .001 | .007 |
| Voids per day | 7 (5–8) | 9 (6–12) | .030 | 10 (7–14) | .011 | .702 |
| History of abuse | 6/42 (14%) | 12/23 (52%) | .003 | 15/23 (65%) | .003 | .999 |
| Dysmenorrhea | 15/35 (43%) | 14/15 (93%) | .003 | 18/19 (95%) | .003 | .999 |
| Endometriosis | 0/42 (0%) | 5/23 (22%) | .003 | 8/23 (35%) | .003 | .972 |
| Fibromyalgia | 0/42 (0%) | 3/23 (13%) | .033 | 2/23 (9%) | .117 | .999 |
| Irritable bowel syndrome | 1/42 (2%) | 3/23 (13%) | .285 | 9/23 (39%) | .003 | .112 |
| Pelvic pain (VAS 10 cm) | 0 (0–0) | 4.1 (2.7–6.5) | .001 | 6.2 (3.4–7.2) | .001 | .554 |
| Days in bed (last 3 months) | - | 4 (0–30) | - | 5 (0–20) | - | .423 |
| Pain duration (years) | - | 3 (2–18) | - | 3.6 (1.4–9.5) | - | .151 |
| CES-D score | 2 (0–4) | 13 (3–21) | .001 | 15 (8–23) | .001 | .191 |
| STAI score | 27 (24–32) | 38 (29–48) | .001 | 43 (36–50) | .001 | .118 |
| SF12 Physical Component | 56 (56–59) | 42 (31– 51) | .001 | 37 (27–47) | .001 | .339 |
| SF12 Mental Component | 55 (49–57) | 46 (36–55) | .001 | 42 (35–51) | .001 | .578 |
Data are specified as median (interquartile range), or as a proportion (percent) for each group. Significance of exploratory analysis differences pain groups vs. pain free groups using a Dunn test with a Holm-Sidak correction or a chi square with a Bonferroni correction.
differences between pain groups p < 0.05. As an exploratory analysis for potential group differences, reported significance should not be construed as formal hypothesis tests.
UW – University of Wisconsin Symptom Scale, ICSI/ICPI – Interstitial Cystitis Symptom Index/Problem Index, CESD - Center for Epidemiologic Studies Depression survey, STAI -State-Trait Anxiety Inventory, VAS – Visual Analog Scale, SF12- Short Form 12 Health Survey.
Bladder Testing Flow Rate
An important consideration in replacing pain measurements obtained with retrograde bladder filling with natural diuresis is that different flow rates could affect sensation or pain report. To determine the impact of natural variation in flow rate on perception we analyzed empirical differences of average flow on sensation and pain. There were no significant differences in flow rate between groups (Healthy: 6.6 [5.6–8.0] ml/min, CPP: 6.6 [5.4–9.0]. PBS: 6.0 [5.0 – 6.8] mL/min, p=0.45). However, there was a significant positive correlation between flow rate and maximum tolerance bladder volume (r=0.73, p<0.001). This is potentially due to the fact that women with low bladder capacity drink less to improve tolerability.15 Also flow rates increased over time. Across all participants, there was a significant increase in flow rate from first sensation (4.3 [2.5–6.6] mL/min) to first urge (6.9 [5.3–9.2], p<0.001) and again from first urge to maximum tolerance (8.7 [7.0–10.8], p<0.001). To account for any potential confounding, we examined tolerances across the groups with a general linear model accounting for flow rate. In the general linear model, there was a significant effect of rate on maximum tolerance (p<0.001), but women with PBS still had a 112 [44 – 181] ml lower volume at maximal capacity compared to control women or women with CPP (p<0.001).
