Abstract
BACKGROUND
Re-hospitalization after discharge for acute decompensated heart failure is a common problem. Low-socioeconomic urban patients suffer high rates of re-hospitalization and often over-utilize the emergency department (ED) for their care. We hypothesized that early consultation with a cardiologist in the ED can reduce re-hospitalization and health care costs for low-socioeconomic urban patients with acute decompensated heart failure.
METHODS
There were 392 patients treated at our center for acute decompensated heart failure who received standardized education and follow-up. Patients who returned to the ED received early consultation with a cardiologist; 392 patients who received usual care served as controls. Thirty- and 90-day re-hospitalization, ED re-visits, heart failure symptoms, mortality, and health care costs were recorded.
RESULTS
Despite guideline-based education and follow-up, the rate of ED re-visits was not different between the groups. However, the rate of re-hospitalization was significantly lower in patients receiving the intervention compared with controls (odds ratio 0.592), driven by a reduction in the risk of readmission from the ED (0.56 vs 0.79, respectively). Patients receiving the intervention accumulated 14% fewer re-hospitalized days than controls and 57% lower 30-day total health care cost. Despite the reduction in health care resource consumption, mortality was unchanged. After accounting for the total cost of intervention delivery, the health care cost savings was substantially greater than the cost of intervention delivery.
CONCLUSION
Early consultation with a cardiologist in the ED as an adjunct to guideline-based follow-up is associated with reduced re-hospitalization and health care cost for low-socioeconomic urban patients with acute decompensated heart failure.
Keywords: Cost-effectiveness, Heart failure, Readmission, Re-hospitalization
Acute decompensated heart failure is the most common reason for hospitalization and re-hospitalization in the US, with underserved patients disproportionately affected.1–3 The Centers for Medicare & Medicaid Services (CMS) penalize centers with excess 30-day re-hospitalizations.4 As target rates are not adjusted for social disparities, penalties are imposed disproportionately on centers that care for under-served patients.1 Attempts to reduce re-hospitalizations have had varying success,5–9 especially in low-socioeconomic urban patients. These patients consume disproportionately more health care resources than more affluent patients and often over-utilize the emergency department (ED) for their care,10–13 leading to fragmented care and elevated health care costs.14
The University of Chicago Medical Center (UCMC) is a tertiary care center on Chicago’s South Side, one of the poorest and most violent regions of the city.15 In accordance with the 2013 American College of Cardiology/American Heart Association Guideline for the Management of Heart Failure,16 patients treated at our center with acute decompensated heart failure beginning in January 2015 received standardized education while admitted and early outpatient follow-up after discharge. In addition, patients who returned to the ED after discharge received early consultation with a cardiologist. The purpose of this study was to determine if guideline-directed education and early follow-up reduced 30-day re-visits to the ED in this population, and if not, to determine whether early consultation with a cardiologist in the ED could reduce readmissions in a cost-efficient manner.
METHODS
Study Design
A retrospective cohort study was performed. Patients treated on the General Cardiology Service at UCMC for a primary diagnosis of acute decompensated heart failure January–December 2015 received the intervention described below (Figure 1). Patients with a ventricular assist device or orthotopic heart transplant and patients who planned to receive either within the study period were excluded. Patients receiving the intervention in 2015 were compared against a nearest neighbor propensity-matched control cohort of patients treated for acute decompensated heart failure at UCMC the prior year who received usual care. The rate of 30-day re-hospitalization after heart failure discharge in our center had been stable at approximately 22% for at least 5 years, and data from CMS indicate that 5% of patients discharged from UCMC are re-hospitalized at outside centers within 30 days. Data were obtained via query of the medical record. The study protocol was approved by the Institutional Review Board at the University of Chicago under a general waiver of consent. The intervention was designed as a quality improvement intervention and approved by the Office of Clinical Effectiveness at the University of Chicago.
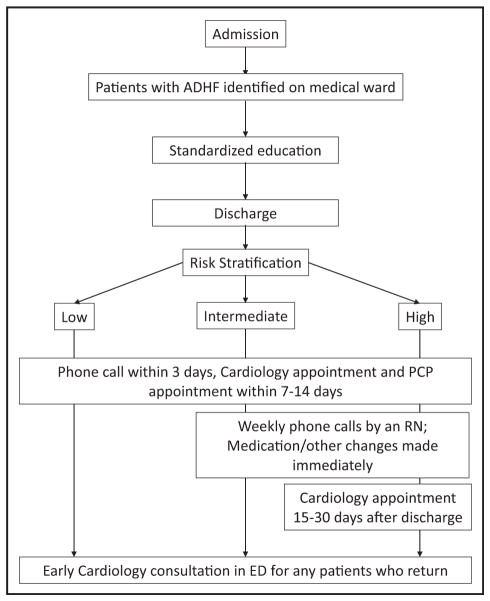
Figure 1.
