Abstract
Purpose
Biomarkers for outcome after immune-checkpoint blockade are strongly needed as these may influence individual treatment selection or sequence. We aimed to identify baseline factors associated with overall survival (OS) following pembrolizumab treatment in melanoma patients.
Experimental design
Serum lactate dehydrogenase (LDH), routine blood count parameters, and clinical characteristics were investigated in 616 patients. Endpoints were OS and best overall response following pembrolizumab. Kaplan-Meier analysis and Cox regression were applied for survival analysis.
Results
Relative eosinophil count (REC) ≥1.5%, relative lymphocyte count (RLC) ≥17.5%, ≤2.5-fold elevation of LDH, and the absence of metastasis other than soft-tissue/lung were associated with favorable OS in the discovery (n=177) and the confirmation (n=182) cohort and had independent positive impact (all P<0.001). Their independent role was subsequently confirmed in the validation cohort (n=257; all P<0.01). The number of favorable factors was strongly associated with prognosis. One-year-OS probabilities of 83.9% vs 14.7% and response rates of 58.3% vs 3.3% were observed in patients with four out of four compared to those with none out of four favorable baseline factors present, respectively.
Conclusions
High REC and RLC, low LDH, and absence of metastasis other than soft-tissue/lung are independent baseline characteristics associated with favorable OS of patients with melanoma treated with pembrolizumab. Presence of four favorable factors in combination identifies a subgroup with excellent prognosis. In contrast, patients with no favorable factors present have a poor prognosis, despite pembrolizumab, and additional treatment advances are still needed. A potential predictive impact needs to be further investigated.
Keywords: Melanoma, pembrolizumab, biomarker, prognosis, LDH, RLC, REC
Introduction
Antibodies targeting programmed cell death protein-1 (PD-1) represent the second breakthrough in immune checkpoint blockade therapy of melanoma after approval of ipilimumab. Two PD-1-blocking antibodies, pembrolizumab and nivolumab have been approved by the FDA/EMEA for melanoma.
Pembrolizumab demonstrated clinical activity in a variety of solid tumors (1, 2) and improved overall survival (OS) of melanoma patients compared to ipilimumab (3). For ipilimumab-naïve patients treated with 10 mg pembrolizumab per kilogram of body weight either every 2 weeks or every 3 weeks, estimated 12-month survival rates were 68% and 74% and the proportion of patients with objective responses was 34% and 33%, respectively (3). Nevertheless, only 21–28% of ipilimumab-pretreated patients exhibit objective responses to pembrolizumab and approximately 40% die within one year (4, 5). In melanoma, nivolumab was associated with improved OS and progression-free survival (6) and higher response rates (7) in comparison to chemotherapy in phase III randomized clinical trials. Moreover, nivolumab alone or combined with ipilimumab resulted in significantly longer progression-free survival than ipilimumab alone among previously untreated patients (8).
Thus far, no biomarkers have yet been established to clearly predict clinical benefit from pembrolizumab. Most studies focused on the immunohistochemical analysis of anti-programmed cell death ligand-1 (PD-L1) on tumor cells and reported an association between high expression and clinical responses to nivolumab (9–11) or pembrolizumab (12). However, PD-L1 expression on tumor cells cannot be used as selection criterion for anti-PD-1 antibody treatment, since clinical activity was also observed in patients with low/negative PD-L1-expressing tumors, and because of differences in the definition of PD-L1 positivity, intra-patient heterogeneity, and limited assay standardization (11, 13, 14).
The absolute lymphocyte count (ALC) has been reported as biomarker candidate for outcome after ipilimumab treatment (15, 16) but not for pembrolizumab. Increase in activated T cells during pembrolizumab or during nivolumab and ipilimumab in combination were reported previously (13, 17). ALC was not obviously changing upon combined immune-checkpoint blockade and low ALC did not preclude clinical activity. Clinical responses were correlated with low levels of circulating myeloid-derived suppressor cells (MDSCs) (17). High NY-ESO-1 and MART-1–specific T cells at baseline or decreases during treatment as well as increases in circulating T regulatory cells (Tregs) were associated with disease progression in patients treated with nivolumab +/− peptide vaccine (11). Preexisting CD8+ T cells in the tumor microenvironment were required for tumor regression after pembrolizumab (18), while the presence of an immune infiltrate in early on-treatment samples was even more predictive of response for PD-1 blockade compared to that in pre-treatment samples in another study (19). Higher numbers of PD1+ T-cells at baseline and increased PD-L1 expression during treatment were associated with clinical response in an analysis of sequential tumor biopsies of 24 melanoma patients treated with PD-1 blockade (20).
The aim of the present study was to examine readily available clinical factors and peripheral blood count parameters to identify pre-treatment factors associated with OS of advanced melanoma patients treated with pembrolizumab.
Patients and Methods
Patients
Data from patients treated with pembrolizumab were provided by 30 clinical sites (Supplementary Table 1). Inclusion criteria were unresectable stage III or stage IV melanoma, treatment with at least one dose of pembrolizumab, and available data of differential blood counts and/or LDH from blood draws taken 0–28 days before the first dose of pembrolizumab. Patients gave their written informed consent for use of clinical data for scientific purposes. This study was approved by the Ethics Committee, University of Tübingen (approvals 524/2012BO2 and 234/2015BO2).
Study design
Differences in OS according to 15 variables were investigated. These were: gender, age, pattern of visceral tumor involvement (distant lymph nodes/soft tissue and/or lung only vs involvement of other organs), and routine blood analyses (LDH, leucocyte count, absolute and relative counts of lymphocytes, monocytes, eosinophils, basophils and neutrophils). LDH was analyzed by means of the LDH-ratio (actual value divided by the upper limit of normal). The analysis of the discovery cohort (recruitment until December 3rd 2014) aimed to identify prognostic factor candidates. Candidate factors and respective cut-off points of all continuous variables were systematically defined by applying an optimization algorithm as similarly described earlier (21). Briefly, differences in OS were analyzed in the discovery cohort using an approach of maximally selected p-values based on log rank tests at different cut-off points. A primary cut-off candidate was that resulting in the lowest significant p-value comparing patients with values above vs below the respective cut-off point. A secondary cut-off candidate was that resulting in the lowest significant p-value comparing patients within the groups defined by the primary cut-off candidate. Cut-off points were only tested if the smaller group comprised at least 10% of all patients of the discovery cohort with available data. More details about the cut-off point selection algorithm are presented in Supplementary Methods.
The analysis of the confirmation cohort (recruitment until December 24th 2014) aimed to confirm the previously defined candidates. Next, a combination model based on confirmed candidates was defined by analysis of all patients of the combined discovery and confirmation cohort. The analysis of an additional validation cohort (recruitment until April 30th 2015) finally aimed to validate the combination model.