Controlled bladder filling elicits greater pain in PBS
Women with PBS reached all cystometric thresholds (first sensation, first desire to void, and maximal capacity) at significantly lower volumes and time-to-threshold than healthy controls (Fig 1A, Table S1;p’s<0.05). The mean bladder volume and time-to-threshold was significantly lower in PBS compared to the CPP group at maximal capacity only. Volume and time-to-threshold at each cystometric threshold did not differ between CPP and healthy controls. However, women with CPP reported more pain than healthy controls at all sensory thresholds (Fig 1B, Table S1;p’s<0.05). Furthermore, women with PBS had higher bladder pain than those with CPP at first sensation and first desire to void (Fig 1B, Table S1;p’s<0.05).
Figure 1.
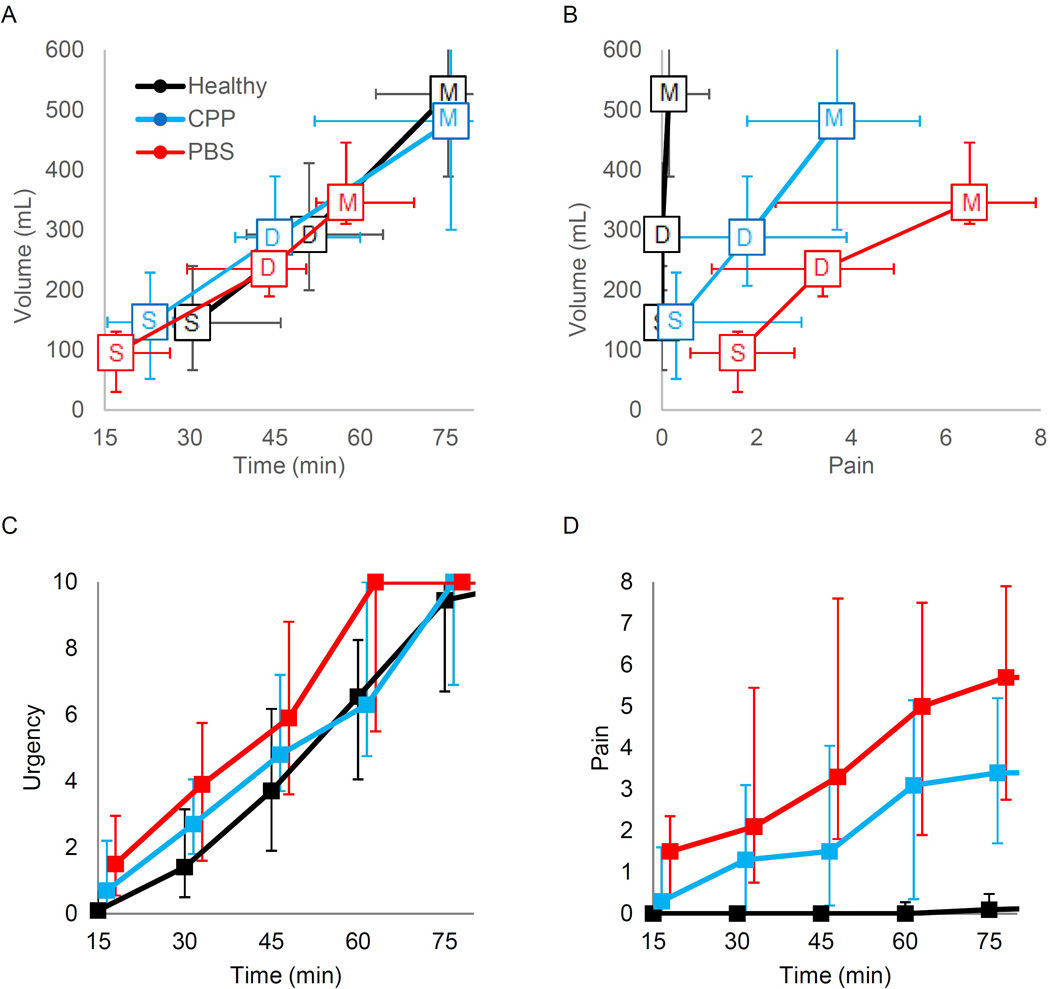
Time course of provoked diuresis and evoked bladder pain by pain diagnostic category. Panel A: The median volume and time are shown for first sensation (S), first desire (D), and maximal capacity (M) for each of the 3 groups with error bars indicating 25–75th percentiles. Panel B shows the relationship between volume and pain. C and D show evolution of reported bladder and urgency (VAS 0–10) obtained every 15 minutes regardless of cystometric thresholds.