Outline of the intervention. Patients with acute decompensated heart failure were identified on the medical ward after admission. All patients received standardized education, medication reconciliation, social needs assessment, and disposition assessment. At the time of discharge, patients were risk stratified. All patients received a telephone call from a nurse within 3 days of discharge and a cardiology and primary care physician (PCP) appointment within 7–14 days of discharge. Intermediate- and high-risk patients additionally received weekly telephone calls from a nurse for 4 weeks. High-risk patients received an additional clinic appointment 15–30 days after discharge. ADHF = acute decompensated heart failure; ED = emergency department.
Intervention Design and Delivery
Patients received 3 one-on-one education sessions with specially trained nurse educators regarding lifestyle modification, self-care, emergency plans, and need for adherence. Patients received nutritional counseling from a dietitian, with a focus on restricting dietary sodium. Pharmacists reviewed medications, side effects, and need for compliance. Rehabilitation specialists educated patients about appropriate exercise. Patients received written material explaining these topics in patient-appropriate language. When available, family members or caregivers were included in educational sessions.
After discharge, patients received a telephone call from a specially trained nurse within 3 days of discharge during which the patient’s status was assessed using a standardized questionnaire (Supplementary Figure 1, available online). Patients with concerning responses (as noted) were contacted by a cardiovascular nurse practitioner (NP), social worker, or pharmacist, with changes made to their medication regimen as necessary. All patients received an appointment with their cardiologist (if available) or cardiovascular NP and an appointment with their primary care provider within 7–14 days of discharge. At each visit, symptoms were assessed, a physical examination was performed, new complaints were addressed, education was reinforced, and medications were adjusted as needed. Patients with a HOSPITAL score17,18 ≥ 5 (intermediate- to high-risk for re-hospitalization) received additional weekly telephone calls, and patients with a HOSPITAL score ≥7 (high risk) also received an additional cardiology appointment 15–30 days after discharge.
For patients who returned to the ED after discharge, immediate consultation with a cardiologist familiar with the patient’s history was obtained before any nonemergent work-up or treatment was begun. The cardiologist provided relevant background of the patient’s baseline symptoms as well as past health care utilization patterns. The cardiologist guided the work-up and treatment of the patient’s complaint by recommending diagnostic testing, medication selection, and follow-up. If the ED physician and cardiologist agreed that the patient could be safely discharged with close outpatient follow-up, the patient was discharged. However, if, after considering the cardiologist’s recommendations, the ED physician felt the patient required readmission, the patient was readmitted.
Measurement of Educational Efficacy
Patients receiving the intervention January–June 2015 completed the Atlanta Heart Failure Knowledge Test prior to and after education. A group of 15 patients hospitalized with acute decompensated heart failure who did not receive the standardized education served as controls.
Measurement of Heart Failure Symptoms
Patients receiving the intervention July–December 2015 completed the Minnesota Living with Heart Failure Questionnaire (MLHFQ)19 at the time of discharge to quantify their heart failure-related symptoms. The MLHFQ was administered again by telephone 30 days later.
Outcomes
The primary outcome was all-cause unplanned re-hospitalization within 30 days of discharge. Planned re-hospitalizations (such as elective procedures and chemotherapy treatments) were not included. Secondary outcomes included a composite endpoint of all-cause unplanned re-hospitalization and death, ED re-visits, a composite endpoint of ED re-visits or death, total re-hospitalized days, total 90-day health care expenditure at our center, and all-cause 90-day mortality.
Calculation of Re-hospitalization Rates
Re-hospitalizations were defined as an unplanned readmission to the hospital (regardless of inpatient or observation status). Unplanned re-visits to the ED that did not result in readmission were tabulated separately. Re-hospitalizations were tabulated as a dichotomous outcome such that patients with one or more re-hospitalizations within 90 days of the index discharge contributed a single re-hospitalization event at the date of earliest re-hospitalization. Planned re-hospitalizations were excluded using the CMS Planned Readmission Algorithm Version 2.120 (Appendix: Methods; available online).
Calculation of Total Re-hospitalized Days
Total re-hospitalized days were calculated as the sum of the individual lengths of stay for all re-hospitalizations following an index discharge.
Cost Analysis
Time-driven activity-based costing21 was performed. Task times were recorded for activities related to the intervention that were not part of usual care. Cost per minute was calculated by dividing the Illinois average salary for each discipline by 124,800, the number of minutes in a work-year. The marginal cost for delivering the complete intervention was calculated as the cost per minute for each discipline multiplied by the number of minutes each staff member spent providing the intervention. The cardiologist (0.5 full-time equivalent) and NP (1.0 full-time equivalent) were considered fixed costs and valued at $250,000 and $100,000 per year, respectively.
Total health care expenditure was calculated by querying billing data at UCMC and summing the net expected pay (Appendix: Methods; available online) from all encounters following the index discharge. Data were adjusted for inflation and reported in $US2016.
