Abstract
Adeno-associated viruses (AAV) contain minimal viral proteins necessary for their replication. During virus assembly, AAV acquire, inherently and submissively, various cellular proteins. Our previous studies identified the association of AAV vectors with the DNA binding protein nucleophosmin (NPM1). Nucleophosmin has been reported to enhance AAV infection by mobilizing AAV capsids into and out of the nucleolus, indicating the importance of NPM1 in the AAV life cycle; however the role of NPM1 in AAV production remains unknown. In this study, we systematically investigated NPM1 function on AAV production using NPM1 knockdown cells and revealing for the first time the presence of G-quadruplex DNA sequences (GQRS) in the AAV genome, the synergistic NPM1-GQRS function in AAV production and the significant enhancement of NPM1 gene knockdown on AAV vector production. Understanding the role of cellular proteins in the AAV life cycle will greatly facilitate high titre production of AAV vectors for clinical use.
Keywords: AAV biology, NPM1, G-quadruplex DNA sequences, shRNA
1. Introduction
Adeno-associated viruses (AAV) have never been associated with any human disease and, thus have been most successfully used as a delivery vector for gene therapies. The first licenced gene therapy product for human use (Bryant et al., 2013) is based on AAV and AAV products have been continuously improved for clinical applications, e.g. for haemophilia B (Nathwani et al., 2011), heart disease (Jessup et al., 2011) and congenital blindness (Brument et al., 2002). Although many advances in AAV vector design have been made, the production of high titre AAV is still a significant challenge as in the case of the first marketed product Glybera, which is required to be administered to patients as a dose of 3 × 1012 vg /kg via 40 or 60 multiple injections (Bryant et al., 2013).
The limitation in high titre AAV production may be due to the AAV dependence on helper viruses and insufficient understanding of factors, viral or cellular, that contribute to AAV replication. It has been well documented that AAV rep gene expression and AAV DNA replication depended on AAV interaction with a helper virus, e.g. adenovirus, herpes-virus or papillomavirus (Chang et al., 1989, Muzyczka, 1992, Nicolas et al., 2012); however, a number of studies have otherwise suggested that none of the helper virus genes was directly involved in AAV replication and that the helper function was to induce cellular factors that were missing or inactivated in a normal cell cycle, emphasising the importance of cellular factors in the AAV life cycle (Muzyczka, 1992, Ni et al., 1998). For example, helper viruses have been demonstrated to induce entry of the host cell into S-phase and lead to changes of cellular milieu which were necessary for AAV assembly (Carter, 2004). Helper-virus independent genotoxic or cytotoxic factors such as SV40 LTAg that transformed or altered the cell cycle have been shown to support AAV replication and assembly (Nicolas et al., 2012, Steinbach et al., 1997, King et al., 2001, Sonntag et al., 2011, Naumer et al., 2012), highlighting the AAV dependence on cellular components. Direct involvement of cellular proteins in AAV replication, e.g. ssDNA binding protein, replication protein (RPA), replication factor C (RFC) and proliferating cell nuclear antigen (PCNA), have been extensively studied and displayed as much replication activity as Adenoviral helper function (Ni et al., 1998). Indirect function of cellular factors in AAV replication required their interactions with AAV proteins (Inagaki et al., 2007, Choi et al., 2010, Hirsch et al., 2013, Pegoraro et al., 2006). For example, the interaction of cellular proteins SP1, YY1, Topors or TATA-binding protein (TBP) with Rep protein resulted in the assemblage of a transcription imitation complex e.g. p5-Rep-SP1-p19, which led to the initiation of AAV DNA replication and expression (Pereira and Muzyczka, 1997, , 2001; Hermonat et al., 1996; Stracker et al., 2004). Early studies have shown that AAV replication required only the AAV leading strand DNA synthesis and was thus independent of α, δ, ε DNA polymerases (Ni et al., 1998, Ward and Berns, 1996), suggesting a potential requirement of novel cellular proteins for AAV replication.
A number of studies have shown that DNA binding proteins played a vital role in AAV life cycle; for example, at the early stage of AAV replication, DNA-binding proteins interacted with the AAV ITR sequence for unwinding AAV dsDNA, nicking ssDNA, facilitating ssDNA association with nuclei and ultimately enhancing viral DNA production and protein expression (Weitzman, 2006). At the late stage of the AAV life cycle, DNA-binding proteins were required for viral DNA accumulation, translocation and the formation of viral DNA-protein complexes to facilitate virus assembly (Weitzman, 2006). Virus-originated DNA binding proteins, e.g. helper Adenovirus E2A and HSV UL29, have been extensively studied showing inherent functions throughout the AAV life cycle; however, some studies have likewise showed that cellular proteins, e.g. total HeLa cell proteins and DNA helicase, could support helper virus-independent replication of AAV virus, indicating an alternative cellular mechanism(s) in the AAV life cycle (Steinbach et al., 1997, King et al., 2001, Sonntag et al., 2011, Naumer et al., 2012). A number of cellular DNA binding proteins, e.g. ssDNA binding protein, ANP32B, NCL, YB1 and NPM1, have been shown to play a role in the AAV life cycle (Ni et al., 1998, Nash et al., 2009; Nicolas et al., 2010; Satkunanathan et al., 2014; Dong et al., 2014).
The G-quadruplex DNA sequence (GQRS) is gaining increasing attention because it is highly represented in selected regions of the genome, e.g. telomeres and gene promoters. The G-quadruplex sequences have been recently identified within the genome of a number of viruses, e.g. HIV, HSV, EBV influenza, papillomavirus and cauliflower mosaic virus (Piekna-Przybylska et al., 2014, Artusi et al., 2015; Tlučková et al., 2013; Métifiot et al., 2014; Lyonnais et al., 2003; Harris and Merrick, 2015). It is generally believed that the viral GQRS structure formed steric road blocks to DNA replication and transcription (Harris and Merrick, 2015, Huppert, 2008). Destruction of G-quadruplex by GQRS ligands, e.g. BRACO-19, could effectively unblock GQRS inhibition of viral DNA replication of HIV and HSV viruses (Harris and Merrick, 2015, Perrone et al., 2014). Furthermore, previous studies have shown that interaction of NPM1 with DNA GQRS could lead to the repression of cellular and viral DNA replication (Scognamiglio et al., 2014, Siddiqui-Jain et al., 2002). There has been so far no report on the presence or function of GQRS structure within the AAV genome.
A previous study has showed that NPM1 existed in close proximity to Cap or Rep proteins and most prevalently with AAV Cap. Agents that augmented AAV infection, such as helper adenovirus, herpesvirus or genotoxic agents were known to redistribute nucleolar proteins, e.g. NPM1 and NCL, away from the nucleolus, indicating potential NPM1 negative regulation on AAV replication (Qiu and Brown, 1999, Matthews, 2001, Johnson and Samulski, 2009a, Johnson and Samulski, 2009b). Following up our previous proteomics study on the function of cellular proteins on AAV production (Satkunanathan et al., 2014), this study focused on the DNA binding protein, Nucleophosmin (NPM1) that showed a clear association with AAV (Nicolas et al., 2010; Satkunanathan et al., 2014; Dong et al., 2014; Gimenez et al., 2012), on its function and cellular-viral interaction during AAV replication. Understanding these mechanism(s) may enable us to manipulate AAV biological processes and to improve AAV vectors as a gene delivery system.
2. Materials and methods
2.1. Gene knockdown with shRNA
Small hairpin RNA (shRNA) for NPM1 knockdown were transfected with the presence of Lipofectamin 2000 (Life Technologies, Baltimore, MD, USA) into 293T cells according to the manufactureer's protocol. Control cells were transfected with empty capsids without a shRNA sequence. Transfection efficiency was evaluated by Western blotting.
2.2. shRNA interference and the establishment of gene knockdown cell lines
Five small hairpin RNA (shRNA) sequences were used for gene knockdown of NPM1 and were expressed from plasmid pLKO.1-puro (Sigma, USA) using a Lentiviral expression system. shRNA and lentiviral vector packaging plasmids were transfected into 293T cells using the CaCl2 transfection method. Control cells were transfected with empty capsids without a shRNA sequence (mock) or a scramble shRNA sequence targeting non-mammalian gene sequences (scramble).