Finally, clinical responses were collected as assessed by the investigators of the respective clinical site and categorized as either complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to RECIST V1.1 criteria (22). The best overall response (bOR) was defined as the best achieved response from the start of pembrolizumab and to the date of progressive disease or start of a new systemic treatment. All tumor assessments within this time period were considered. Based on the bOR we analyzed the proportion of patients who had an objective response (complete or partial response, patients without a disease assessment following initiation of pembrolizumab were considered to be non-responders).
Statistics
Follow-up time was defined from the date of the first dose to the date of last known contact or death. Survival probabilities and median survival with 95% confidence intervals (CI) were estimated according to the Kaplan-Meier method, and compared using log rank tests. Only deaths due to melanoma were considered and other causes of death were regarded as censored events, except for the analysis presented in Supplementary Figure 2. Here, death from any reason was considered as “event”. Cox proportional hazard analyses using stepwise procedures were used to determine the relative impact of confirmed factors. Patients with missing data in variables analyzed in the given Cox regression model were excluded. Results are described by means of hazard ratios (HR) with 95% CIs, and P-values are based on the Wald test. The concordance index (c-index) was calculated for different models as a measure of the discriminatory ability that allows comparison of models. A model with a c-index=0.5 has no predictive value, a model with a c-index =1 would allow a perfect prediction of patient outcome (23). The concordance index was analyzed using the survConcordance function in the survival package for R.
Associations between best overall response and biomarker categories were analyzed by Chi square and Fisher´s exact tests. Throughout the analysis, P-values <0.05 were considered statistically significant. All statistical tests were two-sided. Analyses were carried out using SPSS Version 22 (IBM, USA) and R 3.2.1 (R Foundation for Statistical Computing, Vienna Austria).
Results
Patients and treatments
A total of 616 patients treated with pembrolizumab were included (Table 1). The median age was 60 years, and 40.6% were male. Of 578 patients with sufficient data for classification according to AJCC 2009 (24), 483 were assigned to the M-category M1c (83.6%), 59 to M1b (10.2%), 25 to M1a (4.3%), and eleven patients had unresectable stage III disease (1.9%). Treatment was mainly administered in the early access program (65.3%; NCT02083484) or in the Keynote-002 study (17.7%; NCT01704287) (5). Fifty-four patients were treated with commercial pembrolizumab after FDA marketing approval (8.8%), 42 patients were treated in the Keynote-001 study (6.8%; NCT01295827) (1, 4), and nine were treated in the Keynote-006 study (1.5%; NCT01515189). 97.9% had received at least one prior systemic treatment including ipilimumab, while 2.1% of patients received pembrolizumab as first-line therapy. Of 389 patients with available data on the bOR, 28.3% experienced a clinical response (5.4% CR and 22.9% PR). 19.5% of patients had a bOR of SD, and 36.2% had PD. 15.9% of patients had no radiologic tumor assessment before death due to melanoma progression. Among the whole population, 224 (36.4%) of patients died during follow up. 219 died from melanoma progression, two patients died from voluntary physician-assisted ending of life and another three from cerebral strokes. Median follow-up was 5.5 months for patients who were alive at the last follow-up, and 2.9 months for those who died.
Table 1.
Patient and treatment characteristics.
| Discovery Cohort 1 (n=177) |
Confirmation Cohort 2 (n=182) |
Validation Cohort 3 (n=257) |
Total (n=616) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Gender | Male | 71 | 40.1 | 78 | 42.9 | 101 | 39.3 | 250 | 40.6 |
| Female | 106 | 59.9 | 104 | 57.1 | 156 | 60.7 | 366 | 59.4 | |
| Age | ≤ 50 years | 42 | 23.7 | 54 | 29.7 | 55 | 21.4 | 151 | 24.5 |
| 51 – 60 years | 45 | 25.4 | 50 | 27.5 | 63 | 24.5 | 158 | 25.6 | |
| 61 – 70 years | 44 | 24.9 | 49 | 26.9 | 77 | 30.0 | 170 | 27.6 | |
| ≥ 70 years | 46 | 26.0 | 29 | 15.9 | 62 | 24.1 | 137 | 22.2 | |
| Median age, IQR(years) | 61, 51–71 | 58, 48–67 | 62, 52–70 | 60, 51–69 | |||||
| M category (AJCC 2009) | Unresectable stage III | 3 | 1.7 | 6 | 3.3 | 2 | 0.9 | 11 | 1.9 |
| M1a | 2 | 1.1 | 7 | 3.8 | 16 | 7.3 | 25 | 4.3 | |
| M1b | 24 | 13.6 | 19 | 10.4 | 16 | 7.3 | 59 | 10.2 | |
| M1c | 147 | 83.5 | 150 | 82.4 | 186 | 84.5 | 483 | 83.6 | |
| Unknown | 1 | 37 | 38 | ||||||
| LDH | Normal | 76 | 43.9 | 93 | 51.1 | 96 | 57.8 | 265 | 50.9 |
| Elevated | 97 | 56.1 | 89 | 48.9 | 70 | 42.2 | 256 | 49.1 | |
| Unknown | 4 | 91 | 95 | ||||||
| Visceral involvement | Distant lymph nodes/soft tissue | 4 | 2.3 | 11 | 6.0 | 38 | 14.8 | 53 | 8.6 |
| Lung | 41 | 23.2 | 28 | 15.4 | 47 | 18.3 | 116 | 18.8 | |
| Other organs | 129 | 72.9 | 137 | 75.3 | 170 | 66.1 | 436 | 70.8 | |
| None (unresectable stage III) | 3 | 1.7 | 6 | 3.3 | 2 | 0.8 | 11 | 1.8 | |
| Prior ipilimumab | No | 6 | 3.4 | 3 | 1.6 | 4 | 1.6 | 13 | 2.1 |
| Yes | 171 | 96.6 | 179 | 98.4 | 253 | 98.4 | 603 | 97.9 | |
| Line of treatment | First line | 6 | 3.4 | 3 | 1.6 | 4 | 1.6 | 13 | 2.1 |
| Second line or later | 171 | 96.6 | 179 | 98.4 | 253 | 98.4 | 603 | 97.9 | |
| Treatment schedule of pembrolizumab | 10 mg/kg – 2 weeks | 2 | 1.1 | 1 | 0.5 | 7 | 2.7 | 10 | 1.6 |
| 10 mg/kg – 3 weeks | 1 | 0.6 | 24 | 9.3 | 25 | 4.1 | |||
| 2 mg/kg – 3 weeks | 117 | 66.1 | 134 | 73.6 | 216 | 84.0 | 467 | 75.8 | |
| 2 or 10 mg/kg – 3 weeks | 54 | 30.5 | 45 | 24.7 | 10 | 3.9 | 109 | 17.7 | |
| 10 mg/kg – 2 or 3 weeks | 3 | 1.7 | 2 | 1.1 | 5 | 0.8 | |||
| Treatment background | Commercial | 54 | 21.0 | 54 | 8.8 | ||||
| Early access program | 117 | 66.1 | 134 | 73.6 | 151 | 58.8 | 402 | 65.3 | |
| Keynote-001 | 42 | 16.3 | 42 | 6.8 | |||||
| Keynote-002 | 54 | 30.5 | 45 | 24.7 | 10 | 3.9 | 109 | 17.7 | |
| Keynote-006 | 6 | 3.4 | 3 | 1.6 | 9 | 1.5 | |||
AJCC: American Joint Committee on Cancer; LDH: lactate dehydrogenase; IQR: interquartile range
Identification and confirmation of baseline factors associated with OS
The pattern of visceral metastasis, gender and 13 continuous variables (such as age or blood counts) were initially investigated in the discovery cohort (n=177) using a systematic approach of cut-off optimization for continuous factors (Supplementary Table 2). No correlation with OS was observed in the discovery cohort for gender, age, absolute and relative monocyte counts, and absolute basophil count (all P≥0.05). These factors were no longer considered in subsequent analyses. Significant negative correlations with OS were observed for a high LDH-ratio (P<0.001), high leucocyte count (P=0.02) and high absolute and relative neutrophil counts (both P<0.001). Significant positive correlations with OS were observed for high absolute (P=0.02) and relative (P<0.001) eosinophil counts, high absolute (P=0.002) and relative (P<0.001) lymphocyte counts, and high relative basophil counts (P=0.001).
