Abstract
Objective
Previous genetic lineage tracing studies showed that Sox10+ cells differentiate into vascular mural cells, limited to neural crest-derived blood vessels in craniofacial tissues, aortic arch, pulmonary arch arteries, brachiocephalic, carotid arteries and thymus. The purpose of this study was to investigate the contribution of Sox10+ cells to the vascular development in other tissues and organs and their relationship with neural crest.
Approach and Results
Using genetic lineage tracing technique based on Cre/LoxP system, we examined blood vessels of the adult organs of Sox10-Cre/Rosa-LoxP-RFP and Wnt1-Cre/Rosa-LoxP-RFP mice by immunohistological analysis. In addition to previously reported tissues and organs derived from neural crest, we showed that Sox10+ cells also contributed to vascular mural cells in the lung, spleen and kidney, which are derived from non-neural crest origin as evidenced by RFP- blood vessels in these three organs of Wnt1-Cre/Rosa-LoxP-RFP mice.
Conclusions
This study demonstrates that Sox10+ cells contribute to pericytes and smooth muscle cells in most parts of the body, including those from both neural crest and non-neural crest, which has significant implications in vascular remodeling under physiological and pathological conditions.
Keywords: blood vessel, pericyte, smooth muscle cell
Vascular smooth muscle cells (SMCs) have been reported to have heterogeneity in phenotype, marker expression and their origins.1–3 During development, SMCs are mainly derived from mesodermal and neural crest cells.1–3 Using quail-chick chimera techniques, several research groups found that neural crest cells gave rise to pericytes and SMCs in craniofacial region, aortic arch and pulmonary arch arteries, brachiocephalic and carotid arteries.3–5 In recent years, genetic lineage tracing mouse models based on Cre/LoxP system have been developed to label neural crest cells during development, such as Wnt1-Cre and Sox10-Cre/CreER. Wnt1 and Sox10 are expressed in early and post migratory neural crest cells respectively.6–8 Wnt1 has been generally thought to be a strict marker for neural crest cells and it is not expressed after neural crest cells migrate away from the neural tube.6 In contrast, Sox10 is expressed in both neural crest and other non-neural crest lineages, such as oligodendrocytes and inner ear, during development,7, 9 and its expression continues in later development and adult tissues, mainly in the central and peripheral nervous systems,10 which was demonstrated by several reporter mouse lines, such as Sox10-rtTA,11 Sox10-Venus,12 Sox10-Cre13 and Sox10-CreER14. Studies on vascular development using Sox10-Cre/CreER mice showed that Sox10+ cells differentiated into pericytes and SMCs in the brain,14 eye,15 thymus,16, 17 ascending aorta, aorta arch, carotid arteries13, and the neural crest origin of these cells were verified by the studies on quail-chick chimera or Wnt1-Cre mice.3–5, 16, 18 In addition, the study on Wnt1-Cre mice also showed that neural crest cells give rise to SMCs of coronary arteries in the heart,18 although there is no such report from Sox10-Cre mice.
In our recent studies, Sox10+ adult stem cells were identified in many large blood vessels all over the body after vascular injury or in vitro culture,19–21 which raised questions on whether Sox10+ cells participate in general vascular development and whether they are derived from neural crest. To address these questions, we generated Sox10-Cre/Rosa-LoxP-Red fluorescent protein (RFP) and Wnt1-Cre/Rosa-LoxP-RFP mice and examined RFP+ blood vessels in the adult organs of each mouse line by immunohistological analysis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
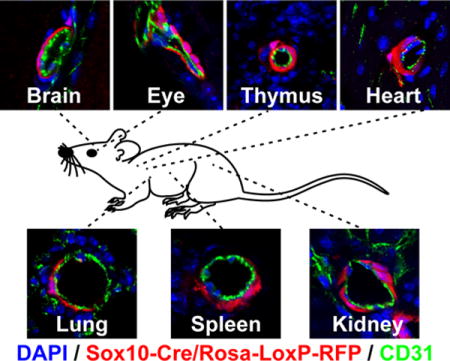
We first examined the blood vessels of Sox10-Cre/Rosa-LoxP-RFP mice. It was consistent with previous reports that RFP labeled SMCs of the carotid artery of Sox10-Cre/Rosa-LoxP-RFP mice, which were immunostained by smooth muscle myosin heavy chain (MYH11), a marker of mature SMCs (Figure 1, A). The SMCs of the descending thoracic aorta (Figure 1, B) and femoral artery (Figure 1, C) of Sox10-Cre/Rosa-LoxP-RFP mice were not labeled by RFP (MYH11+RFP-). In the brain, retina, thymus and heart of Sox10-Cre/Rosa-LoxP-RFP mice, RFP+ cells wrapped the blood vessels of different sizes and expressed NG2, smooth muscle α-actin (ACTA2) and MYH11, suggesting Sox10+ cells or their derivatives differentiated into pericytes and SMCs in these organs (Figure 1, D–G; Supplemental Figure I–II). It was unexpected that RFP also labeled the vascular mural cells of the lung, spleen, kidney (Figure 1, H–J; Supplemental Figure I–II), suggesting that Sox10+ cells or their derivatives participate in vascular development in these organs. Quantification analysis showed that there was a big variation of the percentages of RFP+ vessels in these organs (Figure 1, K), which may suggest distinct contributions of Sox10+ cells to vascular development in different organs. To examine whether these RFP+ perivascular cells were currently expressing Sox10, we performed immunostaining with the antibody against Sox10. We could hardly find any RFP+Sox10+ vascular mural cells (data not shown), implying that they already differentiated and lost Sox10 expression in adult tissues. Few RFP+Sox10+ cells could be found sparsely in the interstitial space of subcutaneous loose connective tissues (Supplemental Figure III). However, these results cannot exclude the possibility that dormant stem cells exist in the tissue and may become Sox10+ upon activation.