2.3. AAV production
Three AAV serotypes, i.e. AAV2, AAV5 and AAV8, were produced and investigated in this study. AAV particles were produced using the Calcium phosphate-BBS method and from 3 plasmids pHelper (Stratagene, USA), pAAV-RC encoding AAV Rep-Cap and pAAV-hrGFP (Stratagene, USA), as detailed in our previous study (Satkunanathan et al., 2014). Vector producer cells were harvested and were subjected to 5 cycles of freeze thaw to release vector particles and the cellular debris was removed by centrifugation (2000 g). AAV vectors were purified by ultra-centrifugation at 4000 rpm for 3 h on a step gradient containing 15%, 25%, 40% and 60% iodixanol.
2.4. Sample preparation for transduction vector genome titration
AAV vectors produced from control, scramble or gene-knockdown cells were used to transduce Hela cells (ATCC, USA) overnight. Cytoplasmic DNA extraction was the same as vector DNA except without Proteinase K digestion of capsid proteins. Genomic DNA was extracted using a Wizard@DNA Genomic purification kit (Promega, USA) before being subjected to quantitation of the housekeeping gene Actin for sample normalisation and the determination of AAV transduction genome titres, using qPCR as described in our previous study (Satkunanathan et al., 2014). Genome titre of AAV vectors was determined using real-time qPCR with AAV2 ITR primers: ITR-F 5’-GGA ACC CCT AGT GAT GGA GTT-3’, ITR- R 5’-CGG CCT CAG TGA GCG A-3’ and ITR-P 5’-CAC TCC CTC TCT GCG CGC TCG-3’and as detailed in our previous study (Werling et al., 2015).
2.5. Immunochemistry
SDS-polyacrylamide gel electrophoresis (10% total acrylamide) was used to resolve protein samples and followed by immunoblotting. Samples for immunoblotting were electrophoresed and electroblotted to Hybond ECL membranes (Amersham, UK). The following primary antibodies were used: mouse anti AAV2 Rep (Fitzgerald, USA) and AAV2 Cap (Abcam, UK), anti NPM1 (Abcam, UK) and anti GAPDH (Abcam, UK). The immunoblots were further incubated with goat anti-mouse, anti-rabbit or rabbit anti-goat horseradish peroxidase conjugates (Sigma, UK). The immuno-reactive proteins were detected using ECL reagents (Amersham, UK). The level of gene knockdown was evaluated using 10 μg total proteins from shRNA virus-transduced cells following the immunoblotting method as described above. AAV2 capsid proteins were quantified using an ELISA kit and following the manufacturer's protocol (Progene, USA) and presented as capsid/mL according to the manufacture's standard curve conversion.
Parental 293T cells and NPM1 knockdown cells, before and after transfection with AAV production plasmids, were fixed with 4% formaldehyde, permeabilised in PBS with 0.1% Triton X-100 (Sigma, USA) and blocked with immunofluorescence buffer before being labelled individually or in combination with the following antibodies: mouse anti NPM1 (Thermo, USA), rabbit anti-NPM1 (Bethyl USA), rabbit anti-AAV Cap (Progen, Germany) and mouse anti-AAV Rep (Fitzgerald, Germany). Antibody-labelled cells were then incubated with AlexaFluor-488 conjugated goat anti-rabbit or DyLight 633 conjugated goat anti-mouse antibodies (ThermoFisher, USA). Nuclei were labelled with Dapi (Sigma, USA). The labelled cells were visualised with a Leica SPX upright laser scanning confocal microscope and analysed using Leica Application Suite X (LASX) software.
Protein-protein interaction assay was performed using G-coupled agarose beads to immunoprecipate anti-NPM1 or mouse anti-Cap antibody interacted proteins in cell lysates before being subjected to immunoblot analysis.
Flow cytometry was performed using a BD FACSCanto II™ machine (BD Biosciences, Oxford, UK). Cells were gated based on forward angle light scatter (FSC) and side angle light scatter (SSC) characteristics and further analysed using BD FACSDiva™ software version 6.1.1 (BD Biosciences).
2.6. G-Quadraplex (GQRS) DNA binding assay
Three biotin-labelled GQRS DNA oligonucleotides (Invitrogene, USA), that is ITRA 5’-TCG GGC GTC GGG CGA CCT TTG GTC GCC TTT TT-Biotin-3’, and ITRB 5’-TGA GGG AGT GGC CAA CTC CAC ACT AGG GGT TCC TTT TT-Biotin-3’, targeting different regions of AAV2 ITR were synthesised and a c-myc specific biotin-oligonucleotide MYC 5’-TGA GGG TGG GGA GGG TGG GGA AGG TTT TT-Biotin-3’ and a non-GQRS biotin-oligonucleotide NGQRS 5’-GCA GCC ATC GCG TCA GAC GCG GAA GCT TCG ATC AAC TAC TTTT-Biotin-3’ were synthesised as a positive control and a negative control, respectively. For each corresponding sequence, a non-biotinylated oligonucleotide was also synthesised for a competition assay. The GQRS DNAs were denatured at 65 °C for 10 min, and cooled to room temperature to allow the formation of GQRS structures. Cell lysates from 293T cells or NPM1 knockdown cells before or after transfection and AAV production were incubated in the presence of GQRS DNA at 4 °C overnight before streptavidin beads were added. The GQRS DNA-protein-bead complex was recovered using a magnet, washed and denatured in Laemmli buffer (SIGMA, USA) at 98 °C for 5 min and finally subjected to immunoblot analysis. Competition assay was performed by adding a dilution series of non-biotin labelled c-myc GQRS DNA to the biotin-labelled ITR GQRS DNA before it being subjected to DNA-protein interaction.
2.7. Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Data was tested for normality using the Kolmogorov-Smirnov test then analysed using a one-way ANOVA followed by Bonferroni's Multiple Comparison Test or a Kruskall Wallis test followed by Dunn's Multiple Comparison Test. A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Establishment of NPM1 knockdown cells
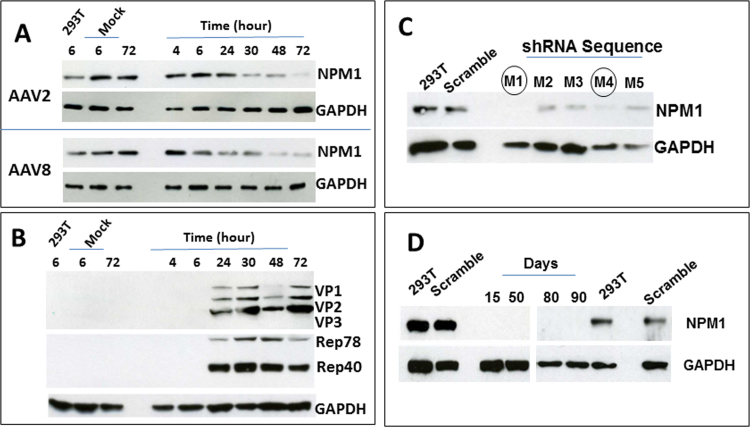
Our previous proteomics and mass spectrometry study has shown that the NPM1 protein was detected in purified AAV vectors (Satkunanathan et al., 2014), suggesting a possible role of NPM1 in AAV assembly. Fig. 1A analysed NPM1 expression dynamics during a complete cycle of AAV2 and AAV8 production, showing that the expression of NPM1 decreases overtime 24 h after the transfection. In correlation, the expression of AAV Rep and Cap (VP1, VP2 and VP3) became detectable at a similar time point when NPM1 expression starts to decline (Fig. 1B), indicating a potential negative regulation of AAV proteins on NPM1 expression. In order to study the function of NPM1 protein in AAV replication and assembly, we used a Lentiviral vector delivery system, as detailed before (Satkunanathan et al., 2014), to establish NPM1 knockdown cells with five NPM1 specific shRNA sequences, i.e. shRNA_M1 to M5 (Fig. 1C). Introducing a shRNA sequence to parental 293T cells led to the significant down-regulation of NPM1 expression (Fig. 1C) compared to NPM1 expression in two control cells that were either with scramble shRNA targeting non-mammalian genes (scramble) or without any shRNA sequences (293T); in particular, shRNA_M1 and M4 sequences (circled, Fig. 1C) led to the most gene knockdown and, the reduction of NPM1 expression (Fig. 1D) sustained for over 90 days after culture in puromycin selection medium, demonstrating the robustness of shRNA gene regulation. Analysis of LV_shRNA integration showed that an average of 1 copy/ cell of NPM1 shRNA sequence integration led to the significant down-regulation of NPM1 expression in knockdown cells.
