Abstract
Over the last 10 years, the use of opto- and chemogenetics to modulate neuronal activity in research applications has increased exponentially. Both techniques involve the genetic delivery of artificial proteins (opsins or engineered receptors) that are expressed on a selective population of neurons. The firing of these neurons can then be manipulated using light sources (for opsins) or by systemic administration of exogenous compounds (for chemogenetic receptors). Opto- and chemogenetic tools have enabled many important advances in basal ganglia research in rodent models, yet these techniques have faced a slow progress in non-human primate (NHP) research. In this review, we present a summary of the current state of these techniques in NHP research and outline some of the main challenges associated to the use of these genetic-based approaches in monkeys. We also explore cutting-edge developments that will facilitate the use of opto- and chemogenetics in NHPs, and help advance our understanding of basal ganglia circuits in normal and pathological conditions.
Keywords: Optogenetics, opsins, chemogenetics, DREADDs, basal ganglia, non-human primates, monkeys
Introduction
In the last 10 years, the use of genetic-based tools to manipulate neuronal activity in research applications has increased significantly. The most common of these are opto- and chemogenetics. Optogenetic techniques involve the genetic delivery of light responsive proteins (opsins) to neurons to manipulate their activity (Fenno et al. 2011). Many different opsins are available for neuroscientific applications, including ion channels and pumps that allow depolarization or hyperpolarization of cell membranes, and consequent excitation or inhibition of neuronal activity, respectively. The most common excitatory tools (i.e., depolarizing opsins), include variants of the cation channel channelrhodopsin (ChR)(Boyden et al. 2005; Nagel et al. 2005); while commonly used hyperpolarizing opsins are proton or chloride pumps, such as archaerodopsin (Arch) (Chow et al. 2010) and halorohodopsin (NpHR)(Gradinaru et al. 2008). When illuminated with the appropriate wavelength, these opsins respond with millisecond time courses, allowing tight temporal control of neuronal activity. An additional, often critical advantage of optogenetic tools, involves the ability to target opsin expression to genetically-defined cell types, through the use of specific regulatory sequences or promoters (many times in combination with a conditional expression system, such as the Cre-recombinase/LoxP system). In sum, these tools have enabled the functional and behavioral dissection of many discrete brain circuits with exquisite precision (Boyden 2011; Deisseroth 2015; Rajasethupathy et al. 2016).
Chemogenetic techniques involve the activation of engineered receptors that are genetically expressed on target neurons, which can then be modulated by systemically administered exogenous compounds that are otherwise biologically inert (Farrell and Roth 2013; Sternson and Roth 2014). The most widely used chemogenetic technique relies on the expression of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) in neurons. DREADDs are synthetic variants of muscarinic acetylcholine receptors coupled to Gi/o, Gq/11, or Gs proteins, providing tools that can increase or decrease the activity of the targeted neuronal population, depending on the intracellular pathway activated (Roth 2016). DREADDs have been used extensively in rodents (Roth 2016), but only to a very limited extent in non-human primates (NHPs) (Eldridge et al. 2016; Grayson et al. 2016; Nagai et al. 2016). As opposed to the rapid-onset responses obtained with optogenetic (or other ion-channel based chemogenetic approaches, see below), the use of engineered GPCRs induce slow-onset and more prolonged modulations of neuronal activity. Chemogenetic approaches have also been used to mediate faster changes in neuronal activity by directly influencing ionic conductances through engineered ligand-gated ion channels (LGICs, Sternson et al. 2016). These chimeric channels are formed by combining a mutated version of the extracellular ligand-binding domain of the α7 nicotinic acetylcholine receptor and the pore domain of other ion channels. The engineered ligand binding domains are termed “pharmacologically selective actuator molecules” (PSAMs), while the respective agonists are known as “pharmacologically selective effector molecules” (PSEMs)(Sternson et al. 2016). The PSAM/PSEM approach has been used to modulate neuronal activity in rodents (Ren et al. 2016; Sternson et al. 2016; Sternson and Roth 2014), however there are no reports yet of use in NHPs.
Our current knowledge of basal ganglia circuits and functions is strongly influenced by recent advances accomplished using optogenetic and chemogenetic methods. For example, these techniques have been used to unravel the contributions of the direct and indirect pathways to movement and reinforcement (Cui et al. 2013; Freeze et al. 2013; Jin and Costa 2015; Jin et al. 2014; Kravitz et al. 2010; Kravitz et al. 2012; Lee et al. 2016; Oldenburg and Sabatini 2015; Tecuapetla et al. 2016; Tecuapetla et al. 2014), provide new descriptions of neuronal heterogeneity within the basal ganglia (Glajch et al. 2016; Saunders et al. 2016; Straub et al. 2016), help us understand the functional roles of nigrostriatal dopamine (DA) neurons (Howe and Dombeck 2016; Straub et al. 2014; Tritsch et al. 2012), and provide new insights about the changes in the plasticity of basal ganglia circuits in pathological conditions (Chu et al. 2015; Fieblinger et al. 2014; Miguelez et al. 2012). However, advances fostered by the use of opto- and chemogenetic tools have been achieved, for the most part, in rodent models; while the use of these techniques in NHPs has remained limited.
In this review, we offer an overview of the current state of the use of optogenetics and chemogenetics in NHPs, describe the main challenges specific to the use of these genetic-based approaches in monkeys, and provide examples of how these techniques can be used in primates to advance our understanding of basal ganglia circuits in normal and pathological conditions.
Use of optogenetics and chemogenetic techniques in non-human primates
To our count, there have been just under 30 papers published in which optogenetic techniques have been used in NHPs, while reports using chemogenetic methods are far sparser, with only 3 papers published so far. Table 1 provides a summary of these publications.
Table 1.
Summary of technical aspects and major contributions of publications to date using optogenetic and chemogenetic techniques in nonhuman primates.
| Publication | Species | Viral Vector
|
Transduction Target | ||
|---|---|---|---|---|---|
| Virus | Promoter | Transgene | |||
| Optogenetic Studies | |||||
|
| |||||
| Han et. al 2009 | Macaca mulatta | Lenti | CaMKIIα | ChR2 | Cortex (PMC and FEF) |
| Diester et. al 2011 | Macaca mulatta | Lenti, AAV5 | hThy1, hSyn | ChR2, eNpHR2, SFO | Cortex (SS, M1) |
| Han et. al 2011 | Macaca mulatta | Lenti | CaMKIIα | ArchT | Cortex (Par., V1) |
| Cavanaugh et. al 2012 | Macaca mulatta | AAV8 | CAG | ArchT | SC |
| Galvan et. al 2012 | Macaca mulatta | Lenti | Ef1α | ChR2 | Thalamus (VL) and putamen |
| Gerits et. al 2012 | Macaca mulatta | AAV5 | CAG | ChR2 | Cortex (Ventral PMC and FEF) |
| Jazayeri et. al 2012 | Macaca mulatta | AAV1 | hSyn | ChR2 | V1 |
| Tamura et. al 2012 | Macaca mulatta & fuscata | Lenti | CMV | ChR2 | Thalamus (MD, VA, VL) |
| Ohayon et. al 2013 | Macaca (*) | AAV5 | hSyn, CAG, CaMKII | ChR2, ArchT, eNpHR3.0 | Cortex (FEF) |
| Ozden et. al 2013 | Macaca mulatta | AAV5 | CaMKIIα | C1V1 | Cortex (SS, PMC) |
| Ruiz et. al 2013 | Macaca mulatta | Lenti, AAV5 | CaMKIIα | ChR2, ArchT, C1V1 | V1 |
| Dai et. al 2014 | Macaca mulatta | AAV5 | CaMKIIα | C1V1 | Cortex (parietal) |
| Huang et. al 2014 | Tupaia belangeri | AAV2/9 | CaMKIIα, hSyn | ChR2 | V1 |
| May et. al 2014 | Macaca mulatta | AAV5 | CaMKIIα | C1V1 | SS |
| Afraz et. al 2015 | Macaca mulatta | AAV8 | CAG | ArchT | STS |
| Inoue et. al 2015 | Macaca mulatta | AAV2 | CMV | ChR2 | Cortex (FEF) |
| Lu et. al 2015 | Macaca (*) | AAV5 | CaMKIIα | C1V1 | Cortex (PMv, M1) |
| Nassi et. al 2015 | Macaca mulatta | Lenti, AAV5 | CaMKIIα | C1V1, eArch3.0 | V1 |
| Acker et. al 2016 | Macaca mulatta | AAV8 | hSyn | Jaws | Cortex (FEF) |
| Galvan et. al 2016 | Macaca mulatta | AAV5 | CaMKIIα | ChR2 | Cortex (PMC, M1) |
| Klein et. al 2016 | Macaca mulatta & fascicularis | AAV5 | CaMKIIα | ChR2 | LGN |
| MacDougall et. al 2016 | Callithrix jacchus | AAV5 | hSyn | ChR2 | Cortex |
| Stauffer et. al 2016 | Macaca mulatta | AAV5/9 | TH, Ef1α | Cre, ChR2 | Midbrain DA neurons |
| Yazdan-Shahmorad et. al 2016 | Macaca mulatta | AAV5 | CaMKIIα | C1V1 | Cotex (M1, SS) |
|
| |||||
| Chemogenetic Studies | |||||
|
| |||||
| Eldridge et. al 2016 | Macaca mulatta | Lenti | hSyn | hM4Di | Cortex (OFC) |
| Grayson et. al 2016 | Macaca mulatta | AAV5 | hSyn | hM4Di | Amygdala |
| Nagai et. al 2016 | Macaca mulatta & fascicularis | Lenti, AAV2 | CMV, hSyn | hM4Di | Caudate |
Abbreviations: AAV, adeno-associated virus; ArchT, archaerhodopsin from Halorubrum strain TP009; C1V1, hybrid of channelrhodopsin 1 and volvox carteri 1; C. Macaques, cynomolgus macaques; CaMKIIα, calcium and calmodulin-dependent protein kinase II α; CAG, cytomegalovirus early enhancer/chicken beta actin promoter; ChR2; channelrhodopsin-2; CMV, cytomegalovirus; DA, dopamine; DREADDs, designer receptor exclusively activated by designer drugs; eArch3.0, enhanced archaerhodopsin 3; Ef1α, elongation factor 1α; eNpHR, enhanced natronomonas pharaonic halorhodopsin; FEF, Frontal eye field; hM4Di, human muscarinic receptor 4 DREADD; hSyn, human synapsin; hThy1, human thymocyte-1; Lenti, lentivirus; LGN, lateral geniculate nucleus of thalamus; M1, primary motor cortex; MD, mediodorsal thalamus; OFC, orbito-frontal cortex; Par., parietal cortex; PMC, premotor cortex; PMv, ventral premotor cortex; R. Macaques, rhesus macaques; SC, superior colliculus; SFO, step-function opsin; SMA, supplementary motor area; SS, somatosensory cortex; STS, superior temporal sulcus; TH, tyrosine hydroxylase; V1, primary visual cortex; VA, ventroanterior thalamus; VL, ventrolateral thalamus.