Statistical Methods
Data were analyzed using SPSS 22.0 (IBM, Armonk, NY). Categorical outcomes were assessed using McNemar’s test or Fisher’s exact test, as appropriate. Because the intervention involved aggressive follow-up during the first 30 days after discharge only, we expected a larger effect on re-hospitalization during this period, and therefore, Kaplan-Meier survival distributions were compared using the generalized Wilcoxon (Breslow) test. Cumulative endpoints were compared using the Mann-Whitney U test. Ordinal variables were compared using the Mann-Whitney U test for unpaired data or the Wilcoxon signed rank test for paired data. The relationship between the intervention, days post discharge, and cumulative cost and re-hospitalized days was assessed using linear regression with an interaction term between intervention and days. Clinical characteristics were compared using an unpaired 2-way Student’s t test for continuous data or Fisher’s exact test for categorical data. Bonferroni correction was used for multiple hypothesis testing. All data are reported as mean ± standard deviation unless otherwise indicated. A P-value of <.05 was considered statistically significant.
RESULTS
There were 392 patients that received the intervention from January to December 2015, and 392 propensity-matched patients who received usual care served as controls. Patients were matched by age, sex, race, left ventricular ejection fraction, household income, comorbidities, health care utilization during the prior year, relevant laboratories, vital signs, readmission risk score, and guideline-based medication usage. Clinical characteristics are shown in Table 1; 80% of patients met the US Census Bureau definition of “low-income,” defined as earning less than twice the federal poverty threshold for a working family of 4, or $45,622. Data related to quality of intervention delivery and heart failure symptoms are reported in (the Appendix: Results, available online).
Table 1.
Clinical Characteristics
| Control | Intervention | P-Value | |
|---|---|---|---|
| Age (y) | 64.8 ± 1.7 | 65.1 ± 1.9 | .782 |
| Female sex (%) | 47 | 47 | 1.000 |
| Black race (%) | 83 | 84 | .773 |
| Hispanic/Latino (%) | 3 | 3 | 1.000 |
| Hospital risk score (%) | .276 | ||
| High risk (≥7) | 19 | 18 | |
| Intermediate risk (5–6) | 33 | 33 | |
| Low risk (≤4) | 48 | 49 | |
| Hospitalizations in prior year (#) | 1.5 ± 1.7 | 1.4 ± 1.9 | .201 |
| ED visits in prior year (#) | 2.1 ± 3.2 | 1.9 ± 3.2 | .362 |
| LVEF (%) | 36.6 ± 10.1 | 36.7 ± 11.4 | .895 |
| BMI | 32.0 ± 10.0 | 32.7 ± 14.5 | .414 |
| Median household income ($) | 35,969 ± 28,969 | 35,969 ± 23,425 | .709 |
| COPD (%) | 13 | 14 | .916 |
| Diabetes (%) | 31 | 29 | .640 |
| Hypertension (%) | 79 | 75 | .206 |
| Dyslipidemia (%) | 31 | 26 | .081 |
| Coronary artery disease (%) | 40 | 38 | .509 |
| Atrial fibrillation (%) | 30 | 30 | .876 |
| Heart rate (beats per minute) | 81.4 ± 13.9 | 81.5 ± 15.2 | .900 |
| SBP (mm Hg) | 115.8 ± 19.7 | 116.6 ± 19.9 | .588 |
| DBP (mm Hg) | 65.7 ± 14.7 | 65.8 ± 13.5 | .968 |
| eGFR | 48.2 ± 48.2 | 45.7 ± 23.6 | .166 |
| LDL | 76.9 ± 28.3 | 81.2 ± 30.2 | .050 |
| HDL | 46.2 ± 15.9 | 45.5 ± 18.6 | .577 |
| Total cholesterol | 143.9 ± 36.1 | 148.5 ± 38.7 | .086 |
| Platelets | 228.9 ± 91.4 | 232.7 ± 87.9 | .556 |
| NT-proBNP | 11,911 ± 19,787 | 13,741 ± 22,978 | .232 |
| Hemoglobin | 11.2 ± 2.3 | 11.2 ± 2.3 | .899 |
| Beta-blocker (%) | |||
| Overall | 56 | 62 | .103 |
| Among eligible patients* | 90 | 92 | .537 |
| ACE/ARB (%) | |||
| Overall | 47 | 46 | .898 |
| Among eligible patients† | 97 | 94 | .111 |
| Aldosterone antagonists (%) | |||
| Overall | 21 | 25 | .104 |
| Among eligible patients‡ | 41 | 49 | .101 |
| Loop diuretic (%) | 67 | 69 | .540 |
| Statin (%) | 28 | 33 | .214 |
| Antiplatelets (%) | 31 | 34 | .493 |
| Warfarin (%) | 17 | 15 | .561 |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; COPD = chronic obstructive pulmonary disease; DBP = diastolic blood pressure; ED = emergency department; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro B-type natriuretic peptide; SBP = systolic blood pressure.
LVEF <40%, without uncontrolled asthma or COPD, bradycardia, hypotension, or prior intolerance.