In the confirmation cohort (n=182), nine factors were again significantly associated with prognosis using the following previously defined cut-off points for categorization: LDH-ratio ≤1.7 vs >1.7 and ≤2.5 vs >2.5, absolute leucocyte count <8750 vs ≥8750, absolute neutrophil count <6050 vs ≥6050, relative neutrophils count <61.5% vs ≥61.5% and <77.5% vs ≥77.5%, absolute eosinophil count <150 vs ≥150, relative eosinophil count <1.5% vs ≥1.5%, relative lymphocyte <17.5% vs ≥17.5% and <26.5% vs ≥26.5%, and relative basophil counts <0.5% vs ≥0.5% (all P<0.05). Patients with unresectable stage III disease or metastases located to distant lymph nodes/soft tissue or lung had a significant longer OS as compared to patients with other visceral metastases in the discovery and confirmation cohorts (both P<0.001).
Definition of a combination model for prognosis after pembrolizumab
Cox regression analysis was performed including all patients of the combined discovery and the confirmation cohort with complete data (n=346) to determine the relative impact of confirmed factors. Complete data were available for 170 out of 177 patients from the discovery cohort and for 176 out of 182 patients from the confirmation cohort. As two cut-off points were confirmed for the LDH-ratio, relative lymphocyte count (RLC) and relative neutrophil count, altogether twelve variable/cut-off combinations were considered in the multivariate model. A LDH-ratio ≤2.5 (HR 2.5; P<0.001), RLC ≥17.5% (HR 1.9; P<0.001), relative eosinophil count (REC) ≥1.5% (HR 2.2; P<0.001), and no involvement of visceral metastasis other than lung (HR 2.5; P<0.001) were factors independently associated with longer OS (Table 2 and Supplementary Table 2). None of the 8 remaining variable/cut-off combinations added further significant independent prognostic information (all P≥0.05).
Table 2.
Cox regression analysis.
| Variable | Categories | Combined discovery & confirmation cohorts (n=346) | Validation cohort (n=166) | All patients (n=512) | |||
|---|---|---|---|---|---|---|---|
| Hazard ratio [95% CI] |
Wald test P-value | Hazard ratio [95% CI] |
Wald test P-value | Hazard ratio [95% CI] |
Wald test P-value | ||
| Visceral involvement | Unresectable stage III, distant lymph nodes/soft tissue, lung | 1.0 | <0.001 | 1.0 | 0.008 | 1.0 | <0.001 |
| Other visceral involvement | 2.5[1.5;4.1] | 3.5[1.4;8.9] | 2.7[1.8;4.2] | ||||
| LDH-ratio | ≤2.5 | 1.0 | <0.001 | 1.0 | <0.001 | 1.0 | <0.001 |
| >2.5 | 2.5 [1.7;3.6] | 6.1[3.1;12.3] | 2.8 [2.0;3.9] | ||||
| Relative lymphocyte count | <17.5% | 1.9 [1.3;2.8] | <0.001 | 2.4 [1.4;4.4] | 0.003 | 2.0 [1.5;2.8] | <0.001 |
| ≥17.5% | 1.0 | 1.0 | 1.0 | ||||
| Relative eosinophil count | <1.5% | 2.2 [1.5;3.1] | <0.001 | 2.2 [1.2;3.9] | 0.006 | 2.0 [1.5;2.8] | <0.001 |
| ≥1.5% | 1.0 | 1.0 | 1.0 | ||||
LDH: lactate dehydrogenase; CI: confidence interval.
Next, we analyzed prognosis according to the number of favorable baseline factors present considering the four independent factors as a combination model. 46 patients (13.3%) had favorable results in all 4 baseline factors and a 1-year survival probability of 87.4%. Patients who had three (n=103; 29.8%) or two (n=99; 28.6%) factors present had a 1-year OS of 68.5% and 46.4%, respectively. In contrast, 1-year OS probability was 12.7% or 15.7% for patients with 1 (n=68; 19.7%) or 0 (n=30; 8.7%) favorable factors, respectively (Supplementary Figure 1A).
Independent validation of the combination model
In the validation cohort (n=257) all four variables were again associated with OS (all P<0.001; Supplementary Table 2) and had again independent impact on prognosis in Cox regression analysis (Table 2) considering 166 of 257 patients with available data for all 4 factors. The risk of death was 2.4-fold (P=0.003) and 2.2-fold (P<0.001) higher for patients in the validation cohort with RLC <17.5% and REC <1.5%, respectively. The presence of visceral metastases other than lung was also associated with OS (HR 3.5; P=0.008). The LDH-ratio >2.5 was the strongest independent factor with a 6.1 times increased risk of death (P<0.001). Overall survival according to the number of favorable baseline factors in the validation cohort is presented in Supplementary Figure 1B.