species of macaca not specified
Optogenetics in NHPs
Although the use of optogenetic techniques to manipulate neuronal firing was established more than 10 years ago (Boyden et al. 2005), the first report of the use of these techniques in NHPs did not appear until 2009. Han et al. showed that virus injections effectively transduced monkey neurons with opsins and, importantly, that neither these injections, nor the opsin expression, induced immune responses in NHPs (Han et al. 2009). This report was followed by other proof of principle studies showing that both excitatory and inhibitory opsins could be successfully used in NHPs (Diester et al. 2011; Han et al. 2011). Subsequent publications were primarily focused on solving technical challenges, such as opsin expression through use of different AAV serotypes (Diester et al. 2011; Dodiya et al. 2010; Galvan et al. 2012; Markakis et al. 2010; Ruiz et al. 2013; Tamura et al. 2012), or alternative designs to deliver the light in the brain (Han 2012; Han et al. 2009; Ozden et al. 2013; Tamura et al. 2012; Wang et al. 2011).
The use of optogenetic techniques has proven effective to study specific neuronal pathways in the NHP (Acker et al. 2016; Inoue et al. 2015; Klein et al. 2016; Lu et al. 2015; Nassi et al. 2015). Some examples of how optogenetics have already been used in NHPs to probe neural circuits include the demonstration that optical stimulation of the primary motor cortex (M1) can induce gamma oscillations that spread beyond the light stimulated area, likely via cortical network interactions (Lu et al. 2015), and that stimulation of opsin-expressing frontal eye field (FEF) terminals modulates neuronal activity in the superior colliculus (Inoue et al. 2015). An important step forward was the demonstration that optogenetics can be used to evoke behavioral changes in NHPs, although these changes are generally less robust than what is observed in rodents (see Technical Challenges). Successful modulation of behavior with optogenetic tools in NHPs has mostly been limited to modulation of eye movements (Acker et al. 2016; Cavanaugh et al. 2012; Gerits et al. 2012; Ohayon et al. 2013), or sensori-visual discrimination (Afraz et al. 2015; Dai et al. 2014). Another study demonstrated that optogenetic stimulation in somatosensory cortex may elicit an artificial sensation in the hand (May et al. 2014). These examples underscore the ongoing transition in the NHP optogenetic field from technical development to the application of these techniques to the study of neuronal circuits and behaviors.
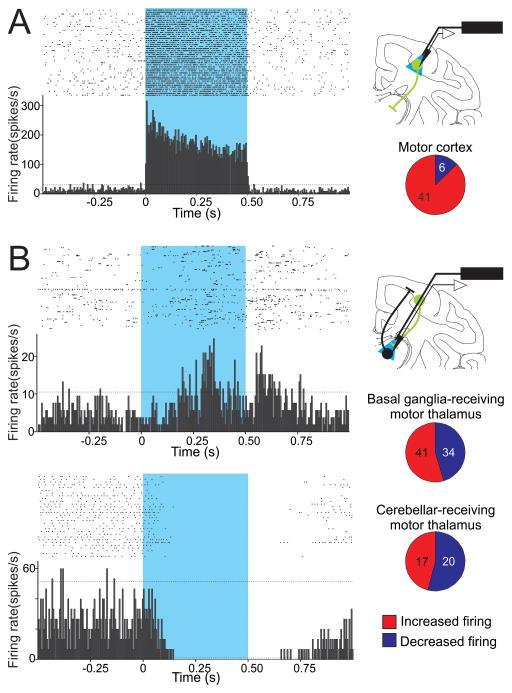
Recently, optogenetic techniques have been successfully applied in NHPs to advance our understanding of the basal ganglia-thalamo-cortical circuits. Using these tools, our group was able to selectively stimulate opsin-expressing corticothalamic terminals in the primate ventral motor thalamus, while simultaneously recording thalamic activity changes in the awake NHP (Galvan et al. 2016). We found that selective light activation of corticothalamic terminals elicits complex patterns of slowly developing excitatory and inhibitory effects in thalamic neurons of the basal ganglia- and cerebellar-receiving regions of the motor thalamus, suggesting a modulatory (instead of a ‘driver’) role of the corticothalamic system in the primate ventral motor thalamus (Fig. 1). In the same year, Stauffer et. al (2016) reported on the use of a cell-type specific promoter and a Cre-dependent approach to achieve selective expression of opsins in midbrain DA neurons in NHPs (see also Future Directions), and used this technique to identify DA neurons during in vivo electrophysiological recordings. Moreover, the authors showed that opsin-mediated stimulation of DA neurons was reinforcing (monkeys were biased to select stimuli that predicted optical stimulation of these neurons), and that pairing of reward with optical stimulation of DA neurons facilitated learning of reward-predicting stimuli (Stauffer et al. 2016).
Figure 1.
Effects of optogenetic stimulation of neurons in motor cortices and cortico-thalamic terminals in motor thalamus. A. Responses of cortical neurons to opsin activation. Left: Raster diagram and peri-stimulus time histogram (PSTH) of a single neuron in primary motor cortex, showing increased firing in responses to 500 ms light pulses. B. Responses of thalamic neurons to activation of opsins on corticothalamic axons. Top: Raster diagram and PSTH of a neuron in the basal ganglia-receiving territory of the motor thalamus that showed an increase in firing during the activation of ChR2. Bottom: A neuron in the cerebellar-receiving motor thalamus showing a decrease in firing during the activation of ChR2. For A and B, raster diagrams and PSTHs are aligned to the start of light pulses, each bin in the PSTH is 5 ms in duration; dashed horizontal lines indicate 2 SD above and below the baseline (based on the firing of the neuron during the 0.5 s prior to the start of each stimulus). The blue rectangles indicate the light pulses. At right: Experimental configuration to activate opsins expressed at the level of somas (top) or terminals (bottom) on cortico-thalamic neurons (green), with simultaneous extracellular recordings of cortical or thalamic neurons (top and bottom, respectively). Pie graphs represent the proportion of neurons in motor regions of cortex and thalamus that increased or decreased firing in response to the light stimulation. Numbers of cases are indicated (figure modified with permission from from Galvan et al. 2016).
Chemogenetics in NHPs
Similar to optogenetics, chemogenetic techniques have seen a slow progression from rodent to NHP models. Indeed, although chemogenetics have been used for more than two decades (Roth 2016), the first reports of their use in primates appeared with three publications in 2016, all of which used the inhibitory DREADD hM4Di (Armbruster et al. 2007) to acutely inactivate brain areas. In the first of these studies, Eldridge et al (2016) showed that hM4Di-mediated silencing of orbitofrontal cortex (OFC), in combination with removal of the rhinal cortex, interfered with reward-based task performance in macaques. Specifically, whereas task error rates were robustly sensitive to reward magnitude under control conditions, this relationship was less apparent during DREADD-assisted functional disconnection of the OFC and rhinal cortex. Based upon these findings, the authors suggested that functional interactions between these two brain regions may be important for retrieving information about stimulus-associated reward value (Eldridge et al. 2016). This report, besides demonstrating that chemogenetics can be used for the study NHP behavior, provided initial information about the pharmacokinetics of clozapine-n-oxide (the DREADD ligand used in this study) after systemic administration. More recently, resting state functional magnetic resonance imaging (fMRI) was used to show that DREADD-mediated inactivation of the amygdala altered the functional connections in amygdalo-cortical and cortico-cortical networks (Grayson et al. 2016). Importantly, this study demonstrated the usefulness of combining DREADD technology with resting-state imaging to explore functional connectivity in NHPs with minimally invasive techniques.