Fig. 1.
Immunoblotting analysis of NPM1 and AAV protein expression in parental 293T cells after transient transfection of AAV packaging and helper plasmids, showing (A) overtime decrease in NPM1 expression in AAV 2 and AAV8 producer cells; (B) detection of AAV capsid (VP1, VP2 and VP3) and Rep proteins from 24 h after transfection; (C) NPM1 knockdown in LV-NPM1-shRNA transduced 293T cells carrying shRNA M1 to M5 sequences targeting NPM1; (D) sustained down-regulation of NPM1 in single cell clones and in culture for up to 90 days. Controls: 293T cell lysate without the transfection of any plasmids (293T), 293T cells with the transfection of pcDNA3.1 plasmids (Mock); 293T cells with a shRNA sequence targeting non-mammalian genes (Scramble) and without any shRNA sequences (293T) were used as gene knockdown controls. Housekeeping gene GAPDH was further used as a loading control.
3.2. Influence of NPM1 knockdown on AAV titre
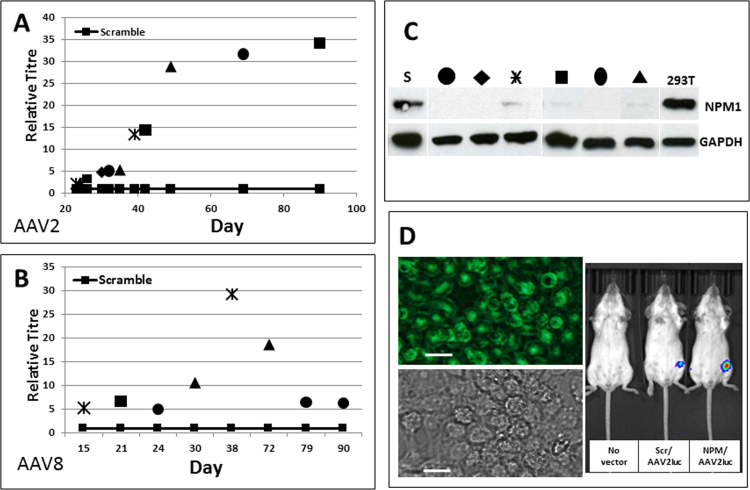
The effect of NPM1 gene knockdown on AAV production was tested for AAV2 and AAV8 carrying a reporter gene GFP. Down-regulation of NPM1 in producer cells led to up to 35 fold increase in AAV titres of AAV2 (Fig. 2A, Table 1) and AAV8 (Fig. 2B, Table 2) compared to control scramble cells. It is noteworthy that Fig. 2A and B show collective data on vectors produced from bulk cells and single cell clones. Each data point is a mean relative titre of triplicate lots of vectors produced from 3 independent experiments of a given cell line at one time point, as detailed previously (Satkunanathan et al., 2014). The reason for us to present collective data on the same figure from different cell lines/clones at different time points is to show not only the relative titres but also the optimal range of production time for NPM1 knockdown cells. On average, NPM1 knockdown cells and parental 293T cells produced AAV2 and AAV8 vectors at around ~ 103 and 102 VG/cell, respectively.
Fig. 2.
Relative genome titres of AAV vectors produced from NPM1 gene knockdown cells compared to that from control scramble cells, showing up to 35 fold and 30 fold increases in (A) AAV2 and (B) AAV8 vector genome titres (VG/mL), respectively, compared to scramble cells and throughout 90 days in culture; (C) NPM1 expression in individual AAV producer cell lines at the last production time points. Different sympols, e.g. square or circle etc, represent independently established NPM1 knockdown cell lines. (D) in vitro transduction on HelaS3 cells (left panel, labelled in green, GFP) and in vivo transduction in Balb C mice (right panel, in blue, luciferase) showing comparable transduction efficiency when using AAV2 vectors produced from control scramble cells (Scr/AAV2luc) or from NPM1 knockdown cells (NPM/AAV2luc). Scale bars are 30 µm.
Table 1.
AAV2 vector genome titres.
| Days | AAV2 | Titre (VG/108cells) | Ratio N/S | |
|---|---|---|---|---|
| Batch 1 | 24 | Scramble | 5.24 ± 0.15 × 109 | |
| NPM | 1.11 ± 0.17 × 1010 | 2.12 | ||
| Batch 2 | 24 | Scramble | 5.24 ± 0.15 × 109 | |
| NPM | 1.24 ± 0.24 × 1010 | 2.37 | ||
| Batch 3 | 26 | Scramble | 1.63 ± 0.25 × 101 | |
| NPM | 4.93 ± 0.31 × 1011 | 3.29 | ||
| Batch 4 | 32 | Scramble | 2.60 ± 0.06 × 1010 | |
| NPM | 1.24 ± 0.14 × 1011 | 4.77 | ||
| Batch 5 | 30 | Scramble | 2.60 ± 0.06 × 1010 | |
| NPM | 1.31 ± 0.17 × 1011 | 5.04 | ||
| Batch 6 | 35 | Scramble | 4.96 ± 2.50 × 1010 | |
| NPM | 2.64 ± 0.41 × 1011 | 5.32 | ||
| Batch 7 | 39 | Scramble | 5.50 ± 1.70 × 1010 | |
| NPM | 7.52 ± 1.12 × 1011 | 13.73 | ||
| Batch 8 | 42 | Scramble | 8.00 ± 0.48 × 109 | |
| NPM | 1.15 ± 0.36 × 1011 | 14.38 | ||
| Batch 9 | 49 | Scramble | 2.73 ± 0.74 × 109 | |
| NPM | 7.88 ± 0.87 × 1010 | 28.86 | ||
| Batch 10 | 69 | Scramble | 2.73 ± 0.74 × 109 | |
| NPM | 8.64 ± 0.27 × 1010 | 31.65 | ||
| Batch 11 | 90 | Scramble | 1.86 ± 0.67 × 109 | |
| NPM | 6.36 ± 0.74 × 1010 | 34.1 |
Table 2.
AAV8 Vector Genome Titres.
| Days | AAV8 | Titre (VG/108cells) | Ratio N/S | |
|---|---|---|---|---|
| Batch 1 | 15 | Scramble | 2.54 ± 0.34 × 107 | |
| NPM | 1.34 ± 0.06 × 108 | 5.26 | ||
| Batch 2 | 21 | Scramble | 2.05 ± 0.19 ×107 | |
| NPM | 1.37 ± 0.14 × 108 | 6.68 | ||
| Batch 3 | 30 | Scramble | 8.28 ± 0.83 × 107 | |
| NPM | 8.80 ± 2.11 × 108 | 10.63 | ||
| Batch 4 | 38 | Scramble | 8.28 ± 0.83 ×107 | |
| NPM | 2.42 ± 0.24 × 109 | 29.28 | ||
| Batch 5 | 72 | Scramble | 8.28 ± 0.83 × 107 | |
| NPM | 1.55 ± 0.85 × 109 | 18.67 | ||
| Batch 6 | 90 | Scramble | 2.54 ± 0.34 × 107 | |
| NPM | 1.60 ± 0.24 × 108 | 6.3 |
Due to the fact that NPM1 knockdown cells cannot be established as single cell clones, all results presented were based on bulk population of NPM1 knockdown cells. Although the NPM1 expression level (Fig. 2C) and the average integration copies of LV_NPM1 shRNA (~2/cell) were comparable among different batches of cell lines, we observed a greater batch-to-batch variation in terms of AAV8 production compared to that of AAV2 production, indicating a serotype specific response to NPM1 knockdown.