Confirmed associations with overall survival in all patients treated with pembrolizumab
In addition to LDH, REC, RLC and the pattern of visceral metastasis as considered in the combination model, we analyzed the other 8 confirmed categorizations in the validation cohort as well. All but AEC (P=0.0763) were again significantly associated with OS (all P<0.05). Univariate analysis considering all 616 patients treated with pembrolizumab is presented in Table 3. The 1-year OS was highest with 81.9% for patients with unresectable stage III melanoma or if distant metastases were limited to soft-tissue and/or lungs. LDH was a strong factor for stratifying patients according to prognosis into three distinct groups. The median survival was 16.1 months for patients with baseline LDH within normal limits or up to 1.7-fold elevation. In contrast, it decreased to 8.7 and 2.3 months for those with >1.7-fold or >2.5-fold elevation above the upper limit of normal, respectively. RLC ≥26.5% identified patients with a survival probability of 77.4% at one year. High absolute or relative eosinophil counts and high relative basophil count were also strongly associated with favorable outcome. High absolute or relative neutrophil counts and high absolute leucocyte count were negatively correlated with OS (all P<0.001).
Table 3.
Overall survival of patients treated with pembrolizumab (cohorts combined) according to confirmed prognostic factors.
| Variable | Total | Categories | n | % | % dead | Univariate Kaplan Meier Estimates | |||
|---|---|---|---|---|---|---|---|---|---|
| Median survival time (months) | 1-year survival rate [95% CI] (%) | Log-rank P-value | |||||||
| All patients | 616 | 100 | 35.6 | 15.5 | 54.5 | [48.8; 60.1] | |||
| Visceral involvement | 616 | Unresectable stage III, distant lymph nodes/soft tissue, lung | 180 | 29.2 | 17.7 | n.r. | 81.9 | [74.4; 89.5] | <0.001 |
| Other visceral involvement | 436 | 70.8 | 42.9 | 8.8 | 40.7 | [33.3; 48.2] | |||
| LDH-ratio | 521 | ≤1.7 | 402 | 77.2 | 28.1 | 16.1 | 61.0 | [53.6; 68.4] | <0.001 |
| ≤2.5 | 38 | 7.3 | 55.3 | 8.7 | 28.0 | [8.9; 47.2] | |||
| >2.5 | 81 | 15.5 | 75.3 | 2.3 | 16.2 | [5.5; 26.9] | |||
| Absolute leucocyte count | 614 | <8750/μL | 423 | 68.9 | 29.3 | 17.6 | 63.2 | [56.8; 69.7] | <0.001 |
| ≥8750/μL | 191 | 31.1 | 49.2 | 7.1 | 34.0 | [23.4; 44.5] | |||
| Absolute neutrophil count | 607 | <6050/μL | 407 | 67.1 | 27.5 | 17.6 | 63.9 | [57.1; 70.7] | <0.001 |
| ≥6050/μL | 200 | 32.9 | 50.5 | 6.4 | 35.6 | [25.5; 45.7] | |||
| Absolute eosinophil count | 607 | <150/μL | 314 | 51.7 | 42.4 | 9.7 | 48.3 | [40.8; 55.9] | <0.001 |
| ≥150/μL | 293 | 48.3 | 27.3 | 17.6 | 61.8 | [53.1; 70.4] | |||
| Relative lymphocyte count | 607 | <17.5% | 287 | 47.3 | 48.4 | 7.0 | 38.9 | [30.6; 47.2] | <0.001 |
| ≥17.5% | 202 | 33.3 | 27.2 | 19.0 | 63.4 | [53.9; 73.0] | |||
| ≥26.5% | 118 | 19.4 | 16.1 | n.r. | 77.4 | [65.2; 89.5] | |||
| Relative neutrophil count | 607 | <61.5% | 144 | 23.7 | 16.0 | n.r. | 76.5 | [65.1; 87.9] | <0.001 |
| ≥61.5% | 299 | 49.3 | 32.8 | 16.0 | 56.9 | [48.8; 65.0] | |||
| ≥77.5% | 164 | 27.0 | 56.1 | 4.9 | 32.2 | [22.2; 42.1] | |||
| Relative eosinophil count | 607 | <1.5% | 225 | 37.1 | 55.1 | 5.8 | 35.3 | [26.8; 43.8] | <0.001 |
| ≥1.5% | 382 | 62.9 | 23.3 | 19.6 | 66.7 | [59.4; 74.0] | |||
| Relative basophil count | 607 | <0.5% | 342 | 56.3 | 45.0 | 8.8 | 45.4 | [38.2; 52.7] | <0.001 |
| ≥0.5% | 265 | 43.7 | 22.3 | n.r. | 67.6 | [58.6; 76.5] | |||
LDH: lactate dehydrogenase; CI: confidence interval; n.r.: not reached
Overall survival and proportion of patients with objective response according to LDH, pattern of distant metastasis, RLC and REC as considered in the combination model
Considering all 512 patients with complete data (Table 2), those with LDH-ratio >2.5 had a HR of 2.8 after initiation of pembrolizumab compared to patients with LDH-ratio ≤2.5 (P<0.001). HR of patients with distant metastasis in visceral organs other than lung/soft-tissue was 2.7 (P<0.001). Moreover, RLC <17.5% was independently associated with poor OS compared to a higher baseline RLC (HR 2.0; P<0.001). Finally, patients presenting REC <1.5% had an increased risk of death (HR 2.0; P<0.001). OS according to the categories of the four single factors is presented in Figure 1. The relative proportion of all possible combination groups and the respective survival analyses accounting for LDH, pattern of visceral metastasis, RLC and REC are presented in Supplementary Table 3.
Figure 1. Overall survival according to confirmed baseline factors independently associated with outcome following pembrolizumab.