The first chemogenetic study in primates to examine basal ganglia-related circuits was recently published by Nagai et al (2016). In this study, a reward-based task was used, similar to that applied by Eldridge et al.(2016), in which the magnitude of the reward (number of drops of juice) varied by trial, and task error rates decreased with larger magnitude rewards. DREADD-mediated inactivation of the rostromedial caudate increased global error rates in this task compared to control conditions, and blunted sensitivity of error rate to reward magnitude. These findings thus led the authors to suggest that the rostromedial caudate is involved in reward valuation (Nagai et al. 2016). In addition, this study used a novel PET-based method to monitor the expression of DREADDs in vivo (as described in Future Directions).
Challenges to the Use of Optogenetics and Chemogenetics in NHPs
Opto- and chemogenetic tools have been transformative for neuroscientific research in rodent models, yet these techniques have faced slow progress in NHP research. To some extent, this lag is because NHP research typically requires additional time and expense compared to rodent studies. Furthermore, the availability of transgenic mouse lines (not widely available yet for most NHP models) have favored more rapid advancements in rodent models. Here we outline other additional challenges associated with the use of genetically targeted tools in NHPs.
Delivery of Opsins or Chemogenetic Constructs and Targeting of Specific Cell Populations
To transfer the genetic sequences of opsins or chemogenetic receptors into neurons or other brain cells in NHPs, researchers are currently limited to the use of replication-defective viral vectors. The most (Lerchner et al. 2014) common types of viral vectors used for this purpose are lenti- and adeno-associated viral vectors (LV and AAV, respectively). LVs are relatively easy to produce, do not induce immunogenicity and can be pseudotyped to display preferential tropism for neurons over glia (Hughes et al. 2015; Teschemacher et al. 2005). In addition, injections of LVs result, in many cases, in a large proportion of cells transduced (high penetrance), albeit in a confined area around the injection site (Lerchner et al. 2014).
On the other hand, AAVs are in many instances preferred because, in contrast to LVs, the genetic material remains episomal and does not integrate with the host DNA, thus reducing the possibility of disrupting normal transcription (Murlidharan et al. 2014; Thomas et al. 2003). AAVs also tend to diffuse further away from the site of injection, allowing for more widespread viral expression patterns (see below). Most frequently, AAVs used in research are recombinant AAV2, which are pseudotyped with capsids of other serotypes (e.g, AAV2/1, AAV2/5, AAV2/9; for simplicity, here we refer to these hybrids by the capsid serotype: AAV1, AAV5, AAV9, etc.).
The efficacy of AAVs may be limited by the possible presence of pre-existing neutralizing antibodies to the wild-type form of the virus in NHP serum or cerebro-spinal fluid (Samaranch et al. 2013; Wang et al. 2010). Although selection of animals based on screening for neutralizing antibodies could minimize this problem (Bevan et al. 2011; Samaranch et al. 2013), immunity could develop after an initial AAV injection (Gray et al. 2013; Kotterman et al. 2015), ultimately limiting the time window to perform multiple AAV injections. Potential solutions to this issue include the use of immunosuppressant drugs before injection of viral solutions (Jiang et al. 2006), or implementation of a different serotype for subsequent injections if neutralizing antibodies develop after the first administration (Gray et al. 2013).
Both LVs and AAVs have a limited DNA packaging capacity, approximately 5 and 8 kilobase pairs, respectively (Davidson and Breakefield 2003; Dong et al. 1996). This is an important consideration because most cell type-specific promoters are too large to be efficiently packaged in AAV or LVs, but recent developments offer alternatives (see Future Directions).
Typically, a brain structure of interest is targeted with direct injections in the brain parenchyma (although injection of the vector in the cerebral ventricles is an option when trying to achieve brain wide transduction (Samaranch et al. 2013)). However, viral vector solutions injected in the brain tissue have a restricted diffusion, and most frequently only cells within a few millimeters of the injection site will be transduced (Davidson and Breakefield 2003). The type of vector strongly influences the spread from the injection site as well as the pattern of transgene expression. Further complicating matters, patterns of expression can also vary depending on the species and the brain region of interest. A small number of studies have rigorously compared AAV serotype-dependent-expression patterns in monkeys. Dodiya et al. (2010) reported that AAV1 and 5 infected a higher number of cells and larger volumes of tissue than AAV8 after injections in the monkey striatum (Dodiya et al. 2010). When injected in the substantia nigra, AAV5 transduced a larger number of cells than AAV1, 2, 3, 4 or 6 (Markakis et al. 2010). Two studies compared various AAV serotypes injected in macaque or marmoset neocortex, and reported that, in general, the area of spread was similar for AAV1, 5, 8, 7 and 9 (Gerits et al. 2015; Watakabe et al. 2014); but the proportion of transduced cells was larger for AAV1 (followed by AAV5, AAV7, AAV9 and AAV8 (Gerits et al. 2015). Infusions of AAV2 resulted in very limited transgene expression (Gerits et al. 2015; Watakabe et al. 2014). In contrast, Lerchner and colleagues (2014) reported that intracortical injections of AAV8 had very limited spread; while the spread of AAV6 and AAV2 was moderate, and AAV9 and 10 achieved a very wide (>10 mm) diffusion (Lerchner et al. 2014). When using the same experimental conditions, LVs achieve a more restricted diffusion (<2 mm from injection site) than AAV2 (Lerchner et al. 2014). The diffusion of viral particles is also influenced by the injection method (Lerchner et al. 2014; Sanftner et al. 2005). In general, the spread of solution is inverse to the speed of injection when using AAVs, but faster injections rates result in a more widespread diffusion in the case of LV solutions (Lerchner et al. 2014).
To overcome the limited spread of viral vector solutions, particularly to cover large brain nuclei (e.g., putamen) researchers have used convection-enhanced delivery (Bankiewicz et al. 2000; Bobo et al. 1994; Hadaczek et al. 2006; White et al. 2012), large injection volumes (Eldridge et al. 2016; Stauffer et al. 2016), and/or the multiple injection tracks (Eldridge et al. 2016). The last two options should be approached cautiously, as delivery of large volumes of fluid or multiple injection penetrations increase the risk of inducing brain tissue damage or puncturing blood vessels (although evidence of this type of damage has not yet been reported). A recent refinement to intracerebral injection of virus solutions is to combine the virus solutions with an MRI contrast agent, such as gadolinium, and conduct the injections using MRI to monitor the diffusion in the brain tissue during and after injection (Fiandaca et al. 2009; Rosenbluth et al. 2011). This technique allows adjustments to injection volume and flow rate to improve the diffusion of the virus solution.
Transgene expression is not solely determined by the spread of the viral vector solution, but also by the directional axonal transport of the viral particle or the transgene. In some cases, viral particles can be internalized by the axon terminals at the site of the injection and retrogradely transported to the soma, resulting in expression of the transgene product in neurons that project to the injection site (Murlidharan et al. 2014). Although such retrograde transport could be detrimental in some experimental conditions, it may provide a useful strategy to selectively target brain pathways (see Future Directions). Often times, the transgene product is transported anterogradely along projection axons and terminals. Expression of the transgene in projection axons can be exploited to achieve selective optogenetic activation of a neuronal pathway, as we have done to activate cortical terminals in the monkey thalamus (Fig. 1, Galvan et al. 2016). However, the possibility that spikes evoked by opsin activation may propagate antidromically should be carefully considered during experimental interpretation (Yizhar et al. 2011). The degree of retrograde or anterograde transport also depends largely on the AAV serotype. In our experience, and as reported by others, AAV5 injections in monkeys resulted mostly in robust anterograde transport of the transgene along the axon terminals (e. g., Diester et al. 2011; Galvan et al. 2016; Gerits et al. 2015), while AAV7, AAV8 and AAV9 produced both anterograde and retrograde transport (Gerits et al. 2015; Masamizu et al. 2011), and AAV6 had mostly retrograde transport in primates (San Sebastian et al. 2013).
Viral tropism (i.e., the preference of the viral vector to transduce one cell type over others), is also determined, to some extent, by viral serotype. For instance, in the monkey substantia nigra and caudate nucleus, injections of AAV1 through 6 packaging GFP driven by the ubiquitous cytomegalovirus (CMV) promoter resulted in glial and neuronal transduction for all serotypes; however, compared to the other serotypes, AAV2 injections transduced more neurons than glial cells (65% of cell transfected were identified as neurons, Markakis et al. 2010). Similarly, in cerebral cortex, glia and neurons were transduced when using LV or AAV1, 5, 8 and 9 in combination with the CMV promoter (Lerchner et al. 2014; Watakabe et al. 2014). However, the expression of the transgene was restricted to neurons when the neuronal promoters synapsin (Syn) or CaMKIIα were used (Lerchner et al. 2014; Watakabe et al. 2014). These findings indicate that cell-type specificity can be enhanced, within the current limits of the packing capacity of AAVs and LVs, using a promoter sequence that regulates the expression of the transgene in a subpopulation of cells. However, the tropism achieved with a particular combination of viral vector and promoter can vary across species (Watakabe et al. 2014). For example, although in rodents the CaMKIIα promoter has been shown to exclusively target projection neurons in cerebral cortex (Nathanson et al. 2009), in primates this promoter, in combination with the AAV1 or AAV9 vectors, led to transgene expression not only in projection neurons but also parvalbumin-positive interneurons (Watakabe et al. 2014). Variability in tropism is also observed with the same viral vector-promoter combination in different brain regions, or even across cortical layers (Diester et al. 2011; Gerits et al. 2015; Jazayeri et al. 2012).