Using an equal genome copy number of purified AAV2/GFP vectors to transduce HelaS1 cells in vitro (Fig. 2D, left panel) or purified AAV2/Luciferase vectors to transduce Balb/C mice intramuscularly (Fig. 2D, right panel), we observed a comparable or slight increased infectivity of the AAV2 produced from NPM1 knockdown cells compared to that produced from control scramble cells, demonstrating the robustness of NPM1 knockdown cells in producing infectious and functional AAV.
Using a quantitative ELISA with an antibody specific for assembled AAV capsid particles, we detected two fold higher amounts of AAV capsid particles in control scramble cells than that in NPM1 knockdown cells, at 1.11 ± 0.15 × 1014 and 5.51 ± 0.26 × 1013 particles/108cells, respectively (Table 3); whereas, the number of complete AAV particles carrying AAV genome ( i.e. AAV genome titre) were up to 35 fold higher in NPM1 knockdown cells than that in scramble cells, indicating a greater production of empty particles without vector genome in scramble cells compared to NPM1 cells.
Table 3.
Production of AAV2 capsid particles.
| ELISA | Particles | % of Total particles | Ratio | |
|---|---|---|---|---|
| per 10⁸ cells | in whole cells | S/N | ||
| Whole cells | Scramble | 1.11 ± 0.15 × 1014 | 100% | 2.01 |
| NPM | 5.51 ± 0.26 × 1013 | 100% | ||
| Cytoplasm | Scramble | 7.49 ± 0.80 × 1012 | 6.77% | 1.2 |
| NPM | 9.02 ± 0.82 × 1012 | 16.35% | ||
| Nuclei | Scramble | 1.03 ± 0.01 × 1014 | 93.23% | 2.23 |
| NPM | 4.61 ± 0.89 × 1013 | 83.64% | ||
| Vectors | Scramble | 4.36 ± 0.77 × 1013 | 39.20% | 1.36 |
| NPM | 2.97 ± 0.74 × 1013 | 53.38% |
3.3. Influence of NPM1 knockdown on Plasmid DNA transfection
To study whether NPM1 knockdown influenced the first step of AAV production, i.e. the entry of plasmid DNA into cells, plasmid DNA inside transfected cells were analysed using qPCR targeting AAV2 rep sequence (Table 4). The reason to choose rep instead of ITR sequence as an indication of DNA transfection efficacy is because rep DNA plasmids, by design, do not have DNA replication capacity in the transfected cells. Table 4 showed that, 8.10% and 9.54% of total input of 6.8× ×1013 copies of rep plasmids DNA, i.e. 5.51 ± 0.43 × 1012 and 6.49 ± 0.19 × 1012 copies, were detected in scramble and NPM1 knockdown cells respectively, representing a ~ 8% and ~ 5 × 104 copies/cell transfection efficiency when using the calcium transfection protocol. The comparable transfection efficiency in scramble and NPM1 knockdown cells indicated that NPM1 knockdown did not significantly influence the transfection efficiency thus the transfection process might not contribute to the increased AAV titre observed in NPM1 knockdown cells. However, NPM1 knockdown influenced the distribution of plasmid DNA, with over 2 fold higher amount of plasmid DNA detected in NPM1 knockdown cells compared to control scramble cells (Table 4).
Table 4.
Influence of NPM1 knockdown on plasmid DNA transfection.
| qPCR | Assessment | Location | Samples | DNA Copies/108cells | % Input | Ratio |
|---|---|---|---|---|---|---|
| Target | N/S | |||||
| input | 6.8x x1013 | 100% | ||||
| DNA Transfection | Whole cells | Scramble | 5.51±0.43 x1012 | 8.10% | ||
| Rep | NPM | 6.49±0.19 x1012 | 9.54% | 1.18 | ||
| Cytoplasm | Scramble | 2.73±0.57 x1012 | ||||
| NPM | 1.57±0.76 x1012 | 0.57 | ||||
| Nuclei | Scramble | 4.49±0.38 x1011 | ||||
| NPM | 1.01±0.25 x1012 | 2.25 |
3.4. Influence of NPM1 knockdown on viral DNA production
To investigate whether NPM1 knockdown influences viral DNA replication, DNA extracted from producer cells 72 h after transfection was quantified by qPCR targeting ITR sequence. Our results showed that 91.7% and 195% of input ITR plasmids at 2.6 × 1013 copies/108cells were detected, that is a total of 2.37 ± 0.73 × 1013 and 5.08 ± 0.38 × 1013 copies of ITR DNA detected in 108 scramble and NPM1 knockdown cells, respectively. Taking into account the fact that the transfection efficiency was ~ 8% using the adopted transfection method (Table 4), the majority of the detected ITR DNA was from AAV DNA replication. In addition, the amount of ITR DNA detected in NPM1 knockdown cells was 2.14 fold higher than that in control scramble cells, indicating that NPM1 knockdown significantly enhanced viral DNA replication, resulting in the significant increase in AAV titres.
3.5. NPM1 Interaction with AAV DNA
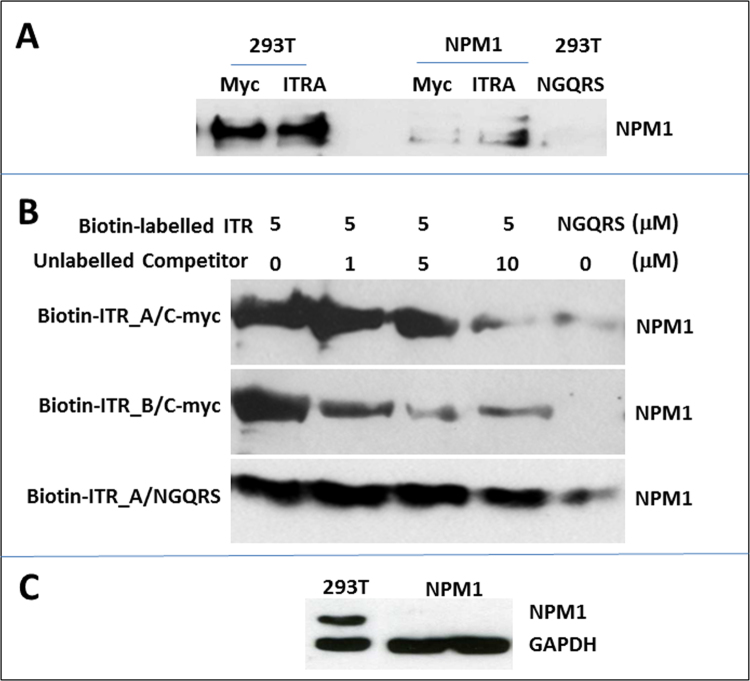
To understand how NPM1 knockdown enhanced AAV DNA replication, the interaction of NPM1 proteins with AAV DNA was systematically investigated. It has been previously reported (Hingorani et al., 2000, Gallo et al., 2012) that NPM1 protein showed high affinity interaction with single strand and complex DNA; in particular, the interaction of NPM1 with a G-quadruplex (GQRS) sequence from the c-myc promoter plays an important role in the onset of acute myeloid leukaemia (AML) disease (Scognamiglio et al., 2014). The interaction of a G-quadruplex structure in the U3 region of HIV-1 genome with transcription factor Sp1 led to HIV-1 reactivation and high-level virus expression. Using software for G-quadruplex sequence prediction and analysis (www.bioinformatics.ramapo.edu/qgrs/analyse.php), we identified a total of 18 GQRS structures within the AAV2/ITR region. Two non-overlapping AAV GQRS sequences, named ITRA and ITRB in this study, were selected and were biotin-labelled to study their interaction with endogenous NPM1 proteins in parental 293T cells. Fig. 3A showed that NPM1 proteins were pulled down by a control c-myc promoter GQRS (Fig. 3A, lane 1) and ITR predicted GQRS sequences ITRA (Fig. 3A, lane 2) when using the DNA binding assay as described in Methods and Materials. There was a trace amount of NPM1 detected in NPM1 knockdown cells when using c-myc or AAV ITRA GQRS (Fig. 3A, lane 4 and 5), reflecting the reduced NPM1 expression in NPM1 knockdown cells. No NPM1 proteins were detected in control 293T cells when using a random non-GQRS DNA oligonucleotide (NGQRS) (Fig. 3A, lane 6) to perform a DNA binding assay.
Fig. 3.