Longitudinal report of bladder urgency and pain ratings are shown in Fig 1C–D, with a significant effect of both time and group observed (p’s<0.001). Nested group×time interactions indicated that women with CPP have significant increasing pain over time compared to healthy participants, and PBS participants have worsening pain over time compared to CPP participants (p’s<0.001).
Potential factors influencing bladder volume tolerance and pain report
In our prior work, full data was not available to evaluate potential predictors of either evoked bladder pain or bladder volume sensitivity. We found some positive associations between pelvic floor mechanical sensitivity and evoked bladder pain report. Pressure pain threshold under the bladder and pain at first sensation was inversely correlated (Table 2, r=−0.46; p<0.01). Pain evoked by clinical bladder exam was also correlated to bladder distension pain at all cystometric thresholds (r=0.51–0.56; p<0.01). All other PPTs (transvaginal or external), as well as pelvic floor anatomy and functional assessments (Table S2) were not associated with bladder distension pain. For predictors of volume sensitivity, we had one planned comparison of average pelvic floor PPT for only women with pain, to determine if mechanical sensitivity was higher in women with volume sensitivity. No difference in pressure thresholds were observed (high volume sensitive < 100 mL first sensation (1.19 kg/cm2 [1.00–1.44];n=21 vs. low volume sensitivity (1.00 kg/cm2 [0.85–1.23], n=25, p = 0.13). Likewise, no significant associations were seen between any volume sensitivity threshold with any PPTs. As with pain, pelvic floor tone, strength, flexibility and voluntary control did not predict volume sensitivity (Table S2). Interestingly, prolonged duration of pain report (aftersensation) following mechanical pressure testing (Table 2) correlated with both bladder distension pain (r=0.50–0.55) and first desire (0.39) and maximal capacity volumes (0.40, all p < 0.05).
Table 2.
Spearman correlations of factors affecting bladder sensitivity, volume, and the Interstitial Cystitis Symptom Index (ICSI).
| Bladder Pain | Bladder Volume | ICSI | |||||
|---|---|---|---|---|---|---|---|
| First sensation |
First desire |
Maximal capacity |
First sensation |
First desire |
Maximal capacity |
||
| ICSI | 0.54* | 0.53* | 0.56* | −0.28 | −0.34 | −0.44* | |
| Pressure Sensitivity | |||||||
| Right PPT | −0.30 | −0.18 | −0.19 | 0.00 | −0.02 | 0.03 | −0.21 |
| Left PPT | −0.26 | −0.15 | −0.16 | 0.14 | 0.06 | 0.09 | −0.17 |
| Bowel PPT | −0.23 | −0.16 | −0.21 | 0.02 | −0.05 | 0.01 | −0.27 |
| Bladder PPT | −0.46* | −0.32 | −0.37 | 0.07 | 0.03 | 0.06 | −0.32 |
| Body PPT | −0.18 | −0.18 | −0.13 | 0.15 | 0.13 | 0.09 | −0.10 |
| Pain Report | |||||||
| Aftersensation | 0.55* | 0.50* | 0.51* | −0.32 | −0.39* | −0.40* | 0.49* |
| Bladder Palpation | 0.56* | 0.51* | 0.55* | −0.01 | −0.10 | −0.17 | 0.60* |
| Psychological | |||||||
| Somatic Symptoms | 0.40* | 0.36 | 0.35 | −0.13 | −0.13 | −0.18 | 0.59* |
| Depression | 0.48* | 0.43* | 0.42* | −0.14 | −0.14 | −0.17 | 0.49* |
| Anxiety | 0.40* | 0.35 | 0.37 | −0.13 | −0.21 | −0.19 | 0.50* |
designates p<0.05 after Holm-Sidak corrections for multiple comparisons.