In summary, as described above, several factors influence the efficacy of a viral vector. However, while the choice of a viral vector and promoter for an optogenetic or chemogenetic experiment in NHPs should be guided by the cell type and brain region of interest, as well as by the experimental design, the expanding list of publications in which these vectors have been used (Table 1) constitutes a valuable starting point for researchers.
Finally, it should be noted that, although AAV and LV vectors are generally considered to be safe, the expression of an exogenous protein has the potential of being detrimental to the cell, either by inducing toxic immune responses (Samaranch et al. 2014), or due to overexpression which can cause protein aggregations or membrane disruption (Gerits and Vanduffel 2013; Rein and Deussing 2012). The choice of a cell-type specific promoter can reduce this possibility, since strong ubiquitous promoters, such as CMV, can be particularly neurotoxic (Watakabe et al. 2014).
Monitoring of transgene expression
Once the injections of viral solutions have been completed, stable transgene expression may require several weeks. Although there is no specific consensus, most authors have reported wait times of 3 to 6 weeks after the virus solution injections to start optogenetic or chemogenetic experiments in monkeys. One of the main difficulties in NHP work is the lack of a minimally invasive in vivo method to assess the temporal course and level of expression of the transgene, and thus researchers most frequently rely on post-mortem examinations of the tissue to verify expression with fluorescence or immunohistochemical approaches. Fluorescent tags commonly fused to the opsin or chemogenetic receptor can be used as an indication of the level of expression, and when the transduction is limited to the superficial cortical layers, the fluorescence on the brain surface can be visualized directly through an optical window (Ruiz et al. 2013). However, this approach is not feasible for deeper structures, such as the basal ganglia. One alternative is to measure in vivo through a fiber optic the fluorescence intensity of the fluorescent tag protein fused to the opsin or chemogenetic receptor. Although this approach has been reported for primates and rodents (Diester et al. 2011; Ozden et al. 2013; Tamura et al. 2012), the usefulness of this method remains limited due to the tissue damage induced by insertion of an optical fiber to detect fluorescence, and the potential for opsin-induced neuronal modulation by fluorescence detection (Dai et al. 2015). An alternative approach is the use of imaging techniques, which have been successfully employed to evaluate in vivo DREADD expression in primates, using PET and a radiolabeled DREADD-agonist (Nagai et al. 2016). As assessed by this method, the expression of the DREADD hM4Di reached a plateau 45 days after viral injection (Nagai et al. 2016). MRI-based approaches may also be an option to monitor transgene expression (Genove et al. 2005), but their use in conjunction with opsins or chemogenetic tools in primates has yet to be established.
Optogenetic-related challenges
Limited Light Delivery
Light activation of opsins in vivo is achieved in most cases by inserting a fiber optic within the brain parenchyma. With larger brains than rodents, light delivered to primate brain tissue will extend to a proportionally smaller territory of the anatomical target. This restricted light delivery could explain, at least in part, the limited ability of optogenetic tools to modify behavior in NHPs (Cavanaugh et al. 2012; Diester et al. 2011; Gerits et al. 2012). Restricted spread of light in the brain tissue may also explain the need to combine electrical and optical stimulation to achieve a behavioral outcome (Ohayon et al. 2013) and the need to use light power intensities several fold higher than those used in rodents. Novel designs in the configuration of the fiber optics can help to maximize intra-brain illumination, including a probe that combines four fibers surrounding a tungsten microelectrode (Tamura et al. 2012), or tapered optic fibers in which the core and cladding are etched to allow light delivery all around the tip of the fiber (Acker et al. 2016). The tapered design not only increases the extent of tissue illuminated using lower light-power intensities, but also reduces tissue damage during brain insertion compared to flat-end fibers (Dai et al. 2014; Dai et al. 2015).
An additional (or complementary) strategy to illuminate a larger tissue area, is the use of opsins with peak excitation at relatively high wavelengths, since red-light shifted wavelengths are less likely to be absorbed by the densely vascularized brain tissue and propagate to a larger area. Experiments in monkeys have successfully used red-shifted excitatory and inhibitory opsins (C1V1 and Jaws respectively) (e.g. Acker et al. 2016; Dai et al. 2015; Galvan et al. 2016; Nassi et al. 2015).
Limitations and Non-specific Effects of Optogenetic Tools
In any optogenetic experiment, one has to consider that activation of opsins expressed on brain cells can lead to additional effects than the intended increase or decrease of neuronal excitability. Some of these effects have been well described from in vitro preparations in rodents, including paradoxical release of neurotransmitter when activating inhibitory opsins at presynaptic terminals (Mahn et al. 2016), changes in extracellular ion concentrations that can affect opsin-negative neurons in the vicinity of the area stimulated (Ferenczi et al. 2016), and short-term plasticity during optical high-frequency stimulation of terminals (Jackman et al. 2014). While some of these issues can be minimized with appropriate light stimulation parameters (e.g., slower stimulation frequencies or longer intervals between light pulses), experiments should be carefully planned based on the known properties of the opsins (Mattis et al. 2012).
Additional concerns in optogenetic experiments involving NHPs include tissue damage induced by light delivery in the brain (i.e. photoxicity), which can arise in part from increased temperature around the optic fiber. The experimental confounds introduced by light-evoked heating may also extend beyond phototoxicity. For example, heating induced by blue light delivery can cause fMRI responses even without opsin activation (Christie et al. 2013). Although in the primate brain heat likely dissipates more easily than in rodents (Acker et al. 2016), the higher light intensities that are commonly used to activate opsins in NHPs can, nevertheless, lead to heating effects. As mentioned above, use of red-light shifted opsins and tapered fiber optics can reduce the light power needed. In addition, one can address the unspecific effects of light stimulation with control experiments in which the fiber optic is inserted in areas that did not receive viral vector injections, or that received viral vectors carrying only the fluorescent protein tag. Finally, repeated brain penetrations with large diameter fiber optics remains a concern, which can be minimized to some extent using thinner and/or tapered fibers (Acker et al. 2016).
Chemogenetic-related Challenges
After systemic administration, clozapine-n-oxide (CNO), the ligand most commonly used to activate DREADDs, can convert to clozapine and N-desmethylclozapine, as has been reported in rats, guinea pigs and humans(Chang et al. 1998; Jann et al. 1994; MacLaren et al. 2016). At nanomolar concentrations, these metabolites activate a variety of endogenous receptors (Lameh et al. 2007; Roth and Driscoll 2014), likely contributing to the behavioral changes observed after CNO administration in animals that have not received DREADDs (MacLaren et al. 2016). Although current reports indicate that CNO conversion to its metabolites may be minimal in monkeys (Eldridge et al. 2016; Nagai et al. 2016), a thorough characterization of CNO pharmacokinetics and metabolism in NHPs is needed. An alternative to CNO is the recently described ‘Compound 21’ a pharmacologically inert ligand that binds DREADDs with high affinity (Chen et al. 2015), but the pharmacokinetics of this compound also remain to be described. Similarly, more information is needed regarding the effects of chronic exposure of DREADDs to their agonists (i.e., ligand desensitization, Roth 2016).
Future Directions
As mentioned above, the use of genetic-based tools in NHPs is expanding but technical challenges remain. Here we discuss some methodological advances that have the potential to expand the use of these methods in NHPs to further the exploration of the basal ganglia and related brain circuits.
Novel Viral Vectors and Promoters for Opto- and Chemogenetics
Recent expansions in the viral vector library are poised to greatly enhance the primate opto- and chemogenetic toolkits. For example, in many cases, investigators may wish to optogenetically target neuronal populations based on their projection patterns, rather than (or in addition to) their molecular phenotype. The resources available to undertake such experiments in primates have been scant, as many viral vectors with proven retrograde transduction capabilities are toxic (such as rabies viral vectors, Ginger et al. 2013; Schnell et al. 2010). New versions of these vectors, however, present reduced toxicity (Reardon et al. 2016). The use of canine adenovirus-2 (CAV-2) as a retrograde viral vector is increasing (Junyent and Kremer 2015), although anecdotal evidence suggests a lack of efficacy in primates (El-Shamayleh et al. 2016), and thus further examination is required. An additional vector worthy of examination in NHP models is the recently described rAAV2-retro, a recombinant AAV with strong retrograde transduction efficiency in rodents (Tervo et al. 2016). It is of substantial interest for future studies to validate the efficacy of CAV-2 and rAAV2-retro vectors in NHPs, which may afford tremendous new opportunities for optogenetic-based functional circuit dissections. Other viral vectors with retrograde capabilities may also be efficient and safe; for example, engineered herpes simples viruses (Enquist et al. 1998; Kay et al. 2001), and the “highly efficient retrograde gene transfer” (HiRet) vector (Kato et al. 2011a; Kato et al. 2011b; Kato et al. 2011c; Oguchi et al. 2015).