Protein-DNA binding assays showing (A) NPM1 interaction with GQRS structure within c-myc (myc, 1st lane) and AAV ITR sequences (ITRA, 2nd lane) in 293T cells after transfection with AAV packaging plasmids and a trace NPM1 interaction with c-myc (myc, 4th lane) and ITR (ITRA, 5th lane) GQRS in NPM1 knockdown cells after AAV plasmid transfection; a non-GQRS DNA oligonucleotide (NGQRS) was included as nonspecific DNA binding control (NGQRS, 6th lane); (B) Competition for AAV_ITR binding to NPM1 proteins using an increased amount of c-myc GQRS (top and middle panels): a progressively diminishing of ITR_A (top panel) or ITR_B GQRS (middle panel) binding to NPM1 proteins; a non-GQRS DNA oligonucleotide (NGQRS, bottom panel) was also used as assay control, showing that non-GQRS DNA oligonucleotides (NGQRS) cannot compete out the AAV ITR_A GQRS binding to NPM1 proteins (bottom panel) and that AAV ITR binding to NPM1 proteins is GQRS specific; (C) NPM1 expression in 293T and NPM1 knockdown cells before DNA binding assays. A non-GQRS DNA oligonucleotide (NGQRS) was included as nonspecific DNA binding control and Housekeeping gene GAPDH was used as a loading control.
To evaluate the specificity of NPM1 binding to AAV GQRS, we carried out a competition assay with the same c-myc promoter GQRS as previously reported (Scognamiglio et al., 2014). Fig. 3B shows that NPM1 binding to biotin-labelled ITR/GQRS can be competed out and a progressively reduced amount of NPM1 were detected when an increased amount of non-biotin-labelled c-myc GQRS was added to 500 μg or 200 μg of total 293T cell lysate proteins, demonstrating a GQRS specific binding of AAV ITR sequences to NPM1 proteins. The expression of NPM1 in parental 293T and NPM1 knockdown cells was confirmed before performing GQRS-binding assays (Fig. 3C). Our study showed for the first time the presence of GQRS in AAV ITR, the interaction of NPM1 protein with the predicted AAV GQRS sequences ITRA and ITRB, and alongside the confirmation of NPM1 interaction with the same c-myc promoter GQRS as previously reported (Scognamiglio et al., 2014).
3.6. Influence of NPM1 knockdown on AAV viral protein expression and localisation
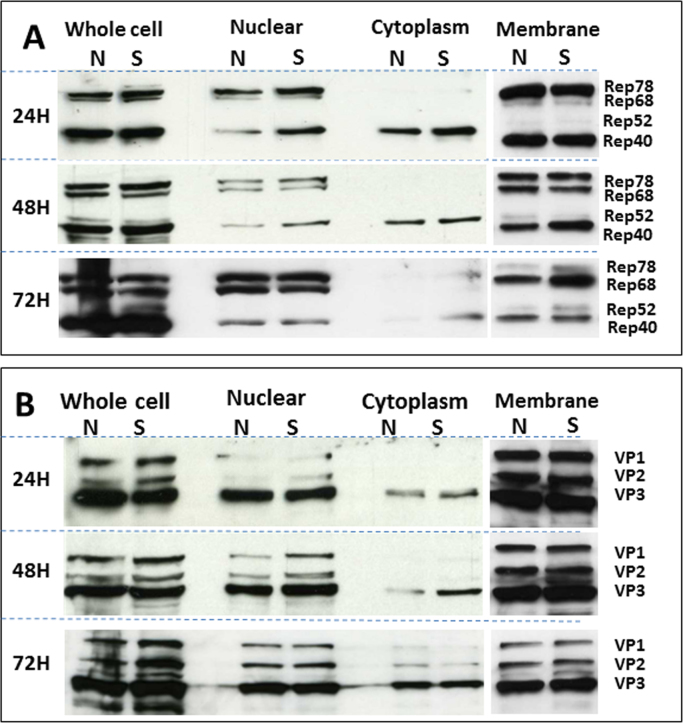
There was no significant difference between NPM1 knockdown (N) and control scramble (S) cells in terms of the expression and the localisation of AAV Rep (Fig. 4A) and Cap (Fig. 4B) proteins; moreover, both Rep and Cap proteins were predominantly associated with the insoluble fraction of cellular/nuclei membranes (Fig. 4). No Rep or Cap proteins were detected in the mock-transfected control 293T cells with pcDNA3.1 plasmids without AAV sequences (data not shown).
Fig. 4.
Immunoblotting analysis of AAV protein showing a comparable (A)rep and (B)cap expression between control scramble cells and NPM1 knockdown cells and comparable distribution of Rep and Cap proteins in cytoplasm, nuclear and insoluble membrane fractions of control scramble (S) and NPM1 knockdown (N) producer cells.
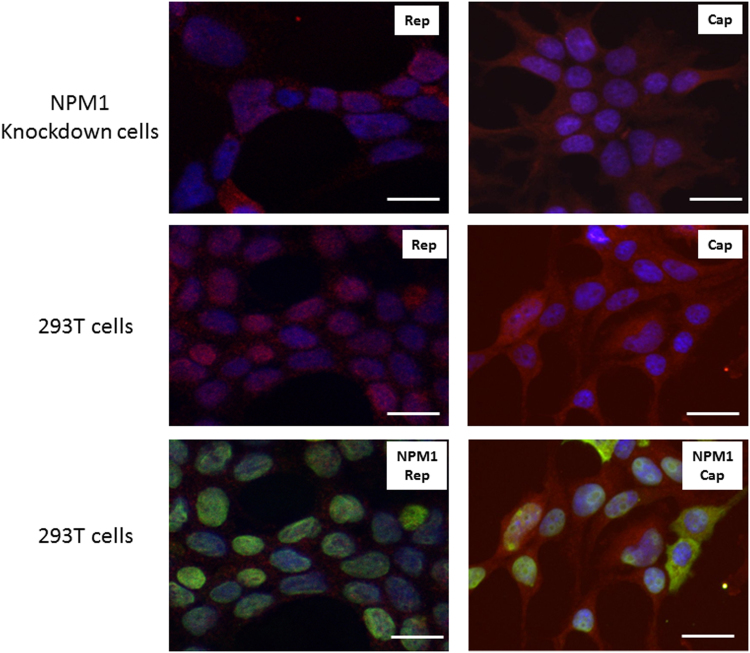
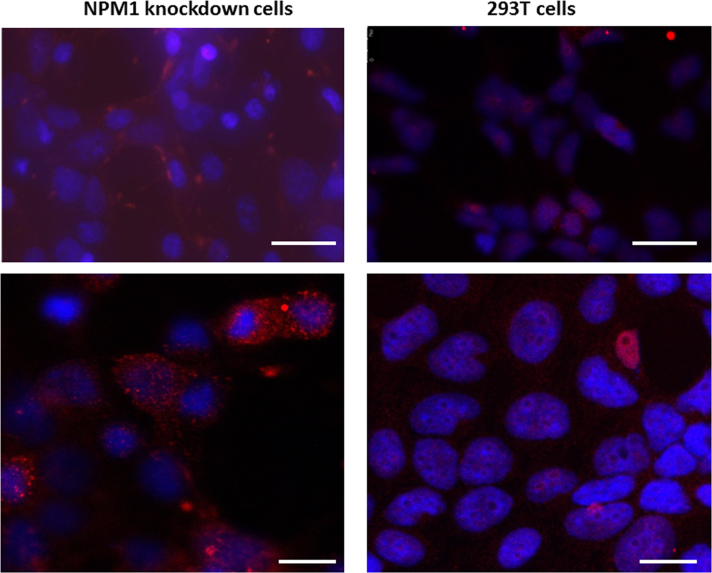
Fig. 5 showed that under fluorescence microscopy, AAV Rep proteins predominantly located in the nuclei (Fig. 5, left panels, labelled in red) and Cap proteins predominantly in cytoplasm (Fig. 5, right panels, labelled in red). In addition, there is no significant difference between NPM1 knockdown (Fig. 5, top panels) and control 293T cells (Fig. 5, middle panels) in the localisation of AAV Rep (Fig. 5, left panel) and Cap (Fig. 5, right panel) proteins. The bottom panel of Fig. 5 showed the nuclear localisation of endogenous NPM1 (Fig. 5, labelled in green) and NPM1 co-localisation with AAV proteins (Fig. 5, labelled in red) in the 293T cells. No NPM1 proteins were detected in NPM1 knockdown cells (data not shown). Fig. 6 showed that using an AAV capsid particle-specific antibody, the assembled AAV capsid were predominantly located in cytoplasm in NPM1 knockdown cells (Fig. 6, left panels, labelled in red), such a cytoplasmic localisation of the capsid particles was less evident in 293T cells (Fig. 6, right panels).