LVEF <40%, without severe renal disease, hyperkalemia, renal artery stenosis, hypotension, or prior intolerance.
LVEF <40%, without severe renal disease, hyperkalemia, or prior intolerance.
Guideline-Based Follow-Up Alone Is Not Associated with Fewer ED Re-visits in Low-Socioeconomic Urban Patients
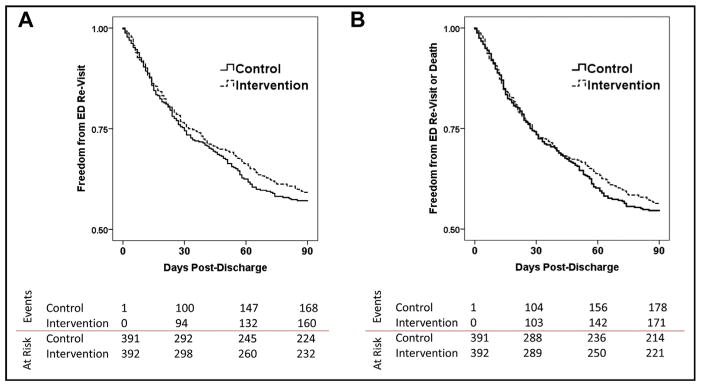
The Kaplan-Meier survival distributions for ED re-visit and the composite endpoint of ED re-visit or death are shown in Figure 2A and B. Despite objectively improved heart failure knowledge, aggressive telephone follow-up, and earlier and more frequent clinic appointments, there was no difference in ED re-visits or the composite endpoint. The Kaplan-Meier survival probability estimates for ED revisit and the composite endpoint of ED revisit or death at 90 days post discharge for intervention patients vs controls were 0.59 ± 0.03 vs 0.57 ± 0.03 and 0.56 ± 0.03 vs 0.55 ± 0.03, respectively, P = NS for both comparisons.
Figure 2.
Emergency department (ED) revisits (A) and the composite endpoint of ED revisits or death (B) within 90 days of discharge were not significantly different between the control and intervention groups.
Early Consultation with a Cardiologist in the ED Is Associated with Reduced Re-hospitalization in Low-Socioeconomic Urban Patients
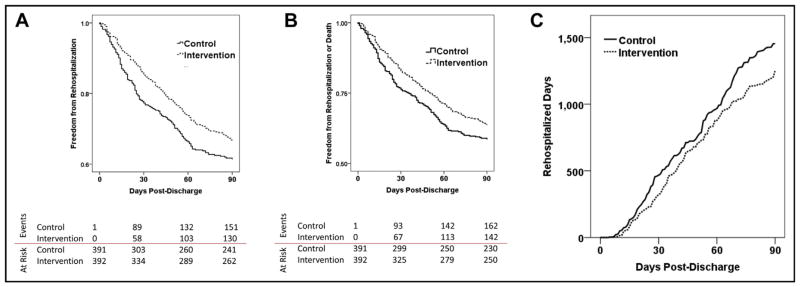
Fifty-eight patients were re-hospitalized within 30 days in the intervention group (14.8%), compared with 89 in the control group (22.7%) (odds ratio 0.592; 95% confidence interval, 0.400–0.867, P <.01). The number needed to treat to prevent one 30-day re-hospitalization was 13 (95% confidence interval, 7.5–40.5). This effect persisted through 90 days post discharge. The Kaplan-Meier survival distributions for all-cause unplanned re-hospitalization and the composite endpoint of all-cause unplanned re-hospitalization or death within 90 days post discharge are shown in Figure 3A and B. The Kaplan-Meier survival probability estimates for all-cause unplanned re-hospitalization and the composite endpoint of all-cause unplanned re-hospitalization or death at 90 days post discharge for intervention patients vs controls were 0.67 ± 0.02 vs 0.62 ± 0.03 and 0.64 ± 0.02 vs 0.59 ± 0.03, respectively; P <.05 for both comparisons. Within 90 days post discharge, intervention patients accumulated 203 fewer re-hospitalized days (14%) than controls (1250 vs 1453 days respectively, Figure 3C), and intervention status was a highly significant moderator of the effect between days post discharge and total re-hospitalized days (t = − 10.9, P <.01), with intervention patients accumulating fewer re-hospitalized days than controls. Within 1 year after discharge, the intervention patients experienced 148 (18%) fewer total re-hospitalizations (690 for intervention vs 838 for control) and 654 (12%) fewer total re-hospitalized days (4840 for intervention vs 5494 for control). Interestingly, whereas total admissions and re-hospitalized days were lower at 1 year, the number of patients readmitted at least once within 1 year was not statistically different (202 intervention vs 216 controls, P = NS).
Figure 3.
The intervention is associated with reduced re-hospitalization or death. Patients receiving the intervention had a significant reduction in (A) re-hospitalization and (B) the composite endpoint of re-hospitalization or death vs control patients and also (C) accumulated fewer re-hospitalized days within 90 days of index discharge.