Importantly, this retrograde targeting approach can be readily extended to chemogenetic studies, with minor additional methodological considerations. A caveat to single retrograde virus labeling is that, for many brain circuits, multiple pathways may be retrogradely transduced, including those that are not of experimental interest. Whereas recruitment of such “off target” pathways could be avoided in optogenetic experiments through appropriate targeting of the light source, systemic administration of a chemogenetic receptor ligand may activate all transduced pathways. Thus, to maintain neural pathway selectivity, the investigator must rely on alternative approaches when using chemogenetic tools, such as the use of a dual viral approach (see below) to express chemogenetic receptors in a single pathway of interest. For example, in a recent study a Hi-Ret vector packaging Cre-recombinase was injected in the caudate nucleus, while a Cre-inducible construct was injected in the lateral prefrontal cortex (LPFC), thus achieving specific expression of the transgene in LPFC neurons that project to the caudate (Oguchi et al. 2015). More straightforwardly, direct microinjections of the chemogenetic receptor ligand can also be targeted to the retrogradely transduced cell bodies of interest (although this approach would void the usefulness of the systemic administration of the chemogenetic ligand).
Of course, viral targeting approaches based on projection patterns will be of little use for selectively targeting interneurons (i.e., in striatum) or subpopulations of spatially intermixed principal cells with overlapping projection patterns (e.g., intratelencephalic and pyramidal tract cortical neurons projecting to striatum). For these cases (and others), one must rely upon the continued development of flexible gene promoter-based targeting approaches. The use of short cis-acting regulatory elements, or fragments of larger gene promoters, have been successful for targeting genetically-defined cell populations in primates (see example in Figure 2 and refs. Dimidschstein et al. 2016; Stauffer et al. 2016). “Mini promoters” provide an additional promising avenue, in which the detection and implementation of short, cell type-specific promoters is guided by bioinformatic searches for candidate regulatory regions for the gene of interest (de Leeuw et al. 2014; Portales-Casamar et al. 2010).
Figure 2.
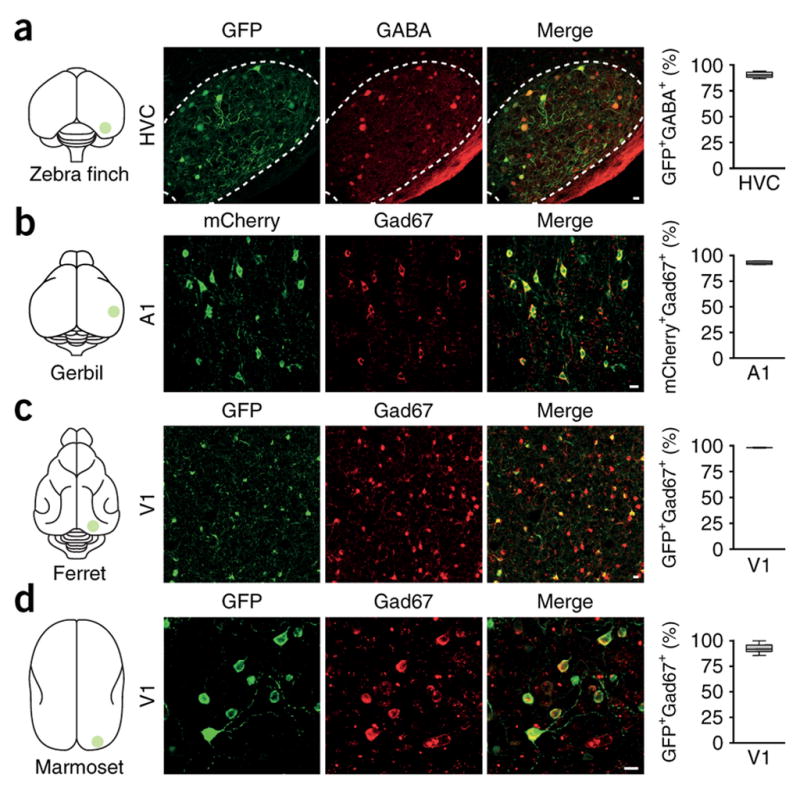
GFP is selectively expressed in telencephalic GABAergic neurons of the HVC in zebra finch, as well as primary visual cortex (V1) of multiple mammalian species (including marmoset) following microinjections of rAAV-GFP placed under an enhancer sequence (mDLx) for the distalless homeobox 5/6 genes. These genes may be expressed in telencephalic GABergic interneurons during embryonic development. For each species, the degree of GFP colocalization with either GABA or GAD67 is provided on the right, in all cases confirming selective and robust GFP expression in GABAergic neurons. The use of well-designed short regulatory elements, as described here, may drive opsin or DREADD expression with exquisite cell type-specificity (figure reproduced with permission from Dimidschstein et al. 2016).
While such novel regulatory elements are under development, an appealing alternative is the dual AAV approach in which the packaging limitations of AAV vectors are partially bypassed through the use of two vectors, one including a recombination enzyme under the gene promoter of interest, and the other encoding recombination-inducible opto- or chemogenetic tools (Gompf et al. 2015; Stauffer et al. 2016). In principle, this technique allows the use of relatively large gene promoters to target specific cell types, particularly as these promoter sequences do not need to be placed alongside large fluorophore genes. Although to date the dual AAV approach has only been reported in NHPs for targeting tyrosine hydroxylase neurons (Stauffer et al. 2016), this method has the potential to allow unprecedented, facile access to genetically-defined neuronal populations in NHPs and other species in which germ-line modifications may not be logistically feasible. Future studies identifying useful gene promoters (both new and previously identified) that are compatible with dual AAV approaches should facilitate the expansion of this toolkit.
Optogenetic Phototagging during In Vivo Extracellular Recordings
In addition to monitoring/inducing neural responses to photostimulation (or inhibition) of select neuronal populations, optogenetic tools may allow for the electrophysiological identification of putative opsin-expressing cells in vivo, a procedure termed “phototagging”. When strict classification criteria are applied, e.g., minimal latency to fire and inter-trial variability (Cohen et al. 2012; Kravitz et al. 2013; Zhang et al. 2013), single unit responses to photostimulation may reliably “tag” neurons expressing excitatory opsins. In this manner, neurons can be identified during in vivo recordings with reference to their molecular identity and/or wiring patterns (e.g., using retrograde viral approaches or detection of optically evoked antidromic potentials), even if the cells of interest are spatially intermingled with other, non-targeted populations. Although increasingly used in rodent studies (Beyeler et al. 2016; Jennings et al. 2013; Nieh et al. 2015), phototagging has, to date, played a minimal role in neurophysiology studies of NHPs. This is, however, changing with the continual refinement of optogenetic toolsets for primates that can provide enhanced opsin targeting specificity for selective neuronal populations (defined by projection specificity, molecular identity, or both). A recent landmark report by Stauffer et al. (2016), using a newly developed AAV encoding ChR2 under a tyrosine hydroxylase promoter fragment, allowed for successful phototagging of DA neurons in the macaque ventral tegmental area/substantia nigra (Stauffer et al. 2016). Following successful and selective opsin targeting, the authors were able to validate earlier proposed electrophysiological criteria for identifying midbrain DA neurons in extracellular recordings, including broader waveforms and lower baseline impulse rates compared to neighboring, non-dopaminergic neurons (Schultz 1986). Future primate optogenetic studies should extend the opportunities provided by phototagging to interrogate neuron-type specific firing dynamics with relation to behavioral and/or ethologically salient events, as well as changes between healthy and disease states. For example, it would be of great interest to phototag striatal direct and indirect pathway neurons in NHPs, as these genetically and functionally distinct cell populations are virtually indistinguishable during traditional in vivo electrophysiological recordings.