Fig. 5.
Fluorescence microscopy of NPM1 and AAV Rep and Cap proteins; showing nuclear localisation of AAV Rep (labelled in red, left panels) and NPM1 proteins (in green, bottom panels) and cytoplasm localisation of AAV Cap protein (in red, right panels) in NPM1 knockdown cells (top panels) and parental 293T cells (middle and bottom panels) after transient transfection of AAV packaging and helper plasmids. Nuclei were labelled with Dapi in blue. Scale bars are 50 µm.
Fig. 6.
Confocal microscopy of assembled AAV2 particles (labelled in red): showing cytoplasm localisation of AAV particles in NPM1 knockdown cells (labelled in red, left panels) compared to nuclear localisation of AAV particles in control 293T cells (labelled in red, right panels). Nuclei were labelled with Dapi in blue. Scale bars are 200 µm for top panel and 15 µm for bottom panel.
4. Discussion
Our results showed for the first time that down-regulation of nucleolar protein Nucleophosmin (NPM1) resulted in up to 35 fold increase in AAV2 and AAV8 vector production. AAV production could be divided into the following stages: stage (1) entry of plasmid DNA into the producer cell nuclei, (2) AAV Rep and Cap protein expression, (3) AAV empty capsid assembly in nuclei (Wistuba et al., 1997), (4) AAV vector DNA amplification, (5) vector DNA-capsid packaging into fully assembled particles, (6) maturation of DNA-packaged particles (full particles) and finally (7) the matured full particles released from nuclei into cytoplasm (Kerr et al., 2006). In this study, NPM1 knockdown showed direct influence on 3 stages of AAV production process, that is, slight increase in AAV plasmid DNA entering nuclei (stage 1), significant increase in AAV DNA amplification (stage 4) and increase in mature AAV particles being released from nuclei to cytoplasm (stage 7), and ultimately led to significant increase in AAV vector production. Our results demonstrated an intrinsic NPM1 negative regulation on AAV replication and production.
The observed influence of NPM1 knockdown on AAV DNA amplification might largely attribute to the NPM1 DNA binding property. Nucleophosmin is a nucleolar protein with many functions including genome stability, DNA duplication and transcriptional regulation (Federici et al., 2010, Box et al., 2016). NPM1 showed high binding affinity to single-stranded nuclei acids and a negative regulation on DNA replication via DNA G-Quadruplex sequence (GQRS) (Gallo et al., 2012, Dumbar et al., 1989). For example, binding of NPM1 to the DNA G-Quadruplex sequence (GQRS) within the c-myc promoter caused a marked down-regulation of c-myc oncogene (Gallo et al., 2012, Perrone et al., 2014). Furthermore, previous studies have also shown that interaction of NPM1 with DNA GQRS could lead to the repression of cellular and viral DNA replication (Scognamiglio et al., 2014, Siddiqui-Jain et al., 2002).
Destruction of G-quadruplex by GQRS ligands, e.g. BRACO-19, could effectively unblock GQRS inhibition of viral DNA replication of HIV and HSV viruses (Harris and Merrick, 2015). In addition to the GQRS functions due to its unique structure, the interaction of DNA GQRS with cellular proteins, e.g. Nucleolin and NPM1, could also lead to the repression of cellular and viral DNA replication (Scognamiglio et al., 2014, Siddiqui-Jain et al., 2002). Federici et al. showed that NPM1 preferentially bound to the GQRS region and induced the formation of GQRS structures in cellular DNA, e.g. SOD2 gene promoter. Studies have also shown that direct NPM1 interaction with adenoviral DNA interfered with adenoviral DNA replication (Hindley et al., 2007, Samad et al., 2012). Mutations of MPN1 that prevented NPM1 interaction with GQRS structure led to oncogene expression and pathogenesis of acute myeloid leukaemia (Banuelos et al., 2013), further demonstrating a GQRS dependent mechanism of NPM1 in gene regulation.
There has been so far no report on the presence or function of the GQRS structure within the AAV genome. Our results showed for the first time that NPM1 interaction with AAV ITR was GQRS specific as NPM1 binding to the two GQRS within AAV ITR could be competed out by c-myc/GQRS. It is likely that the observed increase in AAV vector DNA production in NPM1 knockdown cells might be due to (1) that NPM1 knockdown removed the NPM1 induction of GQRS structure formation and thus reduced the road blocks to AAV vector DNA replication, and (2) NPM1 knockdown removed NPM1 interaction with AAV DNA and thus reduced NPM1 negative regulation of AAV DNA replication. This ultimately led to the significant increase in AAV DNA replication and AAV production in NPM1 knockdown cells, which was consistent with the proposed function of GQRS and NPM1-GQRS interaction in DNA regulation (Lyonnais et al., 2003, Harris and Merrick, 2015, Huppert, 2008).
The well-established NPM1 function as a chaperone protein (Federici et al., 2010, Box et al., 2016) might contribute to the observed influence of NPM1 knockdown on the matured and packaged AAV particles being released from nuclei into cytoplasm. NPM1 regulation of virus life cycle has been so far considered predominantly as a result of direct NPM1 and viral protein-protein interaction (Bevington et al., 2007, Hindley et al., 2007, Ugai et al., 2012, Liu et al., 2012, Gadad et al., 2011). Endogenous NPM1 has been reported to create a barrier at the early stage of AAV infection for subcellular AAV trafficking and for AAV capsid mobilisation from nucleolus to nucleoplasm, highlighting the chaperone property of NPM1 proteins (Liu et al., 2012). Wistuba et al. (1997) has shown that AAV Cap proteins accumulated in the nucleolus in the absence of Rep protein or AAV DNA, suggesting that AAV empty capsid assembly occurred in the nucleolus. Samad et al. (2012) has demonstrated that NPM1 knockdown significantly increased the accumulation of Adenovirus capsids in nuclei and the packaging of viral DNA with Adenovirus core proteins and ultimately influenced the maturation of Adenovirus particles. Although there have been no direct reports of NPM1 function on AAV packaging and maturation, our results showed that NPM1 knockdown led to increased localisation of packaged AAV particles in cytoplasm in NPM1 knockdown cells, supporting the proposed mechanism by Samad et al. (2012) of NPM1 knockdown in virus assembly, maturation and trafficking.
The expression of AAV Rep and Cap proteins and the localisation of un-assembled Cap proteins were not significantly affected by NPM1 knockdown, indicating that the observed NPM1 barrier for subcellular trafficking of AAV proteins during AAV infection was not occurring during AAV production. This might be because the presence of Adenoviral helper function in the AAV production system had redistributed NPM1 away from nucleolus, as reported previously (Johnson and Samulski, 2009a, Johnson and Samulski, 2009b). NPM1 expression has been previously reported to decrease in cells undergoing apoptosis and NPM1 was considered as an anti-apoptotic protein (Frehlick et al., 2007). Such progressive decrease in NPM1 expression was also detected during AAV production in this study, reflecting the induced-apoptosis by the expression of AAV and adenoviral helper virus.
5. Conclusion
In summary, this study systematically investigated NPM1 function on AAV vector production, revealing for the first time the presence of GQRS in the AAV genome, the synergistic NPM1-GQRS function in AAV production and the significant enhancement of NPM1gene knockdown on AAV vector production. Our earlier study has likewise shown a similar enhanced AAV vector production by another DNA binding protein, Y-box protein 1 (YB1) (Satkunanathan et al., 2014), revealing that NPM1 and YB1, which are both DNA binding proteins, might utilise different mechanisms in influencing AAV production. For example, knockdown YB1 removed its competition with Adenovirus E2A and AAV capsid proteins for binding to a specific GGGG(TT) motif in the ITR sequence and thus increased Adenovirus-helped AAV DNA production and packaging (Satkunanathan et al., 2014). NPM1 knockdown removed NPM1-induced formation of GQRS structure and thus reduced the road blocks to AAV vector DNA replication. Although double knockdown of NPM1 and YB1 did not have an add on effect on AAV production (data not shown), our studies highlighted an imperative function of these DNA binding proteins in the AAV life cycle. Understanding the role of cellular proteins in the AAV life cycle would greatly facilitate future production of high titre AAV vectors and may reduce the production of defective empty particles and, ultimately improve the quality and safety of AAV vectors for clinical use.