PPT – pelvic floor pain thresholds
Psychological factors have more impact on bladder symptom reporting than bladder distension pain
Finally, we examined the associations of psychological factors and bladder distension pain or volume sensitivity (Table 2). At first sensation, moderate positive correlations were observed between bladder distension pain, somatic symptom reporting, depression, and anxiety (0.4–0.48 p<0.05), while depression correlated with bladder pain similarly at first urge and maximal capacity. We did not observe significant associations between volume sensitivity and psychological factors, just as we found with mechanical sensitivity and pelvic anatomy factors (Table S2).
Associations between physiological constructs, psychological profiles and disease-specific outcomes measures
We also characterized how the combination of functional bladder pain, capacity and psychosomatic factors influences bladder specific symptom reporting in PBS. Maximal distension pain (r=0.56), maximal capacity volume (r=−.44), palpation evoked bladder pain (r=0.60) and somatic symptoms (r=.59) were associated with clinical bladder symptomatology (ICSI, Table 2). Interestingly, this general somatic symptom report association with bladder symptom report, tis stronger than its relationship with cystometric measures of sensitivity (maximal distension pain r=0.35 and capacity r=−0.18, respectively, p<0.05 Fisher to Z transform). We next explored the distribution of these variables for each group, to better understand the weight of bladder factors vs. somatic symptoms. We plotted the summed standardized values for these three variables for each individual participant adjusting for the general prevalence of CPP and PBS (Fig 2). Although significant bladder distension pain was rare in the general population, half of patients with CPP had bladder distension pain, but not necessarily accompanied by filling sensitivity. In patients with PBS, 19/23 had summed standardized scores for bladder pain, bladder sensation, and somatic symptoms that exceeded 2 standard deviations from median values of a general population. However, there was seemingly little relationship between the amount of bladder pain or enhanced bladder sensation and somatic symptoms among patients with PBS. Multivariate linear regression further identifies that maximal capacity pain, bladder sensitivity, and somatic symptom reporting are each independent factors contributing to ICSI scores (Table S3;R2=0.54, p<0.001). A receiver operating characteristics curve showed that the three factor model generated an area under the curve (AUC) of 0.90 for identifying participants with an ICSI score ≥6, the threshold for PBS diagnosis suggested in the original ICSI validation paper (Fig 2).10 The three factor model was superior to a bladder factors-only (AUC=0.83, p<0.05) or a somatic complaint-only model (AUC=0.74, p<0.01).
Figure 2.
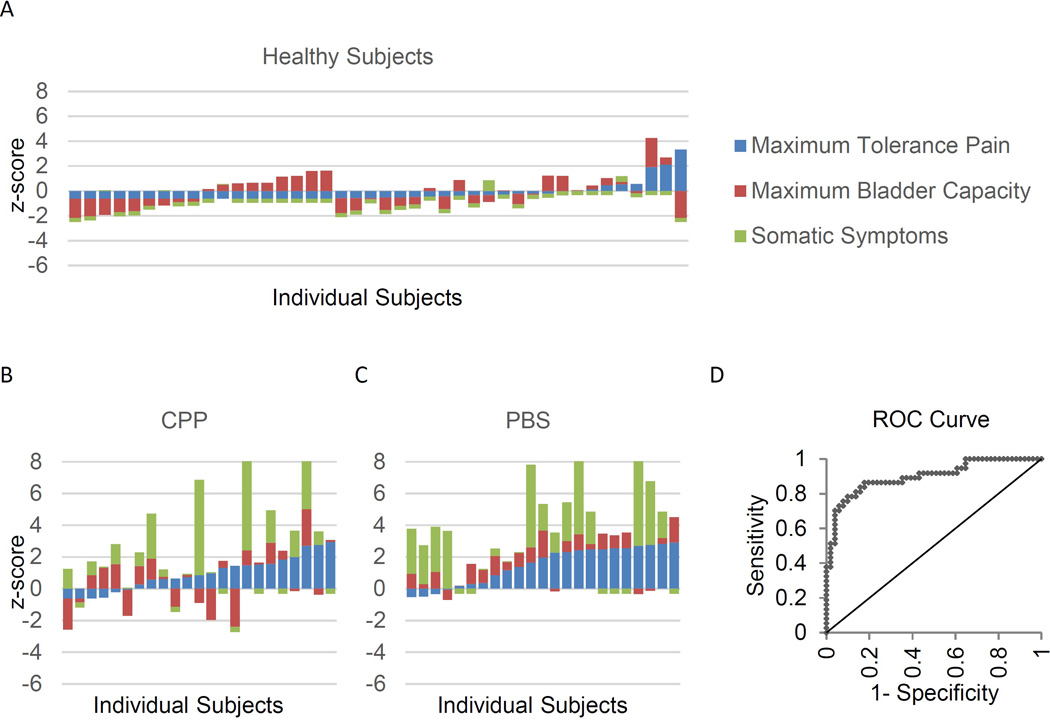
Stacked bar chart of standardized relative severity of bladder pain, bladder capacity and somatic complaint, individual level data. Results scaled for estimated frequency of CPP and PBS in the general population (e.g. 0 = mean, 1 = 1 standard deviation higher than the general population). A–C: Scores of individual participants were z-scored to represent the prevalence using weighted-average scaling assuming 11% CPP only and 4% CPP with PBS.29,30 Each bar represents a single subject. The total height or depth of the bar represents the number of standard deviations above or below the population average. D: ROC curve for using these three parameters to predict an ICSI score ≥6.