Among patients who re-visited the ED, the risk of re-hospitalization from the ED was significantly lower in intervention patients vs controls (0.56 vs 0.69, respectively, P <.05), driven by a reduction in re-hospitalization among patients returning with cardiac-related complaints (Appendix: Results; available online). Length of stay in the ED was not significantly different among intervention patients (5.7 ± 5.8 hours) vs controls (6.6 ± 4.6 hours, P = .27). There was no difference in the risk of 90-day mortality (0.06 for both groups, P = NS).
The Intervention Is Associated with Reduced Total Health Care Expenditure
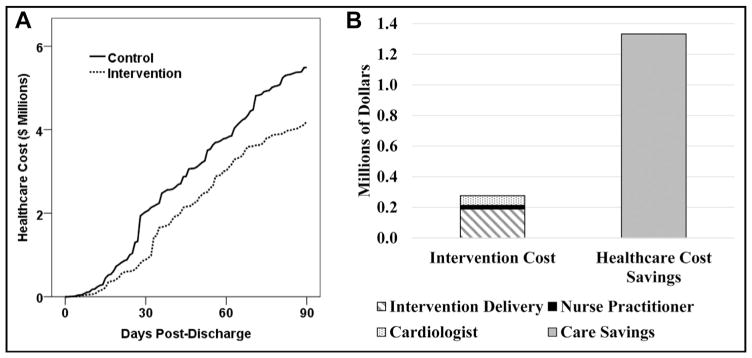
Total health care expenditure was 57% lower at 30 days and 24% lower at 90 days post discharge for intervention patients vs controls (Figure 4A). Among patients with at least one encounter within 30 days of discharge at our center (n = 258 control and 262 intervention patients), 30-day median health care cost was $862 in control patients (interquartile range $237–$6696) and $411 in intervention patients (interquartile range $156–$1444, P <.01), a reduction of 52%. Ninety-day component costs are shown in Supplementary Figure 2 (available online). The per-patient cost of the intervention is presented in Table 2. On average, the cost of delivering the intervention to a single patient on a single admission was $82.25. Given the number-needed-to-treat of 13 above, the marginal cost per readmission prevented was $1069 (exclusive of fixed costs such as the on-call cardiologist and NP). After including the cost of delivering the intervention to all patients and fixed costs for 90 days, the cost of the intervention was $1.1 million less than the 90-day health care cost savings from the intervention (Figure 4B). Intervention status was a highly significant moderator of the effect between days post discharge and health care cost (t = − 13.1, P <.01), with intervention patients consuming less health care cost than controls. One year after index discharge, health care cost consumption was $5.3 million (28%) less in the intervention group vs controls ($13.4M vs $18.8M, respectively).
Figure 4.
The intervention is associated with reduced health care cost. (A) Patients receiving the intervention consumed less total health care cost than control patients. (B) The cost of delivering the intervention to the 392 patients in this study for 90 days was $1.1 million less than the health care cost savings associated with the intervention.
Table 2.
Cost of Intervention Delivery
| Discipline | Minutes | Dollars |
|---|---|---|
| Nursing | 23.32 | $14.13 |
| PT/OT | 14.51 | $9.27 |
| Pharmacy | 24.31 | $23.23 |
| Nutrition | 10.02 | $4.57 |
| Social work | 10.41 | $4.18 |
| Appointment scheduling | 32.95 | $11.03 |
| Educational materials | $5.00 | |
| RN phone calls | 17.88 | $10.84 |
| Total | $82.25 |
OT = occupational therapy; PT = physical therapy; RN = registered nurse.
DISCUSSION
Our findings suggest that early consultation with a cardiologist in the ED is associated with reduced re-hospitalizations, readmitted days, and health care costs for patients who return to the ED after discharge. Heart failure symptoms improved compared with previously reported values, and mortality was unaffected. Interestingly, despite guideline-based education and follow-up, ED re-visits were not reduced, suggesting that intensive follow-up alone may be less effective in reducing health care utilization in a low-socioeconomic urban population. This finding is complementary to work suggesting underserved patients favor the ED for their care despite the availability of lower-cost options such as their primary care physician.11 For patients who return to the ED despite intensified education and follow-up, our results suggest that early consultation with a cardiologist familiar with each patient may reduce re-hospitalization and health care costs substantially without deleterious effects on mortality and heart failure symptoms.
Poor continuity of care is linked with over-testing, medical errors, high cost, and poor outcomes.22 Discharge process standardization, regimented follow-up, and downstream coordination all have been suggested to reduce re-hospitalizations and improve outcomes.23–26 However, the care transition from home back to the ED is especially vulnerable, as the receiving physicians are typically provided little contextual history, and continuity of care is near-completely lost. As low-socioeconomic patients often over-utilize the ED,10–13 they are exposed to this additional risk disproportionally, which may contribute to excess re-hospitalizations and costs in this population. Prior authors suggest that much of the variation in readmission rates among centers is driven by factors outside the hospital’s control27 and therefore, novel interventions that adapt to local cultural use (or misuse) of the health system may be necessary adjuncts to guideline-based care in order to reduce readmissions.