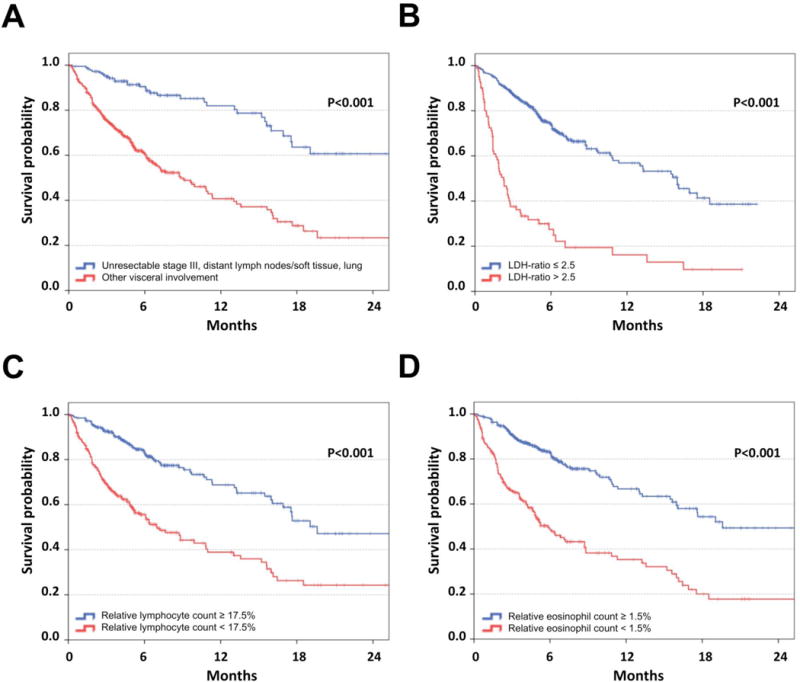
Kaplan-Meier curves of overall survival considering patients of all three cohorts of patients treated with pembrolizumab (n=616) according to the pattern of distant metastasis (A), LDH-ratio (the measured LDH serum concentration divided by the upper limit of normal) (B), relative lymphocyte count (C) and relative eosinophil count (D). Censoring is indicated by vertical lines; P-values were calculated by log rank statistics.
The count of favorable pre-treatment values (LDH-ratio ≤2.5, REC ≥1.5%, RLC ≥17.5% and absence of visceral metastasis other than lung) was strongly associated with long OS after start of pembrolizumab (Figure 2). Four or three favorable baseline values were observed in 70 (13.7%) and 160 (31.3%) patients, respectively. These patients had an very favorable outcome with a 1-year OS probability of 83.9% [70.3%;97.5%] and 68.1% [55.1%;81.1%]. 141 patients (27.5%) had 2 favorable baseline values and a 1-year survival probability of 47.9% [34.8%;61.0%]. In contrast, 1 or no favorable baseline values were present in 109 and 32 patients (21.3%, 6.3%). These patients had a poor prognosis with a 14.3% [3.5%;25.1%] and 14.7% [1.3%;28.2%] probability to survive 1-year, respectively (Figure 2A). The discriminatory ability (c-index) of this model was 0.782.
Figure 2. Overall survival and proportion of patients with objective response following pembrolizumab according to the count of favorable baseline factors.
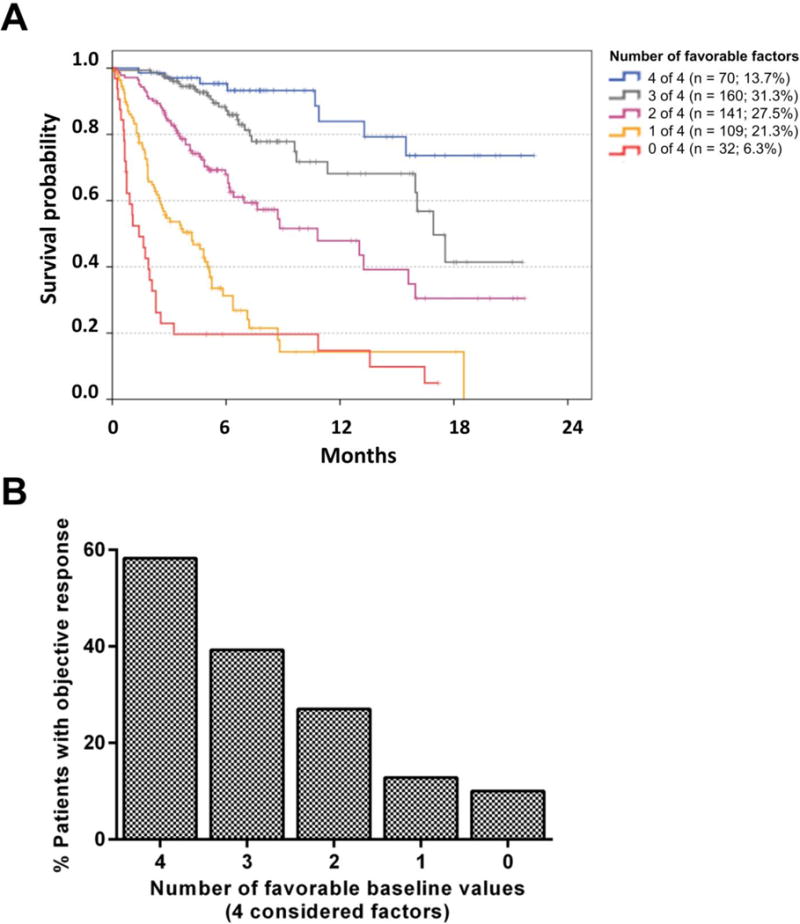
LDH, pattern of distant metastases, relative lymphocyte count and relative eosinophil count) had independent prognostic impact in the combined discovery and confirmation cohort, as well as in the validation cohort. Kaplan Meier curves for OS are presented for all patients according the count of favorable pre-treatment values considering those four factors. Among 512 patients with complete data, the median survival was 16.9, 10.8, 4.2, 1.4 months for patients with 0, 1, 2, or 3 favorable factor(s), respectively. Median survival was not reached for 13.7% of patients who had favorable values in all four factors. Censoring is indicated by vertical lines (A). The proportion of patients with objective response (PR or CR as best overall response according to RECIST V1.1) in 389 of 512 patients with available data is presented according to the number of favorable baseline factors. The differences between the categories were all statistically significant (p<0.05) in pairwise comparisons using Chi-Square/Fisher´s exact tests except the difference between patients with 0 and 1 favorable factor (p=0.674; B).
As five patients died from reasons other than progressive melanoma, we additionally analyzed OS according to the combination model considering death from any reason as “event”. Using this approach, the discriminatory ability was marginally higher (c-index=0.783), and differences in OS remained significant in all pairwise comparisons (all p<0.05; Supplementary Figure 2).
The proportion of patients experiencing an objective response (patients with PR or CR as best overall response according to RECIST V1.1) strongly correlated with the number of favorable baseline values in 389 of 512 patients with available data. An objective response was observed in 58.3%, 38.4%, or 24.3% of patients with 4, 3, or 2 favorable baseline values. In contrast, an objective response was only seen in 7.7% or 3.7% of patients with only 1 or 0 favorable baseline values, respectively (Figure 2B).
The association of the number of favorable baseline values with OS and bOR was also strong if the well-established prognostic markers LDH and the pattern of visceral metastasis were not considered in the combination model: patients with REC ≥1.5% and RLC ≥17.5% had a 1-year survival probability of 70% compared to 49% for patients with either REC ≥1.5% or RLC ≥17.5% and to 22% for those with REC <1.5% and RLC <17.5% (p<0.001 for all pairwise comparisons; Figure 3A). Moreover, significant differences in the proportion of patients with objective responses were observed between the respective groups (42.5%, 23.6%, and 12.5%; 42.5% vs 23.6%: p<0.001; 23.6% vs 12.5%: p=0.042; Figure 3B).