Optogenetics and Functional Neuroimaging
Functional neuroimaging, coupled with optogenetic tools, affords the opportunity to examine whole-brain activity changes that occur in response to selective modulation of a targeted neuronal population (Kolodziej et al. 2014; Lee et al. 2010; Thanos et al. 2013). Functional magnetic resonance imaging with simultaneous optogenetic photostimulation (“opto-fMRI”) is increasingly being employed in rodents to study how recruitment of genetically-identified neurons alters brain-wide hemodynamics, with recent examples involving stimulation of midbrain DA neurons, and D1 and D2 receptor-expressing medium spiny neurons of the dorsal striatum (Bernal-Casas et al. 2017; Lee et al. 2016; Lee et al. 2010). A small number of studies have employed opto-fMRI in NHPs, to varying degrees of success. Whereas Gerits and colleagues (2012) showed fMRI-measured activations in numerous regions following stimulation of ChR2-expressing neurons in the macaque FEF and anterior bank of the arcuate sulcus (including responses both at the stimulation sites and functionally connected areas such as the medial superior temporal area and visual cortex, Gerits et al. 2012), Ohayon et al. (2013) did not identify any fMRI signal changes during optogenetic stimulation of the FEF (Ohayon et al. 2013). The authors of the latter study did find, however, that optogenetic stimulation could augment electrically-evoked fMRI signal changes in the FEF, corroborating physiological and behavioral data demonstrating functional opsin expression. Numerous methodological differences between these studies likely contribute to the disparate results, including the use of an exogenous contrast agent by Gerits et al., which can substantially increase the fMRI signal detection sensitivity compared to the traditionally measured blood-oxygen-level-dependent (BOLD) signal (Kim et al. 2013). An exciting avenue for future opto-fMRI primate studies will be the incorporation of resting state fMRI experiments, in which functional brain networks are identified based on low frequency (>0.1Hz) spontaneous hemodynamic fluctuations (Raichle 2011). This approach has been recently reported in rodents (Ferenczi et al. 2015), and, as mentioned above, also in primates during DREADD-mediated inactivation of the amygdala (Grayson et al. 2016).
Similar to fMRI, positron emission tomography (PET) imaging can also be used to map global brain activity patterns, albeit with reduced spatiotemporal precision. PET imaging is also compatible with awake animal preparations, and even behavioral testing (when employed during the PET tracer uptake period, Thanos et al. 2013). It is thus surprising that there are no published reports coupling optogenetic tools with PET (or single photon emission computed tomography; SPECT) imaging in NHPs. These efforts should be encouraged by early successes in rodent models using DREADD technologies, in which PET imaging following CNO administration has been used to identify network changes evoked by sustained activation of nucleus accumbens direct/indirect pathway neurons (Michaelides et al. 2013), or serotonergic neurons of the dorsal raphe (Urban et al. 2015). Additionally of note is a recently developed 11C-labeled clozapine radioligand that has been validated for use in primates, enabling in vivo mapping of DREADD receptor distribution and occupancy (Nagai et al. 2016).
Optogenetic-Aided Discoveries in Rodent Basal Ganglia: Translation to the Primate
As mentioned earlier, many important discoveries of basal ganglia function have been made in rodents using opto- and/or chemogenetic tools. Although the basal ganglia’s general circuit features have been markedly conserved throughout evolution (Grillner et al. 2013), there are also many examples of cross-species divergence in basal ganglia anatomy and physiology, including differences between rodent and primate (Benhamou et al. 2012; Berger et al. 1991; Garcia-Cabezas et al. 2008; Hardman et al. 2002; Petryszyn et al. 2014; Richfield et al. 1987; Smith et al. 2014). A well-known example is that the primary output nucleus of the motor circuit of the basal ganglia in NHPs and humans is the internal segment of the globus pallidus, while in rodents, the pars reticulata of the substantia nigra transfers most of the motor information out of the basal ganglia. Such interspecies differences are unlikely to be trivial, and are particularly important to consider in the context of modeling basal ganglia diseases and therapeutic interventions in human patients. In this final section, we will describe two now-classic studies in which optogenetic tools were used in rodents to identify putative therapeutically-relevant circuits for Parkinson’s disease (PD). We will argue that replication and extension of this work in NHPs will form a critical bridge to future therapeutic avenues for PD and other basal ganglia-oriented movement disorders.
Although widely used and often with great therapeutic benefit (Bronstein et al. 2011), the mechanism(s) underlying deep brain stimulation (DBS) therapy for Parkinson’s Disease are highly contentious (Chiken and Nambu 2015; Lozano and Lipsman 2013). In one of the earliest papers utilizing optogenetic tools in the basal ganglia, Gradinaru and colleagues (2009) optogenetically mimicked STN-DBS in hemiparkinsonian rats and mice (Gradinaru et al. 2009). Among their main experimental findings, the authors observed the reversal of amphetamine-induced rotational behavior (a common measure of motor deficits in hemiparkinsonian rodents), elicited by high frequency optical stimulation of primary motor cortex or cortico-subthalamic afferents, but not STN neurons. This study and others (Li et al. 2012; Li et al. 2007; Sanders and Jaeger 2016) have raised the intriguing hypothesis that STN-DBS elicits antidromic recruitment of motor cortex, via the hyperdirect pathway. Gradinaru et al. (2009) did not find that optical silencing of STN neurons reduced parkinsonian signs in the rodent model, in contrast to reports that in parkinsonian NHPs and human patients, lesions or pharmacologic inactivation of the STN are antiparkinsonian (Alvarez et al. 2009; Bergman et al. 1990; Levy et al. 2001; Wichmann et al. 1994). Thus, a critical next step will be to replicate and extend upon these rodent findings in a NHP model of parkinsonism.
Optogenetic studies in rodents have also contributed greatly towards our understanding of the direct and indirect pathways of the basal ganglia, including their relation to facilitation and suppression of movement, respectively. Although NHP research played an essential role in the development of this classical model of basal ganglia function (Albin et al. 1989; DeLong 1990), perhaps the most convincing recent evidence for motor antagonism between these pathways has come from rodent optogenetic studies showing increased and reduced locomotion during selective optogenetic stimulation of direct and indirect pathway respectively in BAC transgenic mice (selectively expressing ChR2 in DA D1, D2, or adenosine A2A receptor-expressing striatal projection neurons, Freeze et al. 2013; Gong et al. 2007; Kravitz et al. 2010). Remarkably, Kravitz et al. (2010) were able to show that in mice with bilateral 6-OHDA lesions, optogenetic bilateral stimulation of dorsomedial striatal direct pathway neurons restored normal motor function (Kravitz et al. 2010). Extrapolating from this finding, it is possible that approaches that preferentially recruit the striatal direct pathway may represent a feasible therapeutic avenue for PD motor deficits. Studies of selective, direct pathway stimulation in MPTP-treated monkeys, wherein more sophisticated motor behavioral testing is possible, will be an essential prerequisite towards validating this approach for potential clinical use. Although intracranial optogenetic approaches are unlikely to be clinically viable (at least in the foreseeable future), chemogenetic and/or other gene therapy approaches may offer more realistic therapeutic strategies in this context.
Concluding Remarks
Opto- and chemogenetic tools hold the potential to facilitate a rapid expansion in our understanding of basal ganglia circuits in NHP models. Although rodent studies using these toolsets have shed substantial light on basal ganglia function and dysfunction, one cannot disregard the added complexities of the primate brain, and the need for NHP studies as a key translational bridge from basic research to clinical therapeutics. Fortunately, in the last few years, molecular tools such as opto- and chemogenetics are gaining increasing experimental tractability in NHPs, and are poised to provide unprecedented insights into the basal ganglia and other neural systems.