Home monitoring interventions that require a threshold level of participation and sophistication from the patient are often ineffective,28–30 even when bundled with other interventions.31 While some studies have shown benefit from home monitoring, education, and other interventions, the effect is typically modest, especially in low-socioeconomic groups. Recently, the CHAMPION Study reported a reduction in re-hospitalizations using an implantable pulmonary artery pressure monitor (CardioMEMS; St. Jude Medical, St. Paul, Minn).32 However, this study mostly included white men, and it is unclear how such an intervention would fare in disparate populations. Further, while post hoc analysis suggested a reduction in 30-day re-hospitalization with the CardioMEMS device in an overwhelmingly white (88%), male (76%), and compliant (93%) sub-cohort of Medicare-eligible patients,33 it is unclear how the CardioMEMS device would perform in a more diverse low-socioeconomic population given the daily device interrogation and high level of patient participation required for its use. In these populations, the propensity of patients to favor the ED for their care may diminish the intervention’s efficacy.
Our study has several limitations. Due to the retrospective design, randomization was not possible. However, the re-hospitalization rate had been stable at our center (~22%) for several years and the re-hospitalization rate of other patients in our center remained unchanged. Due to the single-center design, we were unable to capture re-hospitalizations to centers other than UCMC in the controls. However, CMS data indicate that 5% of patients discharged from UCMC are readmitted to outside centers within 30 days, and this number has been stable for at least 3 years. Patients receiving our intervention were also readmitted to outside centers at a self-reported rate of 5%, suggesting the intervention did not redirect readmissions to outside centers. The use of combination isosorbide dinitrate/hydralazine among eligible black patients at the time of hospital discharge was low (5% in controls and 6% in intervention patients, P = NS). Unfortunately, underuse of isosorbide dinitrate/hydralazine is common nationally (4.5% at hospital discharge34 and 0%–7.3% in the clinic35). Similarly, the use of aldosterone antagonists at our center is similar to national practice patterns.36 Finally, while our data suggest no difference in ED re-visits and mortality at 90 days, it is not known if the intervention had a longer-term effect. In order for these findings to be translated into clinical practice, a larger prospective study will be needed.37
In conclusion, early consultation with a cardiologist for patients who return to the ED, as an adjunct to guideline-based follow-up, is associated with reduced re-hospitalizations and health care costs for low-socioeconomic urban patients with acute decompensated heart failure.
Supplementary Material
Telephone questionnaire. Specially trained nurses used this standardized questionnaire to assess each patient’s compliance, symptoms, and plans for follow-up after discharge. Abnormal responses triggered downstream actions as indicated.
Component costs within 90 days of discharge. For all component costs, the intervention was either lower or not significantly different from patients receiving usual care. (A) Inpatient cost; (B) Outpatient cost; (C) ED cost.
CLINICAL SIGNIFICANCE.
Guideline-directed education and intensive outpatient follow-up may not reduce emergency department (ED) re-visits in low-socioeconomic urban patients with acute decompensated heart failure.
Early consultation with a cardiologist in the ED is associated with increased rates of discharge from the ED, reduced re-hospitalizations, and lower health care costs in this population. Clinical outcomes were not compromised.
This strategy may be a cost-efficient method to reduce readmissions in this population.
Acknowledgments
Funding: This work was funded by an Innovation Grant to CET from the University of Chicago Medicine Center for Healthcare Delivery Science and Innovation. There was no pharmaceutical industry funding.
APPENDIX: SUPPLEMENTARY MATERIAL
Methods
Identification of Planned Re-hospitalizations
First, for re-hospitalized patients, all International Classification of Diseases, Ninth Revision (ICD-9, or ICD-10 after October 1, 2015) diagnosis codes and procedure codes for the re-hospitalization encounter were queried. Encounters with an ICD-9/10 diagnosis code that is considered always-planned20 were ignored. Then, procedure codes that are sometimes-planned20 were identified in the re-hospitalized patients’ records. Encounters that had a sometimes-planned procedure code and did not have an acute diagnosis code20 as the primary diagnosis code for the encounter were considered planned re-hospitalizations and were ignored. Encounters with a sometimes-planned procedure code and an acute primary diagnosis code were considered unplanned and included. Encounters without any always-planned or sometimes-planned procedure codes were included.
Calculation of Net Expected Pay
Net Expected Pay is calculated at our center as the sum of Expected Pay and Lump Sum Allocation minus Expected Bad Debt. Expected Pay is populated from a look-up table based on financial class, plan code, discharge year, and admission/discharge codes. Lump Sum Allocation is calculated as each encounter’s contribution to the lump sum payment from Medicare, Medicaid, and some commercial payers. Expected Bad Debt is the percentage of debt that is expected to be uncollected based on patient financial class, admission/discharge codes, and fiscal year.