Figure 3. Overall survival and proportion of patients with objective response following pembrolizumab according to the relative lymphocyte and eosinophil counts.
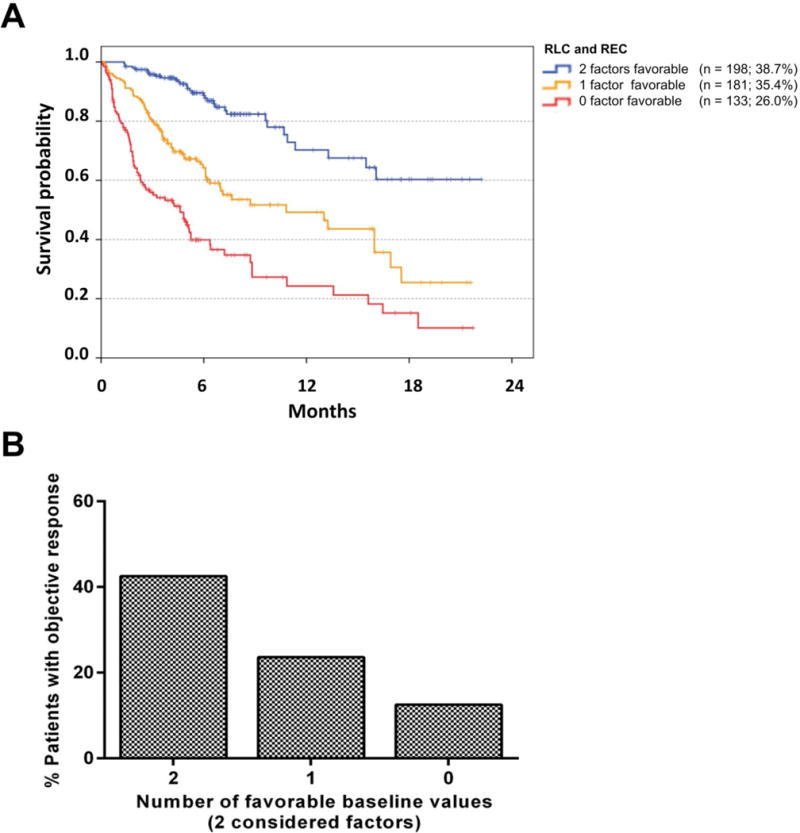
LDH, pattern of distant metastases, relative lymphocyte count and relative eosinophil count had independent prognostic impact in 512 patients with available data in all four baseline factors. Here, the analysis is limited to the impact of relative lymphocyte and eosinophil counts. The pattern of visceral metastasis and LDH are not considered here, as these are well-established prognostic markers. Kaplan Meier curves for OS are presented according the count of favorable pre-treatment values. Censoring is indicated by vertical lines. The differences in OS between categories were significant in all pairwise comparisons (all log rank p<0.001; A). The proportion of patients with objective response (PR or CR as best overall response according to RECIST V1.1) in 389 of 512 patients with available response data is presented according to the number of favorable baseline factors. An objective response was observed in 42.5%, 23.6%, and 12.5% of patients with 2, 1, or 0 favorable baseline values. The differences between the categories were all statistically significant (p=0.042 for 0 vs.1 favorable factor; p<0.001 for 1 vs 2 favorable factors) using Chi-Square/Fishers’s exact tests (B).
The independent prognostic impact of RLC and REC in addition to LDH and the pattern of visceral metastasis was analyzed in a separate Kaplan-Meier analysis after stratification of patients according to the latter two prognostic factors. An independent additional impact was observed in patients with LDH ratio ≤2.5 and no involvement of visceral metastasis other than lung (Supplementary Figure 4A) or if either one condition was true (Supplementary Figure 4B). The additional impact of REC and RLC was not statistically significant for patients with LDH-ratio >2.5 and visceral metastases not limited to the lungs (Supplementary Figure 4C).
This combination model was based on the number of favorable baseline factors and thus did not account for the differences in the relative impact among the four considered factors. Due to the observed differences in HRs ranging from 2.8 (LDH-ratio >2.5) to 2.0 for REC<17.5% and RLC<1.5, other combination models accounting for the relative impact were evaluated as well (Supplementary Figure 4). The discriminatory abilities (c-indices) of the 5 evaluated alternative models ranged between 0.779 and 0.785, and therefore compared similarly to the initial combination model based on the number of favorable baseline factors (c-index=0.782; 95% confidence interval 0.738 – 0.825).
Discussion
In the current study, the LDH-ratio, the pattern of visceral involvement, RLC and REC, represent four baseline factors independently associated with OS of melanoma patients treated with pembrolizumab. Both LDH and pattern of visceral metastases are well established prognostic factors of advanced melanoma patients and are incorporated into the AJCC staging classification.(24) An elevated pretreatment LDH has also been previously described to correlate negatively with OS in patients treated with ipilimumab (15, 16, 25) and pembrolizumab (26). Thus, LDH’s negative association with outcomes following pembrolizumab, as observed here, was not surprising. However, high pre-treatment RLC and REC were also independently associated with improved OS. Differential blood counts have not been investigated as biomarkers for pembrolizumab thus far. For ipilimumab, high ALC (15), low absolute monocyte count, and high RLC (25) at baseline, and increasing ALC during treatment (16), indicate favorable prognosis. No change of ALC or association with responses was reported for patients treated with nivolumab and ipilimumab in combination (17).
Different subsets of T cells targeting tumor-associated- or neo-antigens are involved in immunological melanoma rejection (27, 28). PD-1 is expressed on T cells after initial antigen stimulation (29). After persistent antigen stimulation, PD-1 signaling results in secondary suppression of T cells to prevent sustained proliferation and activation. Engagement occurs after binding its ligands PD-L1 or PD-L2 mainly expressed on stromal cells or antigen presenting cells in the periphery (30, 31). This physiological process can be utilized by cancer cells to escape from T cell rejection by expression of PD-L1 (32, 33). Blocking antibodies targeting PD-1 or its counterpart PD-L1 can prevent this form of T cell inactivation (1, 10, 18, 34) ensuring sustained anti-tumor activity. Of note, the number of tumor mutations and clinical outcome following pembrolizumab were recently reported to correlate in patients with non-small cell lung cancer (NSCLC) suggesting a prominent role of T cells targeting neo-epitopes (35). Thus, T cells represent the main cellular target of pembrolizumab and are required for effective pembrolizumab-induced tumor rejection (18). As T cells represent the majority of lymphocytes this is in agreement with the observed positive correlation of high lymphocyte counts throughout our analysis with OS following pembrolizumab.