Author disclosure statement
No competing financial interests exist for all authors.
Acknowledgements
The project is funded by UK Department of Health.
References
- Artusi S., Nadai M., Perrone R., Biasolo M.A., Palu G., Flamand L., Calistri A., Richter S.N. The Herpes Simplex Virus-1 genome contains multiple clusters of repeated G-quadruplex: implications for the antiviral activity of a G-quadruplex ligand. Antivir. Res. 2015;118:123–131. doi: 10.1016/j.antiviral.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos S., Lectez B., Taneva S.G., Ormaza G., Alonso-Marino M., Calle X., Urbaneja M.A. Recognition of intermolecular G-quadruplexes by full length nucleophosmin. Effect of a leukaemia-associated mutation. FEBS Lett. 2013;587(14):2254–2259. doi: 10.1016/j.febslet.2013.05.055. [DOI] [PubMed] [Google Scholar]
- Bevington J.M., Needham P.G., Verrill K.C., Collaco R.F., Basrur V., Trempe J.P. Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions. Virology. 2007;357(1):102–113. doi: 10.1016/j.virol.2006.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box J.K., Paquet N., Adams M.N., Boucher D., Bolderson E., O'Byrne K.J., Richard D.J. Nucleophosmin: from structure and function to disease development. BMC Mol. Biol. 2016;17(1):19–016-0073-9. doi: 10.1186/s12867-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brument N., Morenweiser R., Blouin V., Toublanc E., Raimbaud I., Cherel Y., Folliot S., Gaden F., Boulanger P., Kroner-Lux G., Moullier P., Rolling F., Salvetti A. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and −5. Mol. Ther.: J. Am. Soc. Gene Ther. 2002;6(5):678–686. doi: 10.1006/mthe.2002.0719. [DOI] [PubMed] [Google Scholar]
- Bryant L.M., Christopher D.M., Giles A.R., Hinderer C., Rodriguez J.L., Smith J.B., Traxler E.A., Tycko J., Wojno A.P., Wilson J.M. Lessons learned from the clinical development and market authorization of Glybera. Human. gene Ther. Clin. Dev. 2013;24(2):55–64. doi: 10.1089/humc.2013.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B.J. Adeno-associated virus helper functions. In: Tijssen P., editor. Vol. 1. CRC Press; Boca Raton, Fla: 2004. pp. 255–295. (Handbook of Parvoviruses). [Google Scholar]
- Chang L.S., Shi Y., Shenk T. Adeno-associated virus P5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J. Virol. 1989;63(8):3479–3488. doi: 10.1128/jvi.63.8.3479-3488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.K., Nash K., Byrne B.J., Muzyczka N., Song S. The effect of DNA-dependent protein kinase on adeno-associated virus replication. PloS One. 2010;5(12):e15073. doi: 10.1371/journal.pone.0015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Duan X., Chow H.Y., Chen L., Lu H., Wu W., Hauck B., Wright F., Kapranov P., Xiao W. Proteomics analysis of co-purifying cellular proteins associated with rAAV vectors. PloS One. 2014;9(2):e86453. doi: 10.1371/journal.pone.0086453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbar T.S., Gentry G.A., Olson M.O. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry. 1989;28(24):9495–9501. doi: 10.1021/bi00450a037. [DOI] [PubMed] [Google Scholar]
- Federici L., Arcovito A., Scaglione G.L., Scaloni F., Lo Sterzo C., Di Matteo A., Falini B., Giardina B., Brunori M. Nucleophosmin C-terminal leukemia-associated domain interacts with G-rich quadruplex forming DNA. J. Biol. Chem. 2010;285(48):37138–37149. doi: 10.1074/jbc.M110.166736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehlick L.J., Eirin-Lopez J.M., Ausio J. New insights into the nucleophosmin/nucleoplasmin family of nuclear chaperones. Bioessay.: News Rev. Mol. Cell. Dev. Biol. 2007;29(1):49–59. doi: 10.1002/bies.20512. [DOI] [PubMed] [Google Scholar]
- Gadad S.S., Rajan R.E., Senapati P., Chatterjee S., Shandilya J., Dash P.K., Ranga U., Kundu T.K. HIV-1 infection induces acetylation of NPM1 that facilitates Tat localization and enhances viral transactivation. J. Mol. Biol. 2011;410(5):997–1007. doi: 10.1016/j.jmb.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Gallo A., Lo Sterzo C., Mori M., Di Matteo A., Bertini I., Banci L., Brunori M., Federici L. Structure of nucleophosmin DNA-binding domain and analysis of its complex with a G-quadruplex sequence from the c-MYC promoter. J. Biol. Chem. 2012;287(32):26539–26548. doi: 10.1074/jbc.M112.371013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez M., Marie S.K., Oba-Shinjo S.M., Uno M., da Silva R., Laure H.J., Izumi C., Otake A., Chammas R., Rosa J.C. Quantitative proteomic analysis and functional studies reveal that nucleophosmin is involved in cell death in glioblastoma cell line transfected with siRNA. Proteomics. 2012;12(17):2632–2640. doi: 10.1002/pmic.201200034. [DOI] [PubMed] [Google Scholar]
- Harris L.M., Merrick C.J. G-quadruplexes in pathogens: a common route to virulence control? PLoS Pathog. 2015;11(2):e1004562. doi: 10.1371/journal.ppat.1004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P.L., Santin A.D., Batchu R.B. The adeno-associated virus Rep78 major regulatory/transformation suppressor protein binds cellular Sp1 in vitro and evidence of a biological effect. Cancer Res. 1996;56(22):5299–5304. [PubMed] [Google Scholar]
- Hindley C.E., Davidson A.D., Matthews D.A. Relationship between adenovirus DNA replication proteins and nucleolar proteins B23.1 and B23.2. J. General. Virol. 2007;88(Pt 12):3244–3248. doi: 10.1099/vir.0.83196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani K., Szebeni A., Olson M.O. Mapping the functional domains of nucleolar protein B23. J. Biol. Chem. 2000;275(32):24451–24457. doi: 10.1074/jbc.M003278200. [DOI] [PubMed] [Google Scholar]
- Hirsch M.L., Li C., Bellon I., Yin C., Chavala S., Pryadkina M., Richard I., Samulski R.J. Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol. Ther.: J. Am. Soc. Gene Ther. 2013;21(12):2205–2216. doi: 10.1038/mt.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaviy H.B., Burley S.K. Thermodynamic analysis of the interaction between YY1 and the AAV P5 promoter initiator element. Chem. Biol. 2001;8(2):179–187. doi: 10.1016/s1074-5521(00)90066-8. [DOI] [PubMed] [Google Scholar]
- Huppert J.L. Four-stranded nucleic acids: structure, function and targeting of G-quadruplexes. Chem. Soc. Rev. 2008;37(7):1375–1384. doi: 10.1039/b702491f. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Lewis S.M., Wu X., Ma C., Munroe D.J., Fuess S., Storm T.A., Kay M.A., Nakai H. DNA palindromes with a modest arm length of greater, similar 20 base pairs are a significant target for recombinant adeno-associated virus vector integration in the liver, muscles, and heart in mice. J. Virol. 2007;81(20):11290–11303. doi: 10.1128/JVI.00963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup M., Greenberg B., Mancini D., Cappola T., Pauly D.F., Jaski B., Yaroshinsky A., Zsebo K.M., Dittrich H., Hajjar R.J., Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.S., Samulski R.J. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J. Virol. 2009;83(6):2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.S., Samulski R.J. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J. Virol. 2009;83(6):2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J.R., Cotmore S.F., Bloom M.E., Linden R.M., Parrish C.R. Hodder Arnold publisher; 2006. Parvoviruses; pp. 305–319. [Google Scholar]