Results
Quality of Intervention Delivery
Patients receiving the intervention demonstrated a significant improvement in their Atlanta Heart Failure Knowledge Test scores (21.7 ± 5.1 prior to education vs 25.5 ± 3.6 after education, P <.001, n = 118), while patients who did not receive the education showed no change (21.1 ± 4.0 vs 21.6 ± 6.3; P = .70, n = 15). Of patients receiving the intervention, 86.2% completed a structured telephone call within 3 days of discharge, compared with 0% of control patients (P <.001). Patients receiving the intervention experienced a 118% increase in completed clinic appointments within 7 days of discharge and a 54% increase in completed clinic appointments within 30 days. The median time to first completed Cardiology clinic appointment was 8 days in the Intervention group (interquartile range 6–19 days) and 18 days in the Control group (interquartile range 9–58 days, P <.001).
Patients Who Received the Intervention Had Improved Heart Failure Symptoms
One hundred six patients completed the Minnesota Living with Heart Failure Questionnaire (MLHFQ) at the time of discharge and 30 days later. Patients receiving the intervention reported significantly improved MLHFQ scores 30 days post discharge compared with pre discharge (44.7 ± 26.4 vs 68.7 ± 20.8 respectively, P <.001). Due to the historical design of this study, the control patients did not complete the MLHFQ. However, the 30-day improvement in patients receiving the intervention was significantly greater than those receiving usual care as reported by Kasper et al9 in a similar urban population (−26 ± 41 vs − 15 respectively, P <.01). In our study, the MLHFQ did not correlate significantly with re-hospitalizations, ED re-visits, or mortality.
Patients Who Re-Visit the ED with Cardiac Complaints Benefit More from Early Cardiology Consultation
While the overall rate of re-visit to the ED within 90 days was not different between the 2 groups (168 in the control group and 160 in the intervention group, P = NS), control patients were slightly more likely to re-visit the ED with cardiac complaints such as chest pain, leg edema, dyspnea of a likely cardiac etiology, or arrhythmias (n = 90/168) than intervention patients (n = 68/160, P = .0474). We acknowledge that this difference is small and is at the margin of statistical significance, which could possibly suggest that the implementation of guideline-based education and follow-up may have decreased cardiac re-visits to the ED somewhat, which were then replaced with noncardiac re-visits. Alternately, this small difference could simply be random chance.
However, among the patients who returned to the ED with a likely cardiac issue, the effect of early cardiology consultation was striking. Among these patients, 25/68 intervention patients were readmitted (37%), compared with 69/90 control patients (77%, P <.001). No significant difference was seen in the readmission rates of patients who re-visited the ED with noncardiac complaints (65/92 intervention and 47/78 control, P = NS).
Further analysis to differentiate between those intervention patients with cardiac complaints who were discharged vs those who were readmitted is a bit anecdotal due to the small N. In general, patients with new oxygen requirements and newly abnormal labs that did not correct after medical optimization and diuresis were readmitted, while those who presented with labs and vitals consistent with their baseline were discharged. Patients presenting with complaints related to a chronic medical problem that was present at the time of index discharge and was being addressed in the outpatient clinic were typically discharged, while patients presenting with new complaints that could not be safely treated as an outpatient (at the discretion of the ED attending physician) were typically admitted. However, the degree to which the consulting cardiologist’s recommendations altered ED physician behavior was not directly addressed in this study.
Footnotes
Conflict of Interest: There were no conflicts of interest.
Authorship: All authors contributed significantly to this manuscript. CET, KTS, CFA, TS, JKL, and RMS conceived, designed, and implemented the intervention. CET, MJC, ASV, and DA performed data analyses. CET, KTS, JKL, and RMS interpreted the data. CET wrote the manuscript. All authors had access to the data presented in this manuscript.
Supplementary appendix and figures accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.amjmed.2017.03.044.