Eosinophils have important functions for tumor surveillance and were described as effectors for tumor rejection in animal models (36–38). For ipilimumab, high REC at baseline was correlated with favorable OS (25, 39) and early increases during treatment was associated with improved clinical responses (40). Nevertheless, a potential role for a beneficial mode of action of pembrolizumab, which may be hypothesized based on the prognostic impact of REC in our study, needs to be investigated in greater detail.
Based on the independent prognostic impact, we analyzed OS stratified by the number of favorable results considering LDH-ratio, pattern of visceral involvement, RLC and REC. The proposed combination model allowed the identification of patient subgroups with extremely different prognoses following pembrolizumab. 13.7% of patients had favorable values in all 4 factors and an 84.2% 1-year OS probability. In strong contrast, prognosis was poor for individuals with 0–1 favorable factors, who had a chance of only 14.8% to survive the first year after start of pembrolizumab and a median survival of 2.6 months.
Our study does not address the key question of whether the number of unfavorable factors according to the proposed combination model is generally prognostic for advanced melanoma patients or specifically predictive for outcome after pembrolizumab. Based on the consideration of LDH and the pattern of visceral metastasis in the combination model, an association with prognosis can be assumed also in other clinical situations. However, the assumed prognostic association of these factors does not exclude the possibility of additional predictive impact for outcome after pembrolizumab. First, after omitting the well-established prognostic markers LDH and the pattern of visceral metastasis, a reduced model limited to RLC and REC was still strongly correlated with prognosis (Figure 3). Second, since lymphocytes are centrally embedded in the mode of action of pembrolizumab, the correlation of high RLC and favorable OS, especially for patients with low visceral tumor burden and low LDH (Supplementary Figure 3A), may ultimately be expected to be related to predictive effects after pembrolizumab. No prognostic role has yet been established for RLC in advanced melanoma patients. Third, a strong correlation of the number of favorable baseline factors with bOR was observed here, in addition to the association with OS. Only patients with partial or complete response were considered as objective responders in the current study. In contrast to the observation of improved OS, which is susceptible for confounding by subsequent treatments, and may be only prognostic, the observation of an objective response is more likely causally linked to the beneficial mode of action of the applied treatment. Thus the correlation of the count of favorable baseline biomarkers with clinical responses and OS, as observed here, underlines a potential predictive role of the biomarker signature in addition to its described prognostic role.
In total, this study included 616 patients from three independent cohorts from 30 sites and six different countries, minimizing the risk that results are confounded by patient selection, regional- or site-specific influences. Nevertheless, such a bias based on the retrospective design cannot be excluded. Potential confounding effects of the treatment line or ipilimumab pretreatment could not be analyzed due to the low number of ipilimumab-naïve patients who received pembrolizumab as first treatment for unresectable disease (n=13). Other factors which might also impact outcome (e.g. the ECOG performance status) were not analyzed here. Finally, a bias induced by patient selection procedures for treatment with pembrolizumab or for provision of data for this study cannot be excluded. Further prospective validation accounting for these limitations of our study is warranted. In strong contrast to the analysis of PD-1/PD-L1 expression in tumor tissue, the assessment of the count of favorable baseline peripheral blood routine factors is feasible and if ultimately shown to be predictive of outcome, could be easily integrated into daily clinical practice.
Nevertheless, based upon these data alone, the proposed combination model should not be used with the purpose to guide treatment decisions at this time. In addition to the inherent study limitations based on the retrospective design, it remains to be clarified as to whether patients with zero or one favorable factor (poor response rate and OS group) still derived some treatment benefit from pembrolizumab that they may not have had with alternative treatment. This question will only be able to be addressed in randomized controlled trials involving pembrolizumab and a comparator (e.g. BRAF/MEK inhibitors, chemotherapy, nivolumab, ipilimumab). In spite of the broad availability of the considered routine factors and the principle possibility to perform such studies in a retrospective setting, the value of analysis of historic cohorts is questionable due to inevitable imbalances between the cohorts and a high risk of bias. In contrast, the analysis of outcome according to baseline biomarkers combinations of patients treated with pembrolizumab compared to a control group in a randomized controlled trial setting may more conclusively clarify if there is predictive impact or not. This is in principle possible in the Keynote-002 trial or Keynote-006 trials. In the Keynote-002 trial, ipilimumab-refractory melanoma patients were randomized to receive two different doses of pembrolizumab or investigator-choice chemotherapy (5). In the Keynote-006 trial, ipilimumab-naive patients were randomized to receive pembrolizumab using two different dosing schedules or ipilimumab (3). Both trials showed a superiority regarding progression free survival (both trials) or OS (Keynote-006) of patients treated with pembrolizumab compared to alternative treatments. As all baseline data required for the proposed combination model including RLC and REC have been collected in the respective trial databases, a retrospective analysis of these data would be possible in principle. We are currently planning to perform such studies in collaboration with the sponsoring company. Ultimately, prospective testing in a randomized controlled trial is necessary to prove or exclude a definite predictive impact.
In conclusion, low pre-treatment values of LDH, limited visceral tumor burden, high REC and high RLC are independently associated with favorable OS of melanoma patients treated with pembrolizumab. Patients with favorable results in all markers have an excellent prognosis, while significant treatment advances are still needed for patients with 3–4 unfavorable baseline values, whose outcome remains poor even in the era of new immunotherapy strategies such as pembrolizumab. Whether the proposed baseline biomarker signature has specific predictive impact for outcome following pembrolizumab or rather represents a prognostic marker combination for advanced melanoma patients is unclear but provides a foundation for investigation in randomized controlled trials.
Supplementary Material
Statement of translational relevance.
This study reports a prognostic model for melanoma patients treated with pembrolizumab involving four baseline factors easily available in clinical practice: the pattern of visceral metastases; serum levels of LDH; relative lymphocyte count; and relative eosinophil count. The probability to survive 12 months after the first dose was 15% in patients with none out of four favorable factors, in contrast to 84% for patients with all four favorable factors present. Our results improve patient counselling. Despite the large differences in outcome according to the number of favorable factors, it remains unclear whether the combination model is predictive of pembrolizumab treatment benefit. This model warrants investigation in randomized controlled trials of pembrolizumab to determine whether it has predictive benefit that may help to guide treatment decisions.