Discussion
Main Findings
We applied a standardized protocol for measuring bladder pain and sensation exploiting the simplicity of provoked diuresis and confirm distension-induced bladder pain is present in both classic PBS as well as in half of CPP patients not meeting PBS criteria. Our non-invasive measure empirically differentiates between relative degrees of visceral sensitivity (CPP vs. PBS) and is less sensitive to non-specific somatic/psychological factors. The simplicity of this measure could make it attractive as a general measure of visceral hypersensitivity, with research applications beyond the urological field.5,6
Another key finding was that pelvic floor pain sensitivity did not predict visceral sensitivity. Our original hypothesized relationship was based on previous clinical observations that targeted pelvic floor physical therapy can improve bladder pain symptoms and can reduce general pelvic floor sensitivity.9,16 In contrast, we found localized mechanical hyperalgesia during palpation or vaginal pressure pain thresholds predicted bladder distension pain and sensation, but only at the bladder site. We can thus conclude that somatovisceral convergence is not a major contributor to bladder filling pain, yet pelvic floor dysfunction could still incrementally influence inputs into brainstem descending inhibition and increase overall bladder pain symptomatology.17 Since as many as 43% of patients do not significantly respond to manual therapy, and questionnaire based studies are vulnerable to somatization, quantitative sensory evaluation of the bladder and pelvic floor may be necessary to elucidate the mechanisms modifiable through physical therapy. One likely possibility is that PBS represents heterogeneous groupings of patients, some with predominantly pelvic floor dysfunction, others mainly expressing dysfunctions in central pain processing, and still others exhibiting predominantly pure bladder mechanical hyperalgesia.18 A factorial approach to unpackaging pain mechanisms has been used to identify patients with diabetic neuropathy that respond to duloxetine, a drug also used off-label to treat PBS.19
Strengths
Several strengths of our study support diuresis-provoked bladder testing as a valid visceral sensitivity task. First, we controlled for sociodemographic features within a reproductive age cohort on bladder distension pain and found no influence. Our extensive demographic characterization, including medical comorbidities (endometriosis, dysmenorrhea, fibromyalgia, abuse exposure) will allow future studies to contrast their findings using these parameters, as our sample is typical of a referral population of significantly distressed women. Second, we controlled for potential psychological confounders with standardized patient reported outcomes instruments, as studies of rectal distension have implied that heightened sensation is entirely mediated by anxiety or somatization.20,21 This allowed us to show that bladder distension pain may be a less biased outcome measure, perhaps reflecting the lack of perceived threat (no catheter or manometry balloon insertion) presented by a noninvasive test. Since self-reported symptom indices are vulnerable to somatization and psychological factors, complete phenotypic assessment of pain states ideally includes symptom-based and unbiased experimental visceral pain assessment.8,22,23 Third, we used a positive control group without significant bladder symptoms to untangle potential pain and urgency relationships on visceral pain sensitivity.