References
- Acker L, Pino EN, Boyden ES, Desimone R. FEF inactivation with improved optogenetic methods. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1610784113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afraz A, Boyden ES, DiCarlo JJ. Optogenetic and pharmacological suppression of spatial clusters of face neurons reveal their causal role in face gender discrimination. Proc Natl Acad Sci U S A. 2015;112:6730–6735. doi: 10.1073/pnas.1423328112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alvarez L, Macias R, Pavon N, Lopez G, Rodriguez-Oroz MC, Rodriguez R, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry. 2009;80:979–985. doi: 10.1136/jnnp.2008.154948. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Experimental neurology. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Benhamou L, Bronfeld M, Bar-Gad I, Cohen D. Globus Pallidus external segment neuron classification in freely moving rats: a comparison to primates. PLoS One. 2012;7:e45421. doi: 10.1371/journal.pone.0045421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neurosciences. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bernal-Casas D, Lee HJ, Weitz AJ, Lee JH. Studying Brain Circuit Function with Dynamic Causal Modeling for Optogenetic fMRI. Neuron. 2017;93:522–532.e525. doi: 10.1016/j.neuron.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober Gordon F, Simonnet C, Calhoon Gwendolyn G, Conyers Garrett F, et al. Divergent Routing of Positive and Negative Information from the Amygdala during. Memory Retrieval Neuron. 2016;90:348–361. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light F1000. Biol Rep. 2011;3 doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep Brain Stimulation for Parkinson Disease. Archives of Neurology. 2011;68 doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, et al. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron. 2012;76:901–907. doi: 10.1016/j.neuron.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW, et al. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:723–739. doi: 10.1016/s0278-5846(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, et al. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci. 2015;6:476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiken S, Nambu A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist. 2015;22:313–322. doi: 10.1177/1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie IN, Wells JA, Southern P, Marina N, Kasparov S, Gourine AV, et al. fMRI response to blue light delivery in the naive brain: implications for combined optogenetic fMRI studies. Neuroimage. 2013;66:634–641. doi: 10.1016/j.neuroimage.2012.10.074. [DOI] [PubMed] [Google Scholar]
- Chu HY, Atherton JF, Wokosin D, Surmeier DJ, Bevan MD. Heterosynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex. Neuron. 2015;85:364–376. doi: 10.1016/j.neuron.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Brooks DI, Sheinberg DL. Optogenetic and electrical microstimulation systematically bias visuospatial choice in primates. Curr Biol. 2014;24:63–69. doi: 10.1016/j.cub.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Dai J, Ozden I, Brooks DI, Wagner F, May T, Agha NS, et al. Modified toolbox for optogenetics in the nonhuman primate. Neurophotonics. 2015;2:031202–031202. doi: 10.1117/1.NPh.2.3.031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- de Leeuw CN, Dyka FM, Boye SL, Laprise S, Zhou M, Chou AY, et al. Targeted CNS delivery using human MiniPromoters and demonstrated compatibility with adeno-associated viral vectors. Mol Ther. 2014;1 doi: 10.1038/mtm.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-V. [DOI] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, et al. An optogenetic toolbox designed for primates. Nat Neurosci. 2011 doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo L, et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci. 2016;19:1743–1749. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya HB, Bjorklund T, Stansell J, 3rd, Mandel RJ, Kirik D, Kordower JH. Differential transduction following basal ganglia administration of distinct pseudotyped AAV capsid serotypes in nonhuman primates. Mol Ther. 2010;18:579–587. doi: 10.1038/mt.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- El-Shamayleh Y, Ni AM, Horwitz GD. Strategies for targeting primate neural circuits with viral vectors. J Neurophysiol. 2016 doi: 10.1152/jn.00087.2016. jn 00087 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, et al. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci. 2016;19:37–39. doi: 10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist LW, Husak PJ, Banfield BW, Smith GA. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- Farrell MS, Roth BL. Pharmacosynthetics: Reimagining the pharmacogenetic approach. Brain Res. 2013;1511:6–20. doi: 10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Vierock J, Atsuta-Tsunoda K, Tsunoda SP, Ramakrishnan C, Gorini C, et al. Optogenetic approaches addressing extracellular modulation of neural excitability. Sci Rep. 2016;6:23947. doi: 10.1038/srep23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2015;351:aac9698-aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandaca MS, Varenika V, Eberling J, McKnight T, Bringas J, Pivirotto P, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2009;47(Suppl 2):T27–35. doi: 10.1016/j.neuroimage.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun. 2014;5:5316. doi: 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of Basal Ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. In vivo optogenetic control of striatal and thalamic neurons in non-human primates. PLoS One. 2012;7:e50808. doi: 10.1371/journal.pone.0050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Effects of Optogenetic Activation of Corticothalamic Terminals in the Motor Thalamus of Awake Monkeys. J Neurosci. 2016;36:3519–3530. doi: 10.1523/JNEUROSCI.4363-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M, Cavada C. Dopamine Innervation in the Thalamus: Monkey versus. Rat Cerebral Cortex. 2008;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Curr Biol. 2012;22:1722–1726. doi: 10.1016/j.cub.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits A, Vancraeyenest P, Vreysen S, Laramee ME, Michiels A, Gijsbers R, et al. Serotype-dependent transduction efficiencies of recombinant adeno-associated viral vectors in monkey neocortex. Neurophotonics. 2015;2:031209. doi: 10.1117/1.NPh.2.3.031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits A, Vanduffel W. Optogenetics in primates: a shining future? Trends Genet. 2013;29:403–411. doi: 10.1016/j.tig.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ginger M, Haberl M, Conzelmann KK, Schwarz MK, Frick A. Revealing the secrets of neuronal circuits with recombinant rabies virus technology. Front Neural Circuits. 2013;7:2. doi: 10.3389/fncir.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajch KE, Kelver DA, Hegeman DJ, Cui Q, Xenias HS, Augustine EC, et al. Npas1+ Pallidal Neurons Target Striatal Projection Neurons. J Neurosci. 2016;36:5472–5488. doi: 10.1523/JNEUROSCI.1720-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Budygin EA, Fuller PM, Bass CE. Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Front Behav Neurosci. 2015;9:152. doi: 10.3389/fnbeh.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Samulski RJ. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, et al. The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron. 2016;91:453–466. doi: 10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Robertson B, Stephenson-Jones M. The evolutionary origin of the vertebrate basal ganglia and its role in action selection. J Physiol. 2013;591:5425–5431. doi: 10.1113/jphysiol.2012.246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- Han X. Optogenetics in the nonhuman primate. Prog Brain Res. 2012;196:215–233. doi: 10.1016/B978-0-444-59426-6.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: Volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol. 2002;445:238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- Howe MW, Dombeck DA. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016 doi: 10.1038/nature18942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Parr-Brownlie LC, Bosch-Bouju C, Schoderboeck L, Sizemore R, Abraham W. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Frontiers in Molecular Neuroscience. 2015;8 doi: 10.3389/fnmol.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takada M, Matsumoto M. Neuronal and behavioural modulations by pathway-selective optogenetic stimulation of the primate oculomotor system. Nat Commun. 2015;6:8378. doi: 10.1038/ncomms9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Beneduce BM, Drew IR, Regehr WG. Achieving high-frequency optical control of synaptic transmission. J Neurosci. 2014;34:7704–7714. doi: 10.1523/JNEUROSCI.4694-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn Ther. 1994;328:243–250. [PubMed] [Google Scholar]
- Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat Neurosci. 2012;15:1368–1370. doi: 10.1038/nn.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Costa RM. Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol. 2015;33:188–196. doi: 10.1016/j.conb.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci. 2014 doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junyent F, Kremer EJ. CAV-2—why a canine virus is a neurobiologist’s best friend. Curr Opin Pharmacol. 2015;24:86–93. doi: 10.1016/j.coph.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Kato S, Kobayashi K, Inoue K, Kuramochi M, Okada T, Yaginuma H, et al. A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein. Hum Gene Ther. 2011a;22:197–206. doi: 10.1089/hum.2009.179. [DOI] [PubMed] [Google Scholar]
- Kato S, Kuramochi M, Kobayashi K, Fukabori R, Okada K, Uchigashima M, et al. Selective neural pathway targeting reveals key roles of thalamostriatal projection in the control of visual discrimination. J Neurosci. 2011b;31:17169–17179. doi: 10.1523/JNEUROSCI.4005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kuramochi M, Takasumi K, Kobayashi K, Inoue K, Takahara D, et al. Neuron-specific gene transfer through retrograde transport of lentiviral vector pseudotyped with a novel type of fusion envelope glycoprotein. Hum Gene Ther. 2011c;22:1511–1523. doi: 10.1089/hum.2011.111. [DOI] [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Kim S-G, Harel N, Jin T, Kim T, Lee P, Zhao F. Cerebral blood volume MRI with intravascular superparamagnetic iron oxide nanoparticles. NMR in Biomedicine. 2013;26:949–962. doi: 10.1002/nbm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Evrard HC, Shapcott KA, Haverkamp S, Logothetis NK, Schmid MC. Cell-Targeted Optogenetics and Electrical Microstimulation Reveal the Primate Koniocellular Projection to Supra-granular Visual. Cortex Neuron. 2016;90:143–151. doi: 10.1016/j.neuron.2016.02.036. [DOI] [PubMed] [Google Scholar]