Acknowledgments
The following people provided data to the project but are not represented in the author list: Steven Goetze: Department of Dermatology, Friedrich Schiller University, Jena, Germany. Cornelia Mauch, Max Schlaak: Department of Dermatology, University Hospital of Cologne, Cologne, Germany. Frank Meiß: Department of Dermatology, University Medical Center Freiburg, Freiburg, Germany. Nadine Went: Department of Dermatology, Hannover Medical School, Hannover, Germany. Dirk Debus, Jasmin Jähner: Department of Dermatology, Klinikum Nord, Nürnberg, Germany. Claudia Pföhler: Department of Dermatology, Saarland University Hospital, Homburg, Germany. Suvada Biserovic, Evelyn Dabrowski, Edgar Dippel: Department of Dermatology, Ludwigshafen Hospital, Ludwigshafen, Germany. Ursula Dietrich: Department of Dermatology, University Medical Center Dresden, Dresden, Germany. Carsten Schulz: Department of Dermatology, University Medical Center Heidelberg, Heidelberg, Germany. Sebastian Haferkamp: Department of Dermatology, University Medical Center Regensburg, Regensburg, Germany. Carsten Weishaupt: Department of Dermatology, University Medical School, Münster, Germany. Patrick Terheyden: Department of Dermatology, University of Lübeck, Lübeck, Germany. Iris Pönitzsch: Department of Dermatology, Medical Faculty of the Leipzig University, Leipzig, Germany. Amir Khammari: University Hospital, Nantes, France. Jennifer Landsberg: University Medical Center Bonn, Bonn, Germany. Marcello Curvietto: Istituto Nazionale Tumori Fondazione Pascale, Naples, Italy. Tabea Wilhelm: Skin Cancer Center Charité, Berlin, Germany.
Financial Support: This work was support by a research grant of the Hiege foundation against skin cancer, Hamburg, Germany and was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
BW reports grants from Merck/MSD, during the conduct of the study; grants and personal fees from Bristol Myers Squibb, personal fees from Philogen, personal fees from Roche, outside the submitted work; JCH reports personal fees from BMS, MSD, Roche, GSK/Novartis, Amgen, outside the submitted work; CaB reports personal fees from AstraZeneca, personal fees from Amgen, personal fees from BMS, personal fees from Roche, personal fees from GSK, personal fees from MSD, personal fees from Novartis, outside the submitted work; MAP reports grants from Bristol-Myers Squibb, personal fees from Bristol-Myers Squibb, outside the submitted work; BS reports other from MSD, outside the submitted work; KK reports personal fees from MSD, personal fees from BMS, during the conduct of the study; LH reports other from MSD, other from BMS, other from GSK, other from Roche, outside the submitted work; and speakers fees from MSD; RG reports grants from Novartis, Pfizer, Roche, Johnson & Johnson, personal fees from Roche, BMS, GSK, Novartis, MerckSerono, MSD, Almirall, Amgen, Galderma, Janssen, Boehringer Ingelheim, Pfizer, personal fees from Roche, BMS, MSD, LEO, GSK, Amgen, Novartis, Almirall, personal fees from Roche; BMS, MSD, outside the submitted work; AB reports personal fees from pharmaceutic industry, outside the submitted work; ChG reports other from BMS, other from GSK, other from Novartis, outside the submitted work; MM reports grants and personal fees from BMS, grants and personal fees from Roche, personal fees from GSK, grants and personal fees from MedImmune, outside the submitted work; CB reports personal fees from MSD, personal fees from BMS, personal fees from GSK, personal fees from Roche, grants and personal fees from Novartis, outside the submitted work; DS reports personal fees from Amgen, GSK, BMS, Novartis, Roche, Amgen, Merck, outside the submitted work; RD reports grants from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Roche, grants and personal fees from GlaxoSmithKline, personal fees from Merck Sharp & Dhome, personal fees from Amgen, outside the submitted work; PAA reports grants and personal fees from Bristol Myers Squibb, grants and personal fees from Roche-Genentech, personal fees from Merck Sharp & Dohme, grants and personal fees from Ventana, personal fees from GSK, personal fees from Novartis, personal fees from Amgen, outside the submitted work; GH reports personal fees and non-financial support from MSD, personal fees and non-financial support from BMS, personal fees and non-financial support from Roche, outside the submitted work; CG reports grants and personal fees from Merck, during the conduct of the study; personal fees from Amgen, grants and personal fees from BMS, personal fees from Novartis, grants and personal fees from Roche, grants and personal fees from GSK, outside the submitted work; JW reports grants from Bristol Myers Squibb, grants from Merck, during the conduct of the study; grants and other from Bristol Myers Squibb, grants and other from Merck, outside the submitted work.
Footnotes
Declaration of interests: AM, KB, ES, JM, AMDG, NB, ER, FM, PM have nothing to disclose.
References
- 1.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 7.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–8. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. ASCO Meeting Abstracts. 2014;32:3005. [Google Scholar]
- 13.Daud AI, Hamid O, Ribas A, Hodi FS, Hwu W-J, Kefford R, et al. Abstract CT104: Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma(MEL): Correlation of tumor PD-L1 expression with outcome. Cancer Research. 2014;74:CT104. [Google Scholar]
- 14.Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–53. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 15.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63:449–58. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 17.Callahan MK, Horak CE, Curran MA, Hollman T, Schaer DA, Yuan J, et al. Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. ASCO Meeting Abstracts. 2013;31:3003. [Google Scholar]
- 18.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PL, Roh W, Reuben A, Spencer CNJH, Lazar A, Davies MMJP, Wani K, Hwu P, Patel S, Woodman S, Glitza I, Hwu WJ, Cooper ZA, Allison J, Sharma P, Wistuba I, Blando JPV, Tetzlaff M, Amaria R, Futreal A, Chin L, Wargo JA. Society for Melanoma Research 2015 Congress. Pigment Cell & Melanoma Research. 2015;28:763. [Google Scholar]
- 20.Vilain RE, Kakavand H, Menzies AM, Madore J, Wilmott J, Dobney R, et al. 3305 PD1 inhibition-induced changes in melanoma and its associated immune infiltrate. European Journal of Cancer. 51:S666. [Google Scholar]
- 21.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens A, Wistuba-Hamprecht K, Geukes Foppen MH, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–61. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30:1835–41. doi: 10.1200/JCO.2011.40.2271. [DOI] [PubMed] [Google Scholar]
- 29.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 31.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–9. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 37.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–13. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 38.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–17. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 39.Schindler K, Harmankaya K, Postow MA, Frantal S, Bello D, Ariyan CE, et al. Pretreatment levels of absolute and relative eosinophil count to improve overall survival (OS) in patients with metastatic melanoma under treatment with ipilimumab, an anti CTLA-4 antibody. ASCO Meeting Abstracts. 2013;31:9024. [Google Scholar]
- 40.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