- King J.A., Dubielzig R., Grimm D., Kleinschmidt J.A. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 2001;20(12):3282–3291. doi: 10.1093/emboj/20.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.D., Chen Y.L., Min Y.L., Zhao B., Cheng C.P., Kang M.S., Chiu S.J., Kieff E., Peng C.W. The nuclear chaperone nucleophosmin escorts an Epstein-Barr Virus nuclear antigen to establish transcriptional cascades for latent infection in human B cells. PLoS Pathog. 2012;8(12):e1003084. doi: 10.1371/journal.ppat.1003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyonnais S., Gorelick R.J., Mergny J.L., Le Cam E., Mirambeau G. G-quartets direct assembly of HIV-1 nucleocapsid protein along single-stranded DNA. Nucleic Acids Res. 2003;31(19):5754–5763. doi: 10.1093/nar/gkg716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.A. Adenovirus protein V induces redistribution of nucleolin and B23 from nucleolus to cytoplasm. J. Virol. 2001;75(2):1031–1038. doi: 10.1128/JVI.75.2.1031-1038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métifiot M., Amrane S., Litvak S., Andreola M.L. G-quadruplexes in viruses: function and potential therapeutic applications. Nucleic Acids Res. 2014;42(20):12352–12366. doi: 10.1093/nar/gku999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Nash K., Chen W., Salganik M., Muzyczka N. Identification of cellular proteins that interact with the adeno-associated virus rep protein. J. Virol. 2009;83(1):454–469. doi: 10.1128/JVI.01939-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C., O'Beirne J., Smith K., Pasi J., Glader B., Rustagi P., Ng C.Y., Kay M.A., Zhou J., Spence Y., Morton C.L., Allay J., Coleman J., Sleep S., Cunningham J.M., Srivastava D., Basner-Tschakarjan E., Mingozzi F., High K.A., Gray J.T., Reiss U.M., Nienhuis A.W., Davidoff A.M. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. New Engl. J. Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumer M., Sonntag F., Schmidt K., Nieto K., Panke C., Davey N.E., Popa-Wagner R., Kleinschmidt J.A. Properties of the adeno-associated virus assembly-activating protein. J. Virol. 2012;86(23):13038–13048. doi: 10.1128/JVI.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni T.H., McDonald W.F., Zolotukhin I., Melendy T., Waga S., Stillman B., Muzyczka N. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J. Virol. 1998;72(4):2777–2787. doi: 10.1128/jvi.72.4.2777-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas B.L., Skipp P., Barton S., Singh D., Bagmane D., Mould R., Angco G., Ward J., Guha-Niyogi B., Wilson S., Howarth P., Davies D.E., Rennard S., O'Connor C.D., Djukanovic R. Identification of lipocalin and apolipoprotein A1 as biomarkers of chronic obstructive pulmonary disease. Am. J. Respir. Crit. care Med. 2010;181(10):1049–1060. doi: 10.1164/rccm.200906-0857OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A., Jolinon N., Alazard-Dany N., Barateau V., Epstein A.L., Greco A., Buning H., Salvetti A. Factors influencing helper-independent adeno-associated virus replication. Virology. 2012;432(1):1–9. doi: 10.1016/j.virol.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Pegoraro G., Marcello A., Myers M.P., Giacca M. Regulation of adeno-associated virus DNA replication by the cellular TAF-I/set complex. J. Virol. 2006;80(14):6855–6864. doi: 10.1128/JVI.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira D.J., Muzyczka N. The cellular transcription factor SP1 and an unknown cellular protein are required to mediate Rep protein activation of the adeno-associated virus p19 promoter. J. Virol. 1997;71(3):1747–1756. doi: 10.1128/jvi.71.3.1747-1756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone R., Butovskaya E., Daelemans D., Palu G., Pannecouque C., Richter S.N. Anti-HIV-1 activity of the G-quadruplex ligand BRACO-19. J. Antimicrob. Chemother. 2014;69(12):3248–3258. doi: 10.1093/jac/dku280. [DOI] [PubMed] [Google Scholar]
- Piekna-Przybylska D., Sullivan M.A., Sharma G., Bambara R.A. U3 region in the HIV-1 genome adopts a G-quadruplex structure in its RNA and DNA sequence. Biochemistry. 2014;53(16):2581–2593. doi: 10.1021/bi4016692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Brown K.E. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257(2):373–382. doi: 10.1006/viro.1999.9664. [DOI] [PubMed] [Google Scholar]
- Samad M.A., Komatsu T., Okuwaki M., Nagata K. B23/nucleophosmin is involved in regulation of adenovirus chromatin structure at late infection stages, but not in virus replication and transcription. J. General. Virol. 2012;93(Pt 6):1328–1338. doi: 10.1099/vir.0.036665-0. [DOI] [PubMed] [Google Scholar]
- Satkunanathan S., Wheeler J., Thorpe R., Zhao Y. Establishment of a novel cell line for the enhanced production of recombinant adeno-associated virus vectors for gene therapy. Human Gene Ther. 2014;25(11):929–941. doi: 10.1089/hum.2014.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scognamiglio P.L., Di Natale C., Leone M., Poletto M., Vitagliano L., Tell G., Marasco D. G-quadruplex DNA recognition by nucleophosmin: new insights from protein dissection. Biochim. Et. Biophys. Acta. 2014;1840(6):2050–2059. doi: 10.1016/j.bbagen.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A., Grand C.L., Bearss D.J., Hurley L.H. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. USA. 2002;99(18):11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag F., Kother K., Schmidt K., Weghofer M., Raupp C., Nieto K., Kuck A., Gerlach B., Bottcher B., Muller O.J., Lux K., Horer M., Kleinschmidt J.A. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J. Virol. 2011;85(23):12686–12697. doi: 10.1128/JVI.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach S., Wistuba A., Bock T., Kleinschmidt J.A. Assembly of adeno-associated virus type 2 capsids in vitro. J. General. Virol. 1997;78(Pt 6):1453–1462. doi: 10.1099/0022-1317-78-6-1453. [DOI] [PubMed] [Google Scholar]
- Stracker T.H., Cassell G.D., Ward P., Loo Y.M., van Breukelen B., Carrington-Lawrence S.D., Hamatake R.K., van der Vliet P.C., Weller S.K., Melendy T., Weitzman M.D. The Rep protein of adeno-associated virus type 2 interacts with single-stranded DNA-binding proteins that enhance viral replication. J. Virol. 2004;78(1):441–453. doi: 10.1128/JVI.78.1.441-453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlučková K., Marusic M., Tothova P., Bauer L., Sket P., Plavec J., Viglasky V. Human papillomavirus G-quadruplexes. Biochemistry. 2013;52(41):7207–7216. doi: 10.1021/bi400897g. [DOI] [PubMed] [Google Scholar]
- Ugai H., Dobbins G.C., Wang M., Le L.P., Matthews D.A., Curiel D.T. Adenoviral protein V promotes a process of viral assembly through nucleophosmin 1. Virology. 2012;432(2):283–295. doi: 10.1016/j.virol.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Berns K.I. In vitro replication of adeno-associated virus DNA: enhancement by extracts from adenovirus-infected HeLa cells. J. Virol. 1996;70(7):4495–4501. doi: 10.1128/jvi.70.7.4495-4501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman M.D. The parvovirus life cycle: an introduction to molecular interactions important for infection. In: Kerr J.R., Cotmore S., Bloom M.E., editors. Parvoviruses. Hodder Arnold; London, UK: 2006. [Google Scholar]
- Werling N.J., Satkunanathan S., Thorpe R., Zhao Y. Systematic comparison and validation of quantitative real-time PCR methods for the quantitation of adeno-associated viral products. Human. gene Ther. Methods. 2015;26(3):82–92. doi: 10.1089/hgtb.2015.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba A., Kern A., Weger S., Grimm D., Kleinschmidt J.A. Subcellular compartmentalization of adeno-associated virus type 2 assembly. J. Virol. 1997;71(2):1341–1352. doi: 10.1128/jvi.71.2.1341-1352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]