- Kolodziej A, Lippert M, Angenstein F, Neubert J, Pethe A, Grosser OS, et al. SPECT-imaging of activity-dependent changes in regional cerebral blood flow induced by electrical and optogenetic self-stimulation in mice. NeuroImage. 2014;103:171–180. doi: 10.1016/j.neuroimage.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Owen SF, Kreitzer AC. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Research. 2013;1511:21–32. doi: 10.1016/j.brainres.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameh J, Burstein ES, Taylor E, Weiner DM, Vanover KE, Bonhaus DW. Pharmacology of N-desmethylclozapine. Pharmacol Ther. 2007;115:223–231. doi: 10.1016/j.pharmthera.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Weitz AJ, Bernal-Casas D, Duffy BA, Choy M, Kravitz AV, et al. Activation of Direct and Indirect Pathway Medium Spiny Neurons Drives Distinct Brain-wide Responses. Neuron. 2016 doi: 10.1016/j.neuron.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Corgiat B, Der Minassian V, Saunders RC, Richmond BJ. Injection parameters and virus dependent choice of promoters to improve neuron targeting in the nonhuman primate brain. Gene Ther. 2014;21:233–241. doi: 10.1038/gt.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Lang AE, Dostrovsky JO, Pahapill P, Romas J, Saint-Cyr J, et al. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain. 2001;124:2105–2118. doi: 10.1093/brain/124.10.2105. [DOI] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan Danny CW, Qian Z-M, Yung Ken KL, Ko H, et al. Therapeutic Deep Brain Stimulation in Parkinsonian Rats Directly Influences Motor Cortex. Neuron. 2012;76:1030–1041. doi: 10.1016/j.neuron.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Lozano Andres M, Lipsman N. Probing and Regulating Dysfunctional Circuits Using Deep Brain Stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Lu Y, Truccolo W, Wagner FB, Vargas-Irwin CE, Ozden I, Zimmermann JB, et al. Optogenetically induced spatiotemporal gamma oscillations and neuronal spiking activity in primate motor cortex. J Neurophysiol. 2015;113:3574–3587. doi: 10.1152/jn.00792.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, et al. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro. 2016;3 doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 2016;19:554–556. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, et al. Comparative transduction efficiency of AAV vector serotypes 1–6 in the substantia nigra and striatum of the primate brain. Mol Ther. 2010;18:588–593. doi: 10.1038/mt.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamizu Y, Okada T, Kawasaki K, Ishibashi H, Yuasa S, Takeda S, et al. Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience. 2011;193:249–258. doi: 10.1016/j.neuroscience.2011.06.080. [DOI] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, Ozden I, Brush B, Borton D, Wagner F, Agha N, et al. Detection of optogenetic stimulation in somatosensory cortex by non-human primates--towards artificial tactile sensation. PLoS One. 2014;9:e114529. doi: 10.1371/journal.pone.0114529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Anderson SAR, Ananth M, Smirnov D, Thanos PK, Neumaier JF, et al. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest. 2013;123:5342–5350. doi: 10.1172/jci72117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguelez C, Morin S, Martinez A, Goillandeau M, Bezard E, Bioulac B, et al. Altered pallido-pallidal synaptic transmission leads to aberrant firing of globus pallidus neurons in a rat model of Parkinson’s disease. J Physiol. 2012;590:5861–5875. doi: 10.1113/jphysiol.2012.241331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlidharan G, Samulski RJ, Asokan A. Biology of adeno-associated viral vectors in the central nervous system. Front Mol Neurosci. 2014;7:76. doi: 10.3389/fnmol.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Kikuchi E, Lerchner W, Inoue KI, Ji B, Eldridge MA, et al. PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun. 2016;7:13605. doi: 10.1038/ncomms13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Avery MC, Cetin AH, Roe AW, Reynolds JH. Optogenetic Activation of Normalization in Alert Macaque Visual Cortex. Neuron. 2015;86:1504–1517. doi: 10.1016/j.neuron.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 2009;161:441–450. doi: 10.1016/j.neuroscience.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh Edward H, Matthews Gillian A, Allsop Stephen A, Presbrey Kara N, Leppla Christopher A, Wichmann R, et al. Decoding Neural Circuits that Control Compulsive Sucrose Seeking. Cell. 2015;160:528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi M, Okajima M, Tanaka S, Koizumi M, Kikusui T, Ichihara N, et al. Double Virus Vector Infection to the Prefrontal Network of the Macaque Brain. PLoS One. 2015;10:e0132825. doi: 10.1371/journal.pone.0132825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon S, Grimaldi P, Schweers N, Tsao DY. Saccade Modulation by Optical and Electrical Stimulation in the Macaque Frontal Eye Field. J Neurosci. 2013;33:16684–16697. doi: 10.1523/jneurosci.2675-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg IA, Sabatini BL. Antagonistic but Not Symmetric Regulation of Primary Motor Cortex by Basal Ganglia Direct and Indirect Pathways. Neuron. 2015;86:1174–1181. doi: 10.1016/j.neuron.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden I, Wang J, Lu Y, May T, Lee J, Goo W, et al. A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J Neurosci Methods. 2013;219:142–154. doi: 10.1016/j.jneumeth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryszyn S, Beaulieu J-M, Parent A, Parent M. Distribution and morphological characteristics of striatal interneurons expressing calretinin in mice: A comparison with human and nonhuman primates. J Chem Neuroanat. 2014;59–60:51–61. doi: 10.1016/j.jchemneu.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Portales-Casamar E, Swanson DJ, Liu L, de Leeuw CN, Banks KG, Ho Sui SJ, et al. A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proc Natl Acad Sci U S A. 2010;107:16589–16594. doi: 10.1073/pnas.1009158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The Restless Brain. Brain Connectivity. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Ferenczi E, Deisseroth K. Targeting Neural Circuits. Cell. 2016;165:524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon TR, Murray AJ, Turi GF, Wirblich C, Croce KR, Schnell MJ, et al. Rabies Virus CVS-N2c(DeltaG) Strain Enhances Retrograde Synaptic Transfer and Neuronal Viability. Neuron. 2016;89:711–724. doi: 10.1016/j.neuron.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein ML, Deussing JM. The optogenetic (r)evolution. Mol Genet Genomics. 2012;287:95–109. doi: 10.1007/s00438-011-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, et al. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci. 2016;19:220–222. doi: 10.1038/nn.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Young AB, Penney JB. Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. The Journal of Comparative Neurology. 1987;262:446–463. doi: 10.1002/cne.902620308. [DOI] [PubMed] [Google Scholar]
- Rosenbluth KH, Eschermann JF, Mittermeyer G, Thomson R, Mittermeyer S, Bankiewicz KS. Analysis of a simulation algorithm for direct brain drug delivery. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BD, Driscoll JPo. [Accessed 20 October 2016];Psychoactive Drug Screening Program. 2014 http://pdsp.med.unc.edu/
- Roth Bryan L. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz O, Lustig BR, Nassi JJ, Cetin A, Reynolds JH, Albright TD, et al. Optogenetics through windows on the brain in the nonhuman primate. J Neurophysiol. 2013;110:1455–1467. doi: 10.1152/jn.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, Salegio EA, San Sebastian W, Kells AP, Bringas JR, Forsayeth J, et al. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Hum Gene Ther. 2013;24:526–532. doi: 10.1089/hum.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, San Sebastian W, Kells AP, Salegio EA, Heller G, Bringas JR, et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther. 2014;22:329–337. doi: 10.1038/mt.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Sebastian W, Samaranch L, Heller G, Kells AP, Bringas J, Pivirotto P, et al. Adeno-associated virus type 6 is retrogradely transported in the non-human primate brain. Gene Ther. 2013;20:1178–1183. doi: 10.1038/gt.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TH, Jaeger D. Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol Dis. 2016;95:225–237. doi: 10.1016/j.nbd.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanftner LM, Sommer JM, Suzuki BM, Smith PH, Vijay S, Vargas JA, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Experimental neurology. 2005;194:476–483. doi: 10.1016/j.expneurol.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Huang KW, Sabatini BL. Globus Pallidus Externus Neurons Expressing parvalbumin Interconnect the Subthalamic Nucleus and Striatal Interneurons. PLoS One. 2016;11:e0149798. doi: 10.1371/journal.pone.0149798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, McGettigan JP, Wirblich C, Papaneri A. The cell biology of rabies virus: using stealth to reach the brain. Nat Rev Microbiol. 2010;8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. Journal of Neurophysiology. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Smith Y, Wichmann T, DeLong MR. Corticostriatal and mesocortical dopamine systems: do species differences matter? Nat Rev Neurosci. 2014;15:63–63. doi: 10.1038/nrn3469-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, et al. Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques. Cell. 2016;166:1564–1571. e1566. doi: 10.1016/j.cell.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An Emerging Technology Framework for the Neurobiology of Appetite. Cell Metab. 2016;23:234–253. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Straub C, Saulnier JL, Begue A, Feng DD, Huang KW, Sabatini BL. Principles of Synaptic Organization of GABAergic Interneurons in the Striatum. Neuron. 2016;92:84–92. doi: 10.1016/j.neuron.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Tritsch NX, Hagan NA, Gu C, Sabatini BL. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci. 2014;34:8557–8569. doi: 10.1523/JNEUROSCI.0589-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Ohashi Y, Tsubota T, Takeuchi D, Hirabayashi T, Yaguchi M, et al. A glass-coated tungsten microelectrode enclosing optical fibers for optogenetic exploration in primate deep brain structures. J Neurosci Methods. 2012;211:49–57. doi: 10.1016/j.jneumeth.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Jin X, Lima Susana Q, Costa Rui M. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell. 2016;166:703–715. doi: 10.1016/j.cell.2016.06.032. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Matias S, Dugue GP, Mainen ZF, Costa RM. Balanced activity in basal ganglia projection pathways is critical for contraversive movements. Nat Commun. 2014;5:4315. doi: 10.1038/ncomms5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschemacher AG, Wang S, Lonergan T, Duale H, Waki H, Paton JF, et al. Targeting specific neuronal populations using adeno- and lentiviral vectors: applications for imaging and studies of cell function. Exp Physiol. 2005;90:61–69. doi: 10.1113/expphysiol.2004.028191. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Robison L, Nestler EJ, Kim R, Michaelides M, Lobo MK, et al. Mapping Brain Metabolic Connectivity in Awake Rats with PET and Optogenetic Stimulation. J Neurosci. 2013;33:6343–6349. doi: 10.1523/jneurosci.4997-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, et al. Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacology. 2015;41:1404–1415. doi: 10.1038/npp.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ozden I, Diagne M, Wagner F, Borton D, Brush B, et al. Approaches to optical neuromodulation from rodents to non-human primates by integrated optoelectronic devices. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:7525–7528. doi: 10.1109/IEMBS.2011.6091855. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res. 2014 doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- White E, Bienemann A, Megraw L, Bunnun C, Wyatt M, Taylor H, et al. Distribution properties of lentiviral vectors administered into the striatum by convection-enhanced delivery. Hum Gene Ther. 2012;23:115–127. doi: 10.1089/hum.2010.185. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, et al. Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science. 2013;340:1232627. doi: 10.1126/science.1232627. [DOI] [PubMed] [Google Scholar]