Limitations
The primary limitation of a naturalistic study is the inability to precisely control the experimental stimulus. However, when we controlled for estimated filling rates, we found no difference between diagnostic groups, and likewise no relationship to distension provoked pain. Although impairments in compliance accounting for limitations in maximal capacity are not associated with PBS, future studies using ultrasonographic elastography could confirm this non-invasively.24,25 We acknowledge this is a modest sized study, but we were adequately powered for our primary contrasts. Future replication of our results in larger cohorts is obviously needed.
Interpretation
How should we investigate the utility of distension-mediated pain as a risk marker for future PBS? We are still quite far from understanding the trajectory that leads from asymptomatic bladder sensitivity to overt bladder pain symptoms. Large scale studies, like the ongoing National Institutes of Health-funded Multidisciplinary Approach to Pelvic Pain network and its predecessors, have not longitudinally characterized the changes in visceral pain sensitivity in healthy participants, perhaps since catheterization elicits more apprehension and pain particularly in younger populations.26,27 Our tool could be used to follow the transition to chronic bladder pain if employed following cases of acute cystitis, focusing selectively on patients exhibiting generalized somatic symptoms. Given that a key role has been identified for somatization in temporomandibular joint disorder risk in a large-scale longitudinal study, assessing visceral sensitivity and somatization simultaneously could be quite enlightening.28
Conclusion
We show preliminary validity for diuresis-induced bladder pain as a visceral pain measure reflecting local mechanical sensitivity. As an assessment tool for mechanism-based study of CPP states, it may be less influenced by psychological factors than patient-reported outcomes. The benefit of our visceral distension measure remains to be fully appreciated, as this task can be studied in ambulatory settings, may be a marker for the emergence of PBS, and potentially could be comingled with other therapeutic trials as an objective marker of disease change following treatment.
Supplementary Material
Acknowledgments
We thank Dr.’s Daniel Clauw, Adam Gafni-Kane, Joel Greenspan, and James W. Griffith for valuable comments on our manuscript.
Funding: F.F.T. was supported by NIH grant K23HD054645, K.M.H. was supported by a Research Career Development Award from the NorthShore University HealthSystem Research Institute, Evanston, IL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Presented in poster format at 15th annual meeting of the International Association for the Study of Pain in Buenos Aires, Argentina October 6–11, 2014
Disclosure of interests: none to report. The ICMJE disclosure forms are available as online supporting information.