Figure 1.
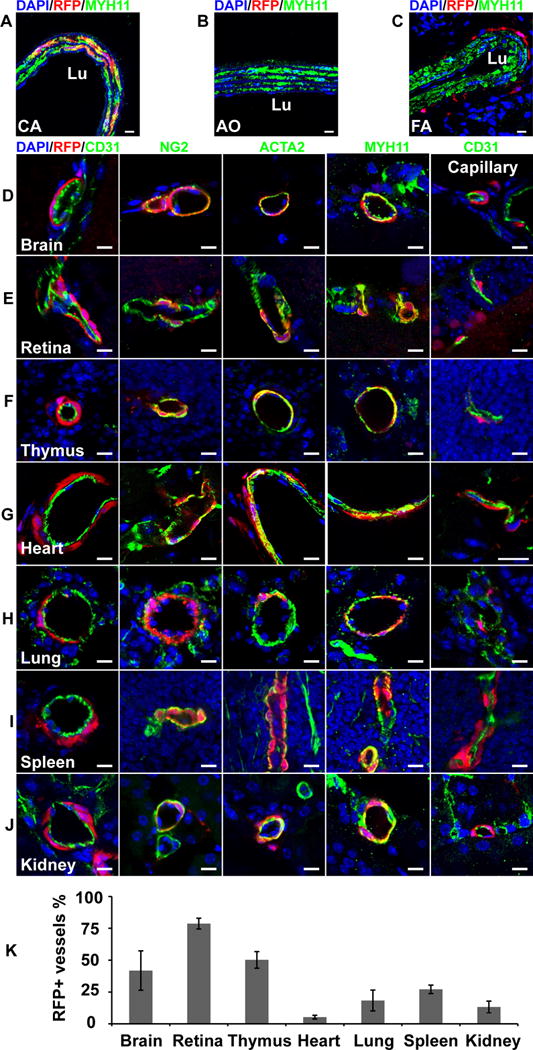
The cross sections of large vessels and different organs of Sox10-Cre/Rosa-LoxP-RFP mice were immunostained by the antibodies against smooth muscle myosin heavy chain (MYH11), CD31, NG2 and smooth muscle a-actin (ACTA2). Cell nuclei were stained by DAPI. The tissues and organs include the carotid artery (A, CA), descending thoracic aorta (B, AO), femoral artery (C, FA), brain (D), retina (E), thymus (F), heart (G), lung (H), spleen (I) and kidney (J). Scale bar, 10 μm. (K) Percentages of RFP+ vessels in different organs (n=6). Data were reported as means ± standard deviation (s.d.).
To investigate whether these RFP+ vascular mural cells of Sox10-Cre/Rosa-LoxP-RFP mice are derived from neural crest, we examined another neural crest reporter line, Wnt1-Cre/Rosa-LoxP-RFP. Immunohistological analysis showed RFP-labeled vascular mural cells in the carotid artery, brain, retina, thymus and heart of Wnt1-Cre/Rosa-LoxP-RFP mice (Figure 2, A, D–G, Supplemental Figure IV). However, no RFP+ vascular mural cells were found in the descending thoracic aorta, femoral artery, lung, spleen and kidney of Wnt1-Cre/Rosa-LoxP-RFP mice (Figure 2, B, C, H–J, Supplemental Figure IV). In addition, we did not find RFP+ blood vessels in the liver of both mouse lines. These results suggest that non-neural crest Sox10+ progenitor cells may differentiate into vascular mural cells in the lung, spleen and kidney of mice during development.
Figure 2.
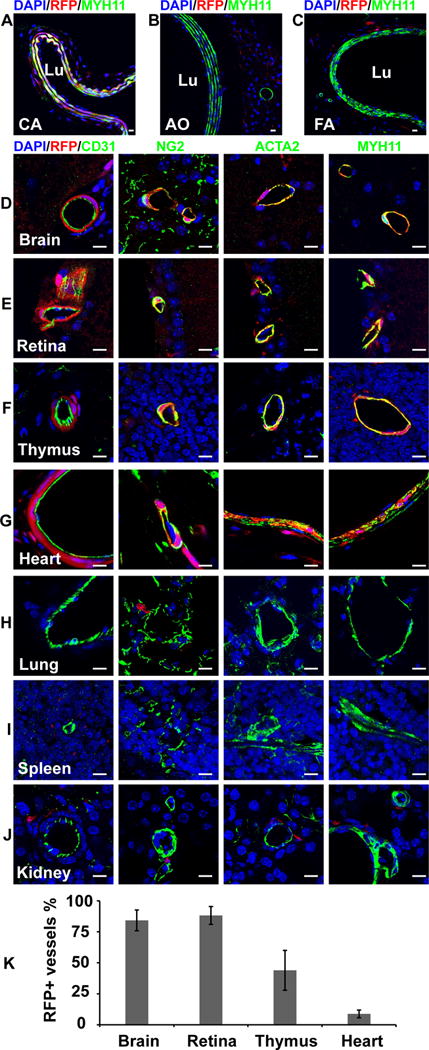
The cross sections of large vessels and different organs of Wnt1-Cre/Rosa-LoxP-RFP mice were immunostained by the antibodies against MYH11, CD31, NG2 and ACTA2. Cell nuclei were stained by DAPI. The tissues and organs include the carotid artery (A, CA), descending thoracic aorta (B, AO), femoral artery (C, FA), brain (D), retina (E), thymus (F), heart (G), lung (H), spleen (I) and kidney (J). Scale bar, 10 μm. (K) Percentages of RFP+ vessels in different organs (n=3). Data were reported as means ± standard deviation (s.d.).
Discussion
Sox10 is expressed in neural crest cells, oligodendrocytes and otic vesicle during development, and its expression is mainly in the central and peripheral nervous systems of adult.3–5, 10–14 In recent years, accumulating studies showed that Sox10+ stem cells exist in multiple adult tissues and participate in tissue remodeling, including spinal cord,12 inferior turbinate,22 periodontal ligament,23 hair follicle,24, 25 mammary epithelium,26 bone marrow,27 blood vessel walls19–21, 28 and subcutaneous loose connective tissues.29 Moreover, Sox10 also plays a role in tumor formation of melanoma.30 Lineage tracing and advanced imaging techniques have been widely used to study cell differentiation during development.31 Here, by using Sox10-Cre/Rosa-LoxP-RFP and Wnt1-Cre/Rosa-LoxP-RFP mice, we found Sox10+ cells or their derivatives could differentiate into pericytes and SMCs in multiple tissues and organs, including those from neural crest: the carotid artery, brain, eye, thymus, heart, and also those from non-neural crest origin: the lung, spleen and kidney. All these studies indicate a broader role of Sox10+ cells in vascular development and the potential role of Sox10+ cells in adult tissue remodeling.
We found a big variation in the percentages of RFP+ vessels in different organs. The high percentages of RFP+ blood vessels in the brain, eye and thymus of Sox10-Cre/Rosa-LoxP-RFP and Wnt1-Cre/Rosa-LoxP-RFP mice are consistent with previous quail-chick chimera studies that these organs are derived from neural crest.3–5 The low percentage of RFP+ blood vessels in the heart of both mouse lines is consistent with previous report that neural crest cells only have a small contribution to coronary vessels.18 Although our results suggest that Sox10+ cells only contribute to low numbers of blood vessels in the lung, spleen and kidney, and have no contribution to liver vasculature, during normal development, the numbers may go up upon cell expansion following injuries or diseases.19–21 It is worth noting that there could be organ-specific regulatory mechanisms. Studies of Sox10 distal cis-regulatory element of the progenitor of vascular mural cells may also provide more insights into vascular remodeling.
Heterogeneity has been reported in pericytes and SMCs, even in the same organs.1, 2, 32 For example, brain pericytes have distinct morphologies, markers and functions along the arteriole-capillary-venule vasculature bed.33 The expression of ACTA2 is weak in the brain pericytes in the middle of capillary bed; however, those in the arteriole-capillary interface express strong ACTA2 and play an important role in blood flow control.33 The brain vascular mural cells in the arteriole-capillary interface have the properties of both pericytes and SMCs, which makes it hard to define their identities as pericytes or SMCs and causes controversies between research groups.33–35 Further study is needed to investigate the contribution of Sox10+ cells to different subpopulations of vascular mural cells, which may help understand the heterogeneity of vascular mural cells.
Another Sox10 reporter mouse line, Sox10-Venus, was developed for real-time monitoring Sox10 expression, which is a useful tool for investigating Sox10+ cells during tissue remodeling especially after injury.12 Our study suggests that Sox10+ cells do not participate in liver vascular development, which is consistent with the results from Sox10-Venus mice.12 The transgene-driven reporter patterns of Sox10-Venus and Sox10-Cre mice are similar as illustrated by previous reports and current study,12, 13 although there may be minor differences as a consequence of position effect, which needs further investigation.
In conclusion, our results suggest that Sox10+ cells contribute to vascular development in most but not all the tissues and organs including those from neural crest and non-neural crest origin. Because this Sox10-Cre mouse is not inducible, we could not determine at which time point Sox10 is activated. Sox10-Venus and inducible Sox10-CreER mice will be valuable for the future studies on the role of Sox10+ cells and their derivatives during vascular development and adult tissue remodeling.
Supplementary Material
Highlights.
Sox10+ cells differentiate into pericytes and smooth muscle cells in multiple tissues and organs, including the carotid artery, brain, eye, thymus, heart, lung, spleen and kidney.
Sox10+ vascular progenitors in the lung, spleen and kidney are derived from non-neural crest origin.
Sox10+ cells contribute to vascular development of different organs to different extent.
Acknowledgments
We thank Mary West for technical assistance. Confocal microscopy was performed at the CIRM/QB3 Shared Stem Cell Facility and Gong Lab at UC Berkeley, and the CNSI Advanced Light Microscopy / Spectroscopy Shared Resource Facility at UCLA.
Source of support:
This work was supported by NIH grants (HL117213 and HL121450 to S.L.), UCLA startup fund (to S.L.) and Siebel Fellowship (to D.W.).
Abbreviations
- SMC
Smooth muscle cell
- RFP
Red fluorescent protein
- ACTA2
Smooth muscle α-actin
- MYH11
Smooth muscle myosin heavy chain
Footnotes
Subject code: [97] Other Vascular biology
Disclosure: None.
References
- 1.Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- 2.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 3.Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- 4.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 5.Korn J, Christ B, Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol. 2002;442:78–88. doi: 10.1002/cne.1423. [DOI] [PubMed] [Google Scholar]
- 6.Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing wnt-1 expression in the developing mouse cns. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- 7.Pusch C, Hustert E, Pfeifer D, Sudbeck P, Kist R, Roe B, Wang Z, Balling R, Blin N, Scherer G. The sox10/sox10 gene from human and mouse: Sequence, expression, and transactivation by the encoded hmg domain transcription factor. Hum Genet. 1998;103:115–123. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- 8.Hall BK. The neural crest and neural crest cells in vertebrate development and evolution. Springer US: 2009. Embryological origins and the identification of neural crest cells. [Google Scholar]
- 9.Breuskin I, Bodson M, Thelen N, Thiry M, Borgs L, Nguyen L, Lefebvre PP, Malgrange B. Sox10 promotes the survival of cochlear progenitors during the establishment of the organ of corti. Dev Biol. 2009;335:327–339. doi: 10.1016/j.ydbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig A, Schlierf B, Schardt A, Nave KA, Wegner M. Sox10-rtta mouse line for tetracycline-inducible expression of transgenes in neural crest cells and oligodendrocytes. Genesis. 2004;40:171–175. doi: 10.1002/gene.20083. [DOI] [PubMed] [Google Scholar]
- 12.Shibata S, Yasuda A, Renault-Mihara F, Suyama S, Katoh H, Inoue T, Inoue YU, Nagoshi N, Sato M, Nakamura M, Akazawa C, Okano H. Sox10-venus mice: A new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain. 2010;3:31. doi: 10.1186/1756-6606-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stine ZE, Huynh JL, Loftus SK, Gorkin DU, Salmasi AH, Novak T, Purves T, Miller RA, Antonellis A, Gearhart JP, Pavan WJ, McCallion AS. Oligodendroglial and pan-neural crest expression of cre recombinase directed by sox10 enhancer. Genesis. 2009;47:765–770. doi: 10.1002/dvg.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon C, Lickert H, Gotz M, Dimou L. Sox10-icreert2 : A mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis. 2012;50:506–515. doi: 10.1002/dvg.22003. [DOI] [PubMed] [Google Scholar]
- 15.Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, Stolt CC, Wegner M, Bogner B, Kaser-Eichberger A, Krefft K, Runge C, Aigner L, Reitsamer HA. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54:7910–7921. doi: 10.1167/iovs.13-12946. [DOI] [PubMed] [Google Scholar]
- 16.Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- 17.Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 19.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Z, Wang A, Wang D, Li S. Smooth muscle cells: To be or not to be? Response to nguyen et al. Circ Res. 2013;112:23–26. doi: 10.1161/CIRCRESAHA.112.281055. [DOI] [PubMed] [Google Scholar]
- 21.Yuan F, Wang D, Xu K, Wang J, Zhang Z, Yang L, Yang GY, Li S. Contribution of vascular cells to neointimal formation. PLoS One. 2017;12:e0168914. doi: 10.1371/journal.pone.0168914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauser S, Widera D, Qunneis F, et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012;21:742–756. doi: 10.1089/scd.2011.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomokiyo A, Maeda H, Fujii S, Monnouchi S, Wada N, Kono K, Yamamoto N, Koori K, Teramatsu Y, Akamine A. A multipotent clonal human periodontal ligament cell line with neural crest cell phenotypes promotes neurocytic differentiation, migration, and survival. J Cell Physiol. 2012;227:2040–2050. doi: 10.1002/jcp.22933. [DOI] [PubMed] [Google Scholar]
- 24.Clewes O, Narytnyk A, Gillinder KR, Loughney AD, Murdoch AP, Sieber-Blum M. Human epidermal neural crest stem cells (hepi-ncsc)–characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev. 2011;7:799–814. doi: 10.1007/s12015-011-9255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Liu JY, Wang S, Xu H, Cui L, Lv S, Xu J, Liu S, Chi G, Li Y. Multipotent neural crest stem cell-like cells from rat vibrissa dermal papilla induce neuronal differentiation of pc12 cells. Biomed Res Int. 2014;2014:186239. doi: 10.1155/2014/186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dravis C, Spike BT, Harrell JC, Johns C, Trejo CL, Southard-Smith EM, Perou CM, Wahl GM. Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep. 2015;12:2035–2048. doi: 10.1016/j.celrep.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wislet-Gendebien S, Laudet E, Neirinckx V, Alix P, Leprince P, Glejzer A, Poulet C, Hennuy B, Sommer L, Shakhova O, Rogister B. Mesenchymal stem cells and neural crest stem cells from adult bone marrow: Characterization of their surprising similarities and differences. Cell Mol Life Sci. 2012;69:2593–2608. doi: 10.1007/s00018-012-0937-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Kennedy E, Mooney CJ, Hakimjavadi R, Fitzpatrick E, Guha S, Collins LE, Loscher CE, Morrow D, Redmond EM, Cahill PA. Adult vascular smooth muscle cells in culture express neural stem cell markers typical of resident multipotent vascular stem cells. Cell Tissue Res. 2014;358:203–216. doi: 10.1007/s00441-014-1937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Wang A, Wu F, Qiu X, Li Y, Chu J, Huang WC, Xu K, Gong X, Li S. Sox10+ adult stem cells contribute to biomaterial encapsulation and microvascularization. Sci Rep. 2017;7:40295. doi: 10.1038/srep40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shakhova O, Zingg D, Schaefer SM, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- 31.Fink J, Andersson-Rolf A, Koo BK. Adult stem cell lineage tracing and deep tissue imaging. BMB Rep. 2015;48:655–667. doi: 10.5483/BMBRep.2015.48.12.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias Moura Prazeres PH, Sena IFG, Borges IdT, de Azevedo PO, Andreotti Julia P, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro dos Santos GS, Mintz A, Delbono O, Birbrair A. Pericytes are heterogeneous in their origin within the same tissue. Developmental Biology. 2017;427:6–11. doi: 10.1016/j.ydbio.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2:041402. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? Journal of Cerebral Blood Flow & Metabolism. 2016;36:451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