References
- 1.Joynt KE, Jha AK. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ Cardiovasc Qual Outcomes. 2011;4(1):53–59. doi: 10.1161/CIRCOUTCOMES.110.950964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, et al. American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Lu N, Huang KC, Johnson JA. Reducing excess readmissions: promising effect of hospital readmissions reduction program in US hospitals. Int J Qual Health Care. 2016;28:53–58. doi: 10.1093/intqhc/mzv090. [DOI] [PubMed] [Google Scholar]
- 5.Drozda JP, Jr, Smith DA, Freiman PC, Pursley J, VanSlette JA, Smith TR. Heart failure readmission reduction: outcomes of a quality improvement initiative implemented by St. John’s Physician Group practice demonstration. Am J Med Qual. 2017;32(2):134–140. doi: 10.1177/1062860616637684. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Mather P, Gupta A, et al. Effect of early intervention with positive airway pressure therapy for sleep disordered breathing on six-month readmission rates in hospitalized patients with heart failure. Am J Cardiol. 2016;117:940–945. doi: 10.1016/j.amjcard.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Donaho EK, Hall AC, Gass JA, et al. Protocol-driven allied health post-discharge transition clinic to reduce hospital readmissions in heart failure. J Am Heart Assoc. 2015;4(12) doi: 10.1161/JAHA.115.002296. http://dx.doi.org/10.1161/JAHA.115.002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackevicius CA, de Leon NK, Lu L, Chang DS, Warner AL, Mody FV. Impact of a multidisciplinary heart failure post-hospitalization program on heart failure readmission rates. Ann Pharmacother. 2015;49:1189–1196. doi: 10.1177/1060028015599637. [DOI] [PubMed] [Google Scholar]
- 9.Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39:471–480. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 10.Pines JM, Russell Localio A, Hollander JE. Racial disparities in emergency department length of stay for admitted patients in the United States. Acad Emerg Med. 2009;16:403–410. doi: 10.1111/j.1553-2712.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- 11.Long T, Genao I, Horwitz LI. Reasons for readmission in an under-served high-risk population: a qualitative analysis of a series of inpatient interviews. BMJ Open. 2013;3:e003212. doi: 10.1136/bmjopen-2013-003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia TC, Bernstein AB, Bush MA. Emergency department visitors and visits: who used the emergency room in 2007? NCHS Data Brief. 2010;(38):1–8. [PubMed] [Google Scholar]
- 13.Sommers AS, Boukus ER, Carrier E. Dispelling myths about emergency department use: majority of Medicaid visits are for urgent or more serious symptoms. Res Brief. 2012;(23):1–10. 1–3. [PubMed] [Google Scholar]
- 14.Adams JG. Emergency department overuse: perceptions and solutions. JAMA. 2013;309:1173–1174. doi: 10.1001/jama.2013.2476. [DOI] [PubMed] [Google Scholar]
- 15.Public Health Statistics - Selected public health indicators by Chicago community area. 2016 Available at: https://data.cityofchicago.org/Health-Human-Services/Public-Health-Statistics-Selected-public-health-in/iqnk-2tcu.
- 16.Writing Committee Members. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 17.Donze J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 18.Donze JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496–502. doi: 10.1001/jamainternmed.2015.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rector TS, Kubo SH, Cohn JN. Patient’s self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Fail. 1987;3:198–209. [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. [Accessed July 1, 2016];Planned readmission algorithm – Versions 2.1 and 3.0. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Planned-Readmission-Algorithm.zip.
- 21.Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82:131–138. 150. [PubMed] [Google Scholar]
- 22.van Walraven C, Mamdani M, Fang J, Austin PC. Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631. doi: 10.1111/j.1525-1497.2004.30082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlay SM, Gheorghiade M, Reid KJ, et al. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12(4):367–374. doi: 10.1093/eurjhf/hfq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DS, Stukel TA, Austin PC, et al. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122(18):1806–1814. doi: 10.1161/CIRCULATIONAHA.110.940262. [DOI] [PubMed] [Google Scholar]
- 25.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111(2):179–185. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 26.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291(11):1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 27.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803–1812. doi: 10.1001/jamainternmed.2015.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition-Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176(3):310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the Telemedical Interventional Monitoring in Heart Failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 31.Desai AS. Home monitoring heart failure care does not improve patient outcomes: looking beyond telephone-based disease management. Circulation. 2012;125(6):828–836. doi: 10.1161/CIRCULATIONAHA.111.031179. [DOI] [PubMed] [Google Scholar]
- 32.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 33.Adamson PB, Abraham WT, Stevenson LW, et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circulation Heart Fail. 2016;9(6) doi: 10.1161/CIRCHEARTFAILURE.115.002600. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.115.002600. [DOI] [PubMed] [Google Scholar]
- 34.Yancy CW, Abraham WT, Albert NM, et al. Quality of care of and outcomes for African Americans hospitalized with heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry. J Am Coll Cardiol. 2008;51(17):1675–1684. doi: 10.1016/j.jacc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Yancy CW, Fonarow GC, Albert NM, et al. Adherence to guideline-recommended adjunctive heart failure therapies among outpatient cardiology practices (findings from IMPROVE HF) Am J Cardiol. 2010;105(2):255–260. doi: 10.1016/j.amjcard.2009.08.681. [DOI] [PubMed] [Google Scholar]
- 36.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161(6):1024e3–1030e3. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. [Accessed February 1, 2017];Care Optimization Through Patient and Hospital Engagement Clinical Trial for Heart Failure (CONNECT-HF) Available at: https://clinicaltrials.gov/ct2/show/NCT03035474.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Telephone questionnaire. Specially trained nurses used this standardized questionnaire to assess each patient’s compliance, symptoms, and plans for follow-up after discharge. Abnormal responses triggered downstream actions as indicated.
Component costs within 90 days of discharge. For all component costs, the intervention was either lower or not significantly different from patients receiving usual care. (A) Inpatient cost; (B) Outpatient cost; (C) ED cost.