Contribution to authorship: FT conceived/designed experiment, performed experiments, analyzed data, wrote the paper; KH conceived/designed experiment, performed experiments, analyzed data, wrote the paper; JK performed experiments, analyzed data, wrote the paper
Details of ethics approval: NorthShore University HealthSystem IRB approval (EH 08-073) 8/1/08
References
- 1.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005 Jun;173(6):1983–1987. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24(7):627–632. doi: 10.1002/nau.20178. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990 May;98(5 Pt 1):1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 5.Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TOC, Evers E, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011 Sep;60(9):1196–1203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song GH, Venkatraman V, Ho KY, Chee MWL, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006 Dec 15;126(1–3):79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain. 2013 Oct;29(10):883–890. doi: 10.1097/AJP.0b013e31827a71a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai HH, North CS, Andriole GL, Sayuk GS, Hong BA. Polysymptomatic, polysyndromic presentation of patients with urological chronic pelvic pain syndrome. J Urol. 2012 Jun;187(6):2106–2112. doi: 10.1016/j.juro.2012.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FitzGerald MP, Anderson RU, Potts J, Payne CK, Peters KM, Clemens JQ, et al. Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2009 Aug;182(2):570–580. doi: 10.1016/j.juro.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Leary MP, Sant GR, Fowler FJ, Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997 May;49(5A Suppl):58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 11.Keller ML, McCarthy DO, Neider RS. Measurement of symptoms of interstitial cystitis. A pilot study. Urol Clin North Am. 1994 Feb;21(1):67–71. [PubMed] [Google Scholar]
- 12.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007 May;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Harden RN. Comparative measurement of pelvic floor pain sensitivity in chronic pelvic pain. Obstet Gynecol. 2007 Dec;110(6):1244–1248. doi: 10.1097/01.AOG.0000289228.23903.cc. [DOI] [PubMed] [Google Scholar]
- 14.Parsons CL, Greenberger M, Gabal L, Bidair M, Barme G. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998 Jun;159(6):1862–1867. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 15.Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005 Jul;174(1):187–189. doi: 10.1097/01.ju.0000162020.10447.31. [DOI] [PubMed] [Google Scholar]
- 16.Oyama IA, Rejba A, Lukban JC, Fletcher E, Kellogg-Spadt S, Holzberg AS, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004 Nov;64(5):862–865. doi: 10.1016/j.urology.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006 Dec;96(6):3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- 18.Warren JW, van de Merwe JP, Nickel JC. Interstitial cystitis/bladder pain syndrome and nonbladder syndromes: facts and hypotheses. Urology. 2011 Oct;78(4):727–732. doi: 10.1016/j.urology.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012 Jun;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead WE, Crowell MD, Davidoff AL, Palsson OS, Schuster MM. Pain from rectal distension in women with irritable bowel syndrome: relationship to sexual abuse. Dig Dis Sci. 1997 Apr;42(4):796–804. doi: 10.1023/a:1018820315549. [DOI] [PubMed] [Google Scholar]
- 21.Piché M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010 Jan;148(1):49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol. 2013 Nov;209(5):422e.1–422.e10. doi: 10.1016/j.ajog.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindler LL, Bennett RM, Jones KD. Central sensitivity syndromes: mounting pathophysiologic evidence to link fibromyalgia with other common chronic pain disorders. Pain Manag Nurs. 2011 Mar;12(1):15–24. doi: 10.1016/j.pmn.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Guan G, Zhang F, Song S, Wang RK, Huang Z, et al. Quantitative elasticity measurement of urinary bladder wall using laser-induced surface acoustic waves. Biomed Opt Express. 2014 Dec 1;5(12):4313–4328. doi: 10.1364/BOE.5.004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinkohl WB, Leach GE. Urodynamic findings in interstitial cystitis. Urology. 1989 Dec;34(6):399–401. doi: 10.1016/0090-4295(89)90453-6. [DOI] [PubMed] [Google Scholar]
- 26.Yiou R, Audureau E, Loche C-M, Dussaud M, Lingombet O, Binhas M. Comprehensive evaluation of embarrassment and pain associated with invasive urodynamics. Neurourol Urodyn. 2015 Feb;34(2):156–160. doi: 10.1002/nau.22521. [DOI] [PubMed] [Google Scholar]
- 27.Suskind AM, Clemens JQ, Kaufman SR, Stoffel JT, Oldendorf A, Malaeb BS, et al. Patient perceptions of physical and emotional discomfort related to urodynamic testing: a questionnaire-based study in men and women with and without neurologic conditions. Urology. 2015 Mar;85(3):547–551. doi: 10.1016/j.urology.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, et al. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 2013 Dec;14(12 Suppl):T75–T90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996 Mar;87(3):321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 30.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011 Aug;186(2):